Sialylated Glycan Bindings from SARS-CoV-2 Spike Protein to Blood and Endothelial Cells Govern the Severe Morbidities of COVID-19
David E Scheim, Paola Vottero, Alessandro D Santin, Allen G Hirsh
International Journal of Molecular Sciences, doi:10.3390/ijms242317039
Consistent with well-established biochemical properties of coronaviruses, sialylated glycan attachments between SARS-CoV-2 spike protein (SP) and host cells are key to the virus's pathology. SARS-CoV-2 SP attaches to and aggregates red blood cells (RBCs), as shown in many pre-clinical and clinical studies, causing pulmonary and extrapulmonary microthrombi and hypoxia in severe COVID-19 patients. SARS-CoV-2 SP attachments to the heavily sialylated surfaces of platelets (which, like RBCs, have no ACE2) and endothelial cells (having minimal ACE2) compound this vascular damage. Notably, experimentally induced RBC aggregation in vivo causes the same key morbidities as for severe COVID-19, including microvascular occlusion, blood clots, hypoxia and myocarditis. Key risk factors for COVID-19 morbidity, including older age, diabetes and obesity, are all characterized by markedly increased propensity to RBC clumping. For mammalian species, the degree of clinical susceptibility to COVID-19 correlates to RBC aggregability with p = 0.033. Notably, of the five human betacoronaviruses, the two common cold strains express an enzyme that releases glycan attachments, while the deadly SARS, SARS-CoV-2 and MERS do not, although viral loads for COVID-19 and the two common cold infections are similar. These biochemical insights also explain the previously puzzling clinical efficacy of certain generics against COVID-19 and may support the development of future therapeutic strategies for COVID-19 and long COVID patients.
Abbreviations The following abbreviations are used in this manuscript:
References
Abassi, Higazi, Kinaneh, Armaly, Skorecki et al., ACE2, COVID-19 Infection, Inflammation, and Coagulopathy: Missing Pieces in the Puzzle, Front. Physiol,
doi:10.3389/fphys.2020.574753Ackermann, Verleden, Kuehnel, Haverich, Welte et al., Pulmonary Vascular Endothelialitis, Thrombosis, and Angiogenesis in COVID-19, N. Engl. J. Med,
doi:10.1056/NEJMoa2015432Ackerstaff, Keunen, Pelt, Swijndregt, Stijnen, Influence of biological factors on changes in mean cerebral blood flow velocity in normal ageing: A transcranial Doppler study, Neurol. Res,
doi:10.1080/01616412.1990.11739941Ahmetaj-Shala, Vaja, Atanur, George, Kirkby et al., Systemic analysis of putative SARS-CoV-2 entry and processing genes in cardiovascular tissues identifies a positive correlation of BSG with age in endothelial cells, bioRxiv,
doi:10.1101/2020.06.23.165324Al-Samkari, Karp Leaf, Dzik, Carlson, Fogerty et al., COVID-19 and coagulation: Bleeding and thrombotic manifestations of SARS-CoV-2 infection, J. Thromb. Haemost,
doi:10.1111/jth.14844Alvarez, Gluck, Fallet, Grégoire, Chevalier et al., Plasma serotonin level after 1 day of fluoxetine treatment: A biological predictor for antidepressant response?, Psychopharmacology,
doi:10.1007/s002130050924Ami, Barshtein, Zeltser, Goldberg, Shapira et al., Parameters of red blood cell aggregation as correlates of the inflammatory state, Am. J. Physiol.-Heart Circ. Physiol,
doi:10.1152/ajpheart.2001.280.5.H1982Aminpour, Cannariato, Safaeeardebili, Preto, Moracchiato et al., In Silico Analysis of the Multi-Targeted Mode of Action of Ivermectin and Related Compounds, Computation,
doi:10.3390/computation10040051Anderson, Brodsky, Mangalmurti, The Evolving Erythrocyte: Red Blood Cells as Modulators of Innate Immunity, J. Immunol,
doi:10.4049/jimmunol.1800565Annunziata, Coppola, Carannante, Simioli, Lanza et al., Home Management of Patients with Moderate or Severe Respiratory Failure Secondary to COVID-19, Using Remote Monitoring and Oxygen with or without HFNC, Pathogens,
doi:10.3390/pathogens10040413Aoki, Iwasawa, Hagiwara, Komatsu, Utsunomiya et al., Pulmonary vascular enlargement and lesion extent on computed tomography are correlated with COVID-19 disease severity, Jpn. J. Radiol,
doi:10.1007/s11604-020-01085-2Atilgan, Goker, Hondur, Kosekahya, Kocer et al., Evaluation of the radial peripapillary capillary density in unilateral branch retinal vein occlusion and the unaffected fellow eyes, Ther. Adv. Ophthalmol,
doi:10.1177/25158414221090092Au Sam, Storey Brian, Moore John, Tang, Chen et al., Clusters of circulating tumor cells traverse capillary-sized vessels, Proc. Natl. Acad. Sci
Aung, Aitken, Teh, Yu, Ofori-Asenso et al., Angiotensin converting enzyme genotypes and mortality from COVID-19: An ecological study, J. Infect,
doi:10.1016/j.jinf.2020.11.012Babalola, Karu, Personal communication
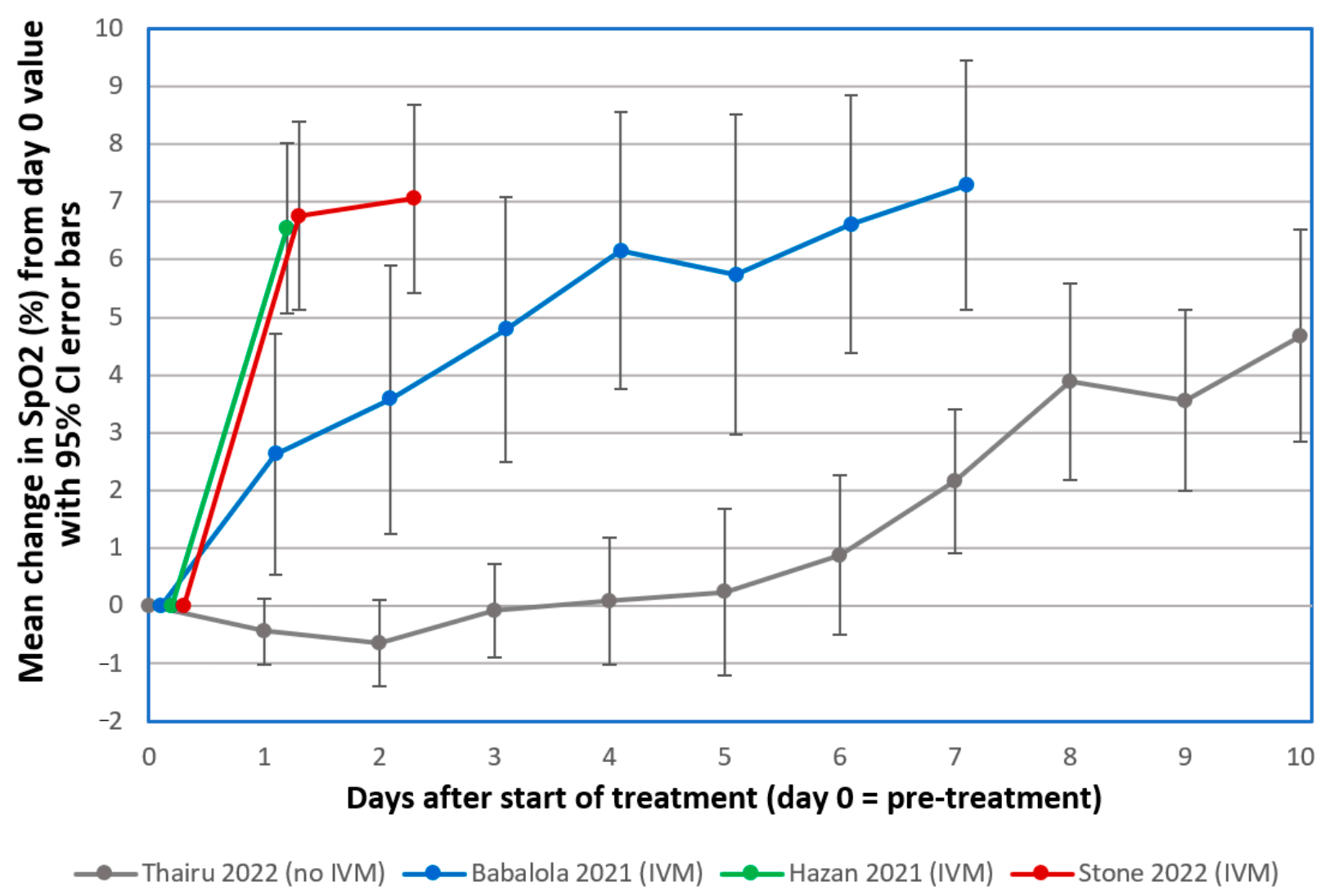
Babalola, Ndanusa, Adesuyi, Ogedengbe, Thairu et al., A Randomized Controlled Trial of Ivermectin Monotherapy Versus HCQ, IVM, and AZ Combination Therapy in COVID-19 Patients in Nigeria, J. Infect. Dis. Epidemiol,
doi:10.23937/2474-3658/1510233Baker, Richards, Guy, Congdon, Hasan et al., The SARS-CoV-2 Spike Protein Binds Sialic Acids and Enables Rapid Detection in a Lateral Flow Point of Care Diagnostic Device, ACS Cent. Sci,
doi:10.1021/acscentsci.0c00855Barshtein, Ben-Ami, Yedgar, Role of red blood cell flow behavior in hemodynamics and hemostasis, Expert Rev. Cardiovasc. Ther,
doi:10.1586/14779072.5.4.743Barshtein, Wajnblum, Yedgar, Kinetics of linear rouleaux formation studied by visual monitoring of red cell dynamic organization, Biophys. J,
doi:10.1016/S0006-3495(00)76791-9Baskurt, Meiselman, Erythrocyte aggregation: Basic aspects and clinical importance, Clin. Hemorheol. Microcirc,
doi:10.3233/CH-2012-1573Baumeier, Aleshcheva, Harms, Gross, Hamm et al., Intramyocardial Inflammation after COVID-19 Vaccination: An Endomyocardial Biopsy-Proven Case Series, Int. J. Mol. Sci,
doi:10.3390/ijms23136940Berndt, Kyle, Ling, The Long Shadow of Patent Expiration: Generic Entry and Rx-to-OTC Switches, Scanner Data and Price Indexes
Berzuini, Bianco, Migliorini, Maggioni, Valenti et al., Red blood cell morphology in patients with COVID-19-related anaemia, Blood Transfus
Bicher, Chapter I: Red cell aggregation in thrombotic disease, trauma and shock
Bicher, Chapter II: Pathological significance of intravascular red cell aggregation
Bicher, Chapter III: Mechanism of red cell aggregation
Bishop, Nance, Popel, Intaglietta, Johnson, Effect of erythrocyte aggregation on velocity profiles in venules, Am. J. Physiol.-Heart Circ. Physiol,
doi:10.1152/ajpheart.2001.280.1.H222Bjoerk, Intonti, Nordlund, Correlation between sludge in the bulbar conjunctiva and the mesentery, Ann. Surg
Bonanad, García-Blas, Tarazona-Santabalbina, Sanchis, Bertomeu-González et al., The Effect of Age on Mortality in Patients With COVID-19: A Meta-Analysis With 611,583 Subjects, J. Am. Med. Dir. Assoc,
doi:10.1016/j.jamda.2020.05.045Boschi, Scheim, Bancod, Militello, Bideau et al., SARS-CoV-2 Spike Protein Induces Hemagglutination: Implications for COVID-19 Morbidities and Therapeutics and for Vaccine Adverse Effects, Int. J. Mol. Sci,
doi:10.3390/ijms232415480Bosco-Lauth, Walker, Guilbert, Porter, Hartwig et al., Susceptibility of livestock to SARS-CoV-2 infection, Emerg. Microbes Infect,
doi:10.1080/22221751.2021.2003724Bosek, Ziomkowska, Pyskir, Wybranowski, Pyskir et al., Relationship between red blood cell aggregation and dextran molecular mass, Sci. Rep,
doi:10.1038/s41598-022-24166-wBramante, Buse, Liebovitz, Nicklas, Puskarich et al., Outpatient treatment of COVID-19 and incidence of post-COVID-19 condition over 10 months (COVID-OUT): A multicentre, randomised, quadruple-blind, parallel-group, phase 3 trial, Lancet Infect. Dis,
doi:10.1016/S1473-3099(23)00299-2Brian, Hogue, Kienzle, The Coronavirus Hemagglutinin Esterase Glycoprotein
Brooks, Evans, Rheology of blood cells
Bua, Messina, Sturiale, Barone, Garozzo et al., Glycomics of Human Erythrocytes, Int. J. Mol. Sci,
doi:10.3390/ijms22158063Buryachkovskaya, Lomakin, Melkumyants, Docenko, Ermishkin et al., Enoxaparin dose impacts blood cell phenotypes during mild SARS-CoV-2 infection: The observational single-center study, Rev. Cardiovasc. Med,
doi:10.31083/j.rcm2204176Bussani, Schneider, Zentilin, Collesi, Ali et al., Persistence of viral RNA, pneumocyte syncytia and thrombosis are hallmarks of advanced COVID-19 pathology, eBioMedicine,
doi:10.1016/j.ebiom.2020.103104Campbell, Boilard, Rondina, Is there a role for the ACE2 receptor in SARS-CoV-2 interactions with platelets?, J. Thromb. Haemost,
doi:10.1111/jth.15156Campbell, History of avermectin and ivermectin, with notes on the history of other macrocyclic lactone antiparasitic agents, Curr. Pharm. Biotechnol,
doi:10.2174/138920112800399095Cantuti-Castelvetri, Ojha, Pedro, Djannatian, Franz et al., Neuropilin-1 facilitates SARS-CoV-2 cell entry and infectivity, Science,
doi:10.1126/science.abd2985Carlisle, False individual patient data and zombie randomised controlled trials submitted to Anaesthesia, Anaesthesia,
doi:10.1111/anae.15263Carneiro, Cook, Murphy, Blakely, Interactions between integrin alphaIIbbeta3 and the serotonin transporter regulate serotonin transport and platelet aggregation in mice and humans, J. Clin. Investig,
doi:10.1172/JCI33374Casalino, Gaieb, Goldsmith, Hjorth, Dommer et al., Beyond Shielding: The Roles of Glycans in the SARS-CoV-2 Spike Protein, ACS Cent. Sci,
doi:10.1021/acscentsci.0c01056Cattin-Ortolá, Welch, Maslen, Papa, James et al., Sequences in the cytoplasmic tail of SARS-CoV-2 Spike facilitate expression at the cell surface and syncytia formation, Nat. Commun,
doi:10.1038/s41467-021-25589-1Cecchi, Ferraris, Desludging Action of Hydroxychloroquine in R.A, Acta Rheumatol. Scand
Celada, Dolera, Alvarez, Artigas, Effects of acute and chronic treatment with fluvoxamine on extracellular and platelet serotonin in the blood of major depressive patients. Relationship to clinical improvement, J. Affect. Disord,
doi:10.1016/0165-0327(92)90082-HChamie, Hibberd, Scheim, COVID-19 Excess Deaths in Peru's 25 States in 2020: Nationwide Trends, Confounding Factors, and Correlations With the Extent of Ivermectin Treatment by State, Cureus,
doi:10.7759/cureus.43168Chan, Kok, Zhu, Chu, To et al., Genomic characterization of the 2019 novel human-pathogenic coronavirus isolated from a patient with atypical pneumonia after visiting Wuhan, Emerg. Microbes Infect,
doi:10.1080/22221751.2020.1719902Chang, Crispin, Aricescu, Harvey, Nettleship et al., Glycoprotein structural genomics: Solving the glycosylation problem, Structure,
doi:10.1016/j.str.2007.01.011Chang, Lindstrom, Olson, Belew, Analysis of HIV Wild-Type and Mutant Structures via in Silico Docking against Diverse Ligand Libraries, J. Chem. Inf. Model,
doi:10.1021/ci700044sCharfeddine, Ibnhadjamor, Jdidi, Torjmen, Kraiem et al., Sulodexide Significantly Improves Endothelial Dysfunction and Alleviates Chest Pain and Palpitations in Patients With Long-COVID-19: Insights From TUN-EndCOV Study, Front. Cardiovasc. Med,
doi:10.3389/fcvm.2022.866113Chatterjee, Bhattacharya, Nag, Dhama, Chakraborty, A Detailed Overview of SARS-CoV-2 Omicron: Its Sub-Variants, Mutations and Pathophysiology, Clinical Characteristics, Immunological Landscape, Immune Escape, and Therapies, Viruses,
doi:10.3390/v15010167Chen, Hui, Ren, Luo, Shu et al., The N-glycosylation sites and Glycan-binding ability of S-protein in SARS-CoV-2 Coronavirus, bioRxiv,
doi:10.1101/2020.12.01.406025Chen, Liu, Guo, Emerging coronaviruses: Genome structure, replication, and pathogenesis, J. Med. Virol,
doi:10.1002/jmv.25681Chien, Sung, Physicochemical basis and clinical implications of red cell aggregation, Clin. Hemorheol. Microcirc,
doi:10.3233/CH-1987-7108Chiu, Chen, Hsu, Hua, Tseng et al., Changes of ECG parameters after BNT162b2 vaccine in the senior high school students, Eur. J. Pediatr,
doi:10.1007/s00431-022-04786-0Choi, Cao, Frank, Woo, Park et al., Structure, Dynamics, Receptor Binding, and Antibody Binding of the Fully Glycosylated Full-Length SARS-CoV-2 Spike Protein in a Viral Membrane, J. Chem. Theory Comput,
doi:10.1021/acs.jctc.0c01144Coghlan, Gilligan, Humphries, Mckenna, Dooley et al., Campylobacter pylori and recurrence of duodenal ulcers-A 12-month follow-up study, Lancet,
doi:10.1016/S0140-6736(87)91545-5Cohen, Varki, Chapter Three-Modulation of Glycan Recognition by Clustered Saccharide Patches
Cosic, Cosic, Loncarevic, RRM Prediction of Erythrocyte Band3 Protein as Alternative Receptor for SARS-CoV-2 Virus, Appl. Sci,
doi:10.3390/app10114053Craddock, Mahajan, Spikes, Krishnamachary, Ram et al., Persistent circulation of soluble and extracellular vesicle-linked Spike protein in individuals with postacute sequelae of COVID-19, J. Med. Virol,
doi:10.1002/jmv.28568Cullen, Swank, Intravascular Aggregation and Adhesiveness of the Blood Elements Associated with Alimentary Lipemia and Injections of Large Molecular Substances, Circulation,
doi:10.1161/01.CIR.9.3.335D'agnillo, Walters, Xiao, Sheng, Scherler et al., Lung epithelial and endothelial damage, loss of tissue repair, inhibition of fibrinolysis, and cellular senescence in fatal COVID-19, Sci. Transl. Med,
doi:10.1126/scitranslmed.abj7790Da Silva, Finamor, Andrade, Lima, Zett et al., Vascular retinal findings after COVID-19 vaccination in 11 cases: A coincidence or consequence?, Arq. Bras. Oftalmol
Dasgupta, Sen, Bakashi, Dasgupta, Nsp7 and Spike Glycoprotein of SARS-CoV-2 Are Envisaged as Potential Targets of Vitamin D and Ivermectin, Preprints.Org,
doi:10.20944/preprints202005.0084.v1Dayer, Coronavirus (SARS-CoV-2) Deactivation via Spike Glycoprotein Shielding by Old Drugs: Molecular Docking Approach, J. Epigenet
De Back, Kostova, Klei, Beuger, Van Zwieten et al., RBC Adhesive Capacity Is Essential for Efficient 'Immune Adherence Clearance' and Provide a Generic Target to Deplete Pathogens from Septic Patients, Blood,
doi:10.1182/blood.V128.22.1031.1031Dierckx, De Backer, Lins, De Meyer, Ides et al., CT-derived measurements of pulmonary blood volume in small vessels and the need for supplemental oxygen in COVID-19 patients, J. Appl. Physiol,
doi:10.1152/japplphysiol.00458.2022Dill, Hu, Berman, Pavia, Lacombe, One-and two-dimensional NMR studies of the N-terminal portion of glycophorin A at 11.7 Tesla, J. Protein Chem,
doi:10.1007/BF01025303Dinenno, Jones, Seals, Tanaka, Limb Blood Flow and Vascular Conductance Are Reduced With Age in Healthy Humans, Circulation,
doi:10.1161/01.CIR.100.2.164Ditzel, Angioscopic Changes in the Smaller Blood Vessels in Diabetes Mellitus and their Relationship to Aging, Circulation,
doi:10.1161/01.CIR.14.3.386Ditzel, Sagild, Morphologic and hemodynamic changes in the smaller blood vessels in diabetes mellitus. II. The degenerative and hemodynamic changes in the bulbar conjunctiva of normotensive diabetic patients, N. Engl. J. Med,
doi:10.1056/NEJM195404082501401Duan, Zheng, Zhang, Niu, Lou et al., The SARS-CoV-2 Spike Glycoprotein Biosynthesis, Structure, Function, and Antigenicity: Implications for the Design of Spike-Based Vaccine Immunogens, Front. Immunol,
doi:10.3389/fimmu.2020.576622Duerschmied, Suidan, Demers, Herr, Carbo et al., Platelet serotonin promotes the recruitment of neutrophils to sites of acute inflammation in mice, Blood,
doi:10.1182/blood-2012-06-437392Edwards, Pierangeli, Liu, Barker, Anderson et al., Hydroxychloroquine Reverses Thrombogenic Properties of Antiphospholipid Antibodies in Mice, Circulation,
doi:10.1161/01.CIR.96.12.4380Ekman, Flower, Mahler, Gould, Barnard et al., In silico molecular dynamics of human glycophorin A (GPA) extracellular structure, Ann. Blood,
doi:10.21037/aob-20-51Engeset, Stalker, Matheson, Effects of Dextran 40 on Red Cell Aggregation in Rabbits, Cardiovasc. Res,
doi:10.1093/cvr/1.4.379Engeset, Stalker, Matheson, Objective measurement of the dispersing effect of dextran 40 on red cells from man, dog, and rabbit, Cardiovasc. Res,
doi:10.1093/cvr/1.4.385Erogul, Gobeka, Dogan, Akdogan, Balci et al., Retinal microvascular morphology versus COVID-19: What to anticipate? Photodiagn, Photodyn. Ther,
doi:10.1016/j.pdpdt.2022.102920Eslick, Tilden, Arora, Torres, Clancy, Clinical and economic impact of "triple therapy" for Helicobacter pylori eradication on peptic ulcer disease in Australia, Helicobacter,
doi:10.1111/hel.12751Facente, Reiersen, Lenze, Boulware, Klausner, Fluvoxamine for the Early Treatment of SARS-CoV-2 Infection: A Review of Current Evidence, Drugs,
doi:10.1007/s40265-021-01636-5Fahmy, Daas, Salunkhe, Petrey, Cosar et al., Is Microthrombosis the Main Pathology in Coronavirus Disease 2019 Severity?-A Systematic Review of the Postmortem Pathologic Findings, Crit. Care Explor,
doi:10.1097/CCE.0000000000000427Fajers, Gelin, Kidney-, liver-and heart-damages from trauma and from induced intravascular aggregation of blood-cells: An experimental study, Acta Pathol. Microbiol. Scand,
doi:10.1111/j.1699-0463.1959.tb00322.xFajgenbaum, June, Storm, None, N. Engl. J. Med
Favaron, Ince, Hilty, Ergin, Van Der Zee et al., Capillary Leukocytes, Microaggregates, and the Response to Hypoxemia in the Microcirculation of Coronavirus Disease 2019 Patients, Crit. Care Med,
doi:10.1097/CCM.0000000000004862Feenstra, Shapiro, None
Fleming, On the Antibacterial Action of Cultures of a Penicillium, with Special Reference to their Use in the Isolation of B. influenzae, Br. J. Exp. Pathol,
doi:10.1093/clinids/2.1.129Fox, Akmatbekov, Harbert, Li, Quincy Brown et al., Pulmonary and cardiac pathology in African American patients with COVID-19: An autopsy series from New Orleans, Lancet Respir. Med,
doi:10.1016/S2213-2600(20)30243-5Fukuda, Dell, Oates, Fukuda, Structure of branched lactosaminoglycan, the carbohydrate moiety of band 3 isolated from adult human erythrocytes, J. Biol. Chem,
doi:10.1016/S0021-9258(17)39722-3Fullegar, Memorandum Explaining Basis for Declining Request for Emergency Use Authorization of Fluvoxamine Maleate
Gao, Zeng, Jia, Stavenhagen, Matsumoto et al., SARS-CoV-2 Spike Protein Interacts with Multiple Innate Immune Receptors, bioRxiv,
doi:10.1101/2020.07.29.227462Gattinoni, Gattarello, Steinberg, Busana, Palermo et al., COVID-19 pneumonia: Pathophysiology and management, Eur. Respir. Rev,
doi:10.1183/16000617.0138-2021Gautret, Lagier, Parola, Hoang, Meddeb et al., Hydroxychloroquine and azithromycin as a treatment of COVID-19: Results of an open-label non-randomized clinical trial, Int. J. Antimicrob. Agents,
doi:10.1016/j.ijantimicag.2020.105949Gedik, Bozdogan, Yavuz, Durmaz, Erol, The assesment of retina and optic disc vascular structures in people who received CoronaVac vaccine, Photodiagn. Photodyn. Ther,
doi:10.1016/j.pdpdt.2022.102742Gedik, Erol, Suren, Yavuz, Kucuk et al., Evaluation of retinal and optic disc vascular structures in individuals before and after Pfizer-BioNTech vaccination, Microvasc. Res,
doi:10.1016/j.mvr.2023.104500Gendrot, Andreani, Jardot, Hutter, Delandre et al., In Vitro Antiviral Activity of Doxycycline against SARS-CoV-2, Molecules,
doi:10.3390/molecules25215064Goshua, Pine, Meizlish, Chang, Zhang et al., Endotheliopathy in COVID-19-associated coagulopathy: Evidence from a single-centre, cross-sectional study, Lancet Haematol,
doi:10.1016/S2352-3026(20)30216-7Gotzsche, Deadly Medicines and Organised Crime: How Big Pharma Has Corrupted Healthcare
Graham, Lew, Klein, Evans, Evans et al., Effect of treatment of Helicobacter pylori infection on the long-term recurrence of gastric or duodenal ulcer. A randomized, controlled study, Ann. Intern. Med,
doi:10.7326/0003-4819-116-9-705Grobbelaar, Venter, Vlok, Ngoepe, Laubscher et al., SARS-CoV-2 spike protein S1 induces fibrin(ogen) resistant to fibrinolysis: Implications for microclot formation in COVID-19, Biosci. Rep,
doi:10.1042/BSR20210611Gundry, Abstract 10712: Observational Findings of PULS Cardiac Test Findings for Inflammatory Markers in Patients Receiving mRNA Vaccines, Circulation,
doi:10.1161/circ.144.suppl_1.10712Guo, Lakshminarayanan, Rodriguez-Palacios, Salata, Xu et al., Glycan Nanostructures of Human Coronaviruses, Int. J. Nanomed,
doi:10.2147/IJN.S302516Gupta, Maciorowski, Zak, Kulkarni, Herbert et al., Heparin: A simplistic repurposing to prevent SARS-CoV-2 transmission in light of its in-vitro nanomolar efficacy, Int. J. Biol. Macromol,
doi:10.1016/j.ijbiomac.2021.04.148Gupta, Neavin, Liu, Biernacka, Hall-Flavin et al., TSPAN5, ERICH3 and selective serotonin reuptake inhibitors in major depressive disorder: Pharmacometabolomics-informed pharmacogenomics, Mol. Psychiatry,
doi:10.1038/mp.2016.6Gómez, Albaiceta, García-Clemente, López-Larrea, Amado-Rodríguez et al., Angiotensin-converting enzymes (ACE, ACE2) gene variants and COVID-19 outcome, Gene,
doi:10.1016/j.gene.2020.145102Haider, Bengs, Schade, Wijnen, Portmann et al., Myocardial 18F-FDG Uptake Pattern for Cardiovascular Risk Stratification in Patients Undergoing Oncologic PET/CT, J. Clin. Med,
doi:10.3390/jcm9072279Halawa, Pullamsetti, Bangham, Stenmark, Dorfmüller et al., Potential long-term effects of SARS-CoV-2 infection on the pulmonary vasculature: A global perspective, Nat. Rev. Cardiol,
doi:10.1038/s41569-021-00640-2Hammi, Perrotin, Guillet, Boynard, Determination of red blood cell aggregation in young and elderly subjects evaluated by ultrasound, Clin. Hemorheol. Microcirc,
doi:10.3233/CH-1994-14115Harrison, Buckley, Rivera-Caravaca, Zhang, Lip, Cardiovascular risk factors, cardiovascular disease, and COVID-19: An umbrella review of systematic reviews, Eur. Heart J.-Qual. Care Clin. Outcomes
Haseeb, Solyman, Abushanab, Abo Obaia, Elhusseiny, Ocular Complications Following Vaccination for COVID-19: A One-Year Retrospective, Vaccines,
doi:10.3390/vaccines10020342Hazan, Dave, Gunaratne, Dolai, Clancy et al., Effectiveness of ivermectin-based multidrug therapy in severely hypoxic, ambulatory COVID-19 patients, Future Microbiol,
doi:10.2217/fmb-2022-0014Helms, Tacquard, Severac, Leonard-Lorant, Ohana et al., High risk of thrombosis in patients with severe SARS-CoV-2 infection: A multicenter prospective cohort study, Intensive Care Med,
doi:10.1007/s00134-020-06062-xHolck, Wolkowitz, Mellon, Reus, Nelson et al., Plasma serotonin levels are associated with antidepressant response to SSRIs, J. Affect. Disord,
doi:10.1016/j.jad.2019.02.063Huang, Dong, Milewska, Golda, Qi et al., Human Coronavirus HKU1 Spike Protein Uses O-Acetylated Sialic Acid as an Attachment Receptor Determinant and Employs Hemagglutinin-Esterase Protein as a Receptor-Destroying Enzyme, J. Virol,
doi:10.1128/JVI.00854-15Huertas, Montani, Savale, Pichon, Tu et al., Endothelial cell dysfunction: A major player in SARS-CoV-2 infection (COVID-19)?, Eur. Respir. J,
doi:10.1183/13993003.01634-2020Hulswit, De Haan, Bosch, Chapter Two-Coronavirus Spike Protein and Tropism Changes
Hulswit, Lang, Bakkers, Li, Li et al., Human coronaviruses OC43 and HKU1 bind to 9-O-acetylated sialic acids via a conserved receptor-binding site in spike protein domain A, Proc. Natl. Acad. Sci,
doi:10.1073/pnas.1809667116Hussien, Abdelaziz, Molecular docking suggests repurposing of brincidofovir as a potential drug targeting SARS-CoV-2 ACE2 receptor and main protease, Netw. Model. Anal. Health Inform. Bioinform,
doi:10.1007/s13721-020-00263-6Hysi, Saha, Kolios, Photoacoustic ultrasound spectroscopy for assessing red blood cell aggregation and oxygenation, J. Biomed. Opt,
doi:10.1117/1.JBO.17.12.125006Hyvärinen, Meri, Jokiranta, Disturbed sialic acid recognition on endothelial cells and platelets in complement attack causes atypical hemolytic uremic syndrome, Blood,
doi:10.1182/blood-2015-11-680009Jacot, Greub, Jaton, Opota, Viral load of SARS-CoV-2 across patients and compared to other respiratory viruses, Microbes Infect,
doi:10.1016/j.micinf.2020.08.004Jaskiewicz, Jodłowska, Kaczmarek, Zerka, Erythrocyte glycophorins as receptors for Plasmodium merozoites, Parasites Vectors,
doi:10.1186/s13071-019-3575-8Johnson, Cheap Antidepressant Shows Promise Treating Early COVID-19, Yahoo News
Juarez, Schcolnik-Cabrera, Dueñas-Gonzalez, The multitargeted drug ivermectin: From an antiparasitic agent to a repositioned cancer drug, Am. J. Cancer Res
Junxiu, Yu, Shaodan, Yi, Yin et al., Microvascular pathological features and changes in related injury factors in a rat acute blood stasis model, J. Tradit. Chin. Med,
doi:10.1016/S0254-6272(17)30034-1Kalhor, Sadeghi, Abolhasani, Kalhor, Rahimi, Repurposing of the approved small molecule drugs in order to inhibit SARS-CoV-2 S protein and human ACE2 interaction through virtual screening approaches, J. Biomol. Struct. Dyn,
doi:10.1080/07391102.2020.1824816Kasinrerk, Tokrasinwit, Phunpae, CD147 monoclonal antibodies induce homotypic cell aggregation of monocytic cell line U937 via LFA-1/ICAM-1 pathway, Immunology,
doi:10.1046/j.1365-2567.1999.00653.xKaur, Shekhar, Sharma, Sarma, Prakash et al., Ivermectin as a potential drug for treatment of COVID-19: An in-sync review with clinical and computational attributes, Pharmacol. Rep,
doi:10.1007/s43440-020-00195-yKim, Popel, Intaglietta, Johnson, Aggregate formation of erythrocytes in postcapillary venules, Am. J. Physiol.-Heart Circ. Physiol,
doi:10.1152/ajpheart.00690.2004Ko, Harigopal, Gehlhausen, Bosenberg, Mcniff et al., Discordant anti-SARS-CoV-2 spike protein and RNA staining in cutaneous perniotic lesions suggests endothelial deposition of cleaved spike protein, J. Cutan. Pathol,
doi:10.1111/cup.13866Kong, Montano, Corley, Helmy, Kobayashi et al., Neuropilin-1 Mediates SARS-CoV-2 Infection of Astrocytes in Brain Organoids, Inducing Inflammation Leading to Dysfunction and Death of Neurons, mBio,
doi:10.1128/mbio.02308-22Korhonen, Haahtela, Pirkola, Parkkinen, A N-acetyllactosamine-specific cell-binding activity in a plant pathogen, Erwinia rhapontici, FEBS Lett,
doi:10.1016/0014-5793(88)80307-7Koutsiaris, Riri, Boutlas, Panagiotou, Kotoula et al., COVID-19 hemodynamic and thrombotic effect on the eye microcirculation after hospitalization: A quantitative case-control study, Clin. Hemorheol. Microcirc,
doi:10.3233/CH-221554Krause, Buisson, Bertrand, Corringer, Galzi et al., Ivermectin: A positive allosteric effector of the alpha7 neuronal nicotinic acetylcholine receptor, Mol. Pharmacol,
doi:10.1124/mol.53.2.283Krauson, Casimero, Siddiquee, Stone, Duration of SARS-CoV-2 mRNA vaccine persistence and factors associated with cardiac involvement in recently vaccinated patients, NPJ Vaccines,
doi:10.1038/s41541-023-00742-7Krejza, Mariak, Walecki, Szydlik, Lewko et al., Transcranial color Doppler sonography of basal cerebral arteries in 182 healthy subjects: Age and sex variability and normal reference values for blood flow parameters, AJR Am. J. Roentgenol,
doi:10.2214/ajr.172.1.9888770Kristjansdottir, Lewerin, Lerner, Waern, Johansson et al., High Serum Serotonin Predicts Increased Risk for Hip Fracture and Nonvertebral Osteoporotic Fractures: The MrOS Sweden Study, J. Bone Miner. Res,
doi:10.1002/jbmr.3443Kumar, Nyodu, Maurya, Saxena, Morphology, Genome Organization, Replication, and Pathogenesis of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2)
Lam, Reilly, Rux, Murphy, Kuri-Cervantes et al., Erythrocytes identify complement activation in patients with COVID-19, Am. J. Physiol. Lung Cell Mol. Physiol,
doi:10.1152/ajplung.00231.2021Lardone, Garay, Parodi, De La Fuente, Angeloni et al., How glycobiology can help us treat and beat the COVID-19 pandemic, J. Biol. Chem,
doi:10.1016/j.jbc.2021.100375Lee, Fusco, Saphire, An efficient platform for screening expression and crystallization of glycoproteins produced in human cells, Nat. Protoc,
doi:10.1038/nprot.2009.29Lee, Shirshin, Rovnyagina, Yaya, Boujja et al., Dextran adsorption onto red blood cells revisited: Single cell quantification by laser tweezers combined with microfluidics, Biomed. Opt. Express,
doi:10.1364/BOE.9.002755Lehrer, Rheinstein, Ivermectin Docks to the SARS-CoV-2 Spike Receptor-binding Domain Attached to ACE2, Vivo,
doi:10.21873/invivo.12134Lenze, Mattar, Zorumski, Stevens, Schweiger et al., Fluvoxamine vs Placebo and Clinical Deterioration in Outpatients With Symptomatic COVID-19: A Randomized Clinical Trial, JAMA,
doi:10.1001/jama.2020.22760Li, Chen, Zhao, Lung, Ye et al., Intravenous Injection of Coronavirus Disease 2019 (COVID-19) mRNA Vaccine Can Induce Acute Myopericarditis in Mouse Model, Clin. Infect. Dis,
doi:10.1093/cid/ciab707Li, Deng, Li, Dorken Gallastegi, Mantzoros et al., Multiphysics and multiscale modeling of microthrombosis in COVID-19, PLoS Comput. Biol,
doi:10.1371/journal.pcbi.1009892Li, Hulswit, Widjaja, Raj, Mcbride et al., Identification of sialic acid-binding function for the Middle East respiratory syndrome coronavirus spike glycoprotein, Proc. Natl. Acad. Sci,
doi:10.1073/pnas.1712592114Lins, Vandevenne, Thillai, Lavon, Lanclus et al., Assessment of Small Pulmonary Blood Vessels in COVID-19 Patients Using HRCT, Acad. Radiol,
doi:10.1016/j.acra.2020.07.019Liu, Han, Blair, Kenst, Qin et al., SARS-CoV-2 Infects Endothelial Cells In Vivo and In Vitro, Front. Cell. Infect. Microbiol,
doi:10.3389/fcimb.2021.701278Liukkonen, Haataja, Tikkanen, Kelm, Finne, Identification of N-acetylneuraminyl alpha 2-->3 poly-Nacetyllactosamine glycans as the receptors of sialic acid-binding Streptococcus suis strains, J. Biol. Chem,
doi:10.1016/S0021-9258(19)36803-6Lobanovska, Pilla, Penicillin's Discovery and Antibiotic Resistance: Lessons for the Future?, Yale J. Biol. Med
Lodigiani, Lapichino, Carenzo, Cecconi, Ferrazzi et al., Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy, Thromb. Res,
doi:10.1016/j.thromres.2020.04.024Lowe, Thrombosis and hemorheology
López-Farfán, Irigoyen, Gómez-Díaz, Exploring SARS-CoV-2 and Plasmodium falciparum coinfection in human erythrocytes, Front. Immunol,
doi:10.3389/fimmu.2023.1120298López-Medina, López, Hurtado, Dávalos, Ramirez et al., Effect of Ivermectin on Time to Resolution of Symptoms Among Adults With Mild COVID-19: A Randomized Clinical Trial, JAMA,
doi:10.1001/jama.2021.3071Maeda, Seike, Kon, Shiga, Erythrocyte Aggregation as a Determinant of Blood Flow: Effect of pH, Temperature and Osmotic Pressure
Magro, Mulvey, Berlin, Nuovo, Salvatore et al., Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: A report of five cases, Transl. Res,
doi:10.1016/j.trsl.2020.04.007Magro, Mulvey, Laurence, Seshan, Crowson et al., Docked severe acute respiratory syndrome coronavirus 2 proteins within the cutaneous and subcutaneous microvasculature and their role in the pathogenesis of severe coronavirus disease 2019, Hum. Pathol,
doi:10.1016/j.humpath.2020.10.002Maier, Truong, Auld, Polly, Tanksley et al., COVID-19-associated hyperviscosity: A link between inflammation and thrombophilia?, Lancet,
doi:10.1016/S0140-6736(20)31209-5Makary, The Real Data Behind the New COVID Vaccines the White Houseis Pushing
Manetta, Aloulou, Varlet-Marie, Mercier, Brun, Partially opposite hemorheological effects of aging and training at middle age, Clin. Hemorheol. Microcirc
Mansanguan, Charunwatthana, Piyaphanee, Dechkhajorn, Poolcharoen et al., Cardiovascular Manifestation of the BNT162b2 mRNA COVID-19 Vaccine in Adolescents, Trop. Med. Infect. Dis,
doi:10.3390/tropicalmed7080196Matrosovich, Herrler, Klenk, Sialic Acid Receptors of Viruses
Maurya, A Combination of Ivermectin and Doxycycline Possibly Blocks the Viral Entry and Modulate the Innate Immune Response in COVID-19 Patients, ChemRxiv,
doi:10.26434/chemrxiv.12630539.v1Mccarthy, Naggie, Boulware, Lindsell, Stewart et al., Effect of Fluvoxamine vs Placebo on Time to Sustained Recovery in Outpatients With Mild to Moderate COVID-19: A Randomized Clinical Trial, JAMA,
doi:10.1001/jama.2022.24100Mccloskey, Postolache, Vittone, Nghiem, Monsale et al., Selective serotonin reuptake inhibitors: Measurement of effect on platelet function, Transl. Res,
doi:10.1016/j.trsl.2007.10.004Meekins, Gaudreault, Richt, Natural and Experimental SARS-CoV-2 Infection in Domestic and Wild Animals, Viruses,
doi:10.3390/v13101993Mei, Van Wijk, Pham, Marin, Role of von Willebrand Factor in COVID-19 Associated Coagulopathy, J. Appl. Lab. Med,
doi:10.1093/jalm/jfab042Melkumyants, Buryachkovskaya, Lomakin, Antonova, Docenko et al., Effect of Sulodexide on Circulating Blood Cells in Patients with Mild COVID-19, J. Clin. Med,
doi:10.3390/jcm11071995Melkumyants, Buryachkovskaya, Lomakin, Antonova, Serebruany, Mild COVID-19 and Impaired Blood Cell-Endothelial Crosstalk: Considering Long-Term Use of Antithrombotics?, Thromb. Haemost,
doi:10.1055/a-1551-9911Menter, Haslbauer, Nienhold, Savic, Hopfer et al., Postmortem examination of COVID-19 patients reveals diffuse alveolar damage with severe capillary congestion and variegated findings in lungs and other organs suggesting vascular dysfunction, Histopathology,
doi:10.1111/his.14134Metwally, Basha, Zaitoun, Abdalla, Nofal et al., Clinical and radiological imaging as prognostic predictors in COVID-19 patients. Egypt, J. Radiol. Nucl. Med,
doi:10.1186/s43055-021-00470-9Mikami, Miyashita, Yamada, Harrington, Steinberg et al., Risk Factors for Mortality in Patients with COVID-19 in New York City, J. Gen. Intern. Med,
doi:10.1007/s11606-020-05983-zMillion, Cortaredona, Delorme, Colson, Levasseur et al., Early Treatment with Hydroxychloroquine and Azithromycin: A 'Real-Life' Monocentric Retrospective Cohort Study of,
doi:10.1101/2023.04.03.23287649Morris, Pershad, Kang, Ridenour, Lavon et al., Altered pulmonary blood volume distribution as a biomarker for predicting outcomes in COVID-19 disease, Eur. Respir. J,
doi:10.1183/13993003.04133-2020Morz, A Case Report: Multifocal Necrotizing Encephalitis and Myocarditis after BNT162b2 mRNA Vaccination against COVID-19, Vaccines,
doi:10.3390/vaccines10101651Muhl, He, Sun, Andaloussi Mäe, Pietilä et al., The SARS-CoV-2 receptor ACE2 is expressed in mouse pericytes but not endothelial cells: Implications for COVID-19 vascular research, Stem Cell Rep,
doi:10.1016/j.stemcr.2022.03.016Nader, Nougier, Boisson, Poutrel, Catella et al., Increased blood viscosity and red blood cell aggregation in patients with COVID-19, Am. J. Hematol,
doi:10.1002/ajh.26440Nakahara, Iwabuchi, Miyazawa, Tonda, Shiga et al., Assessment of Myocardial (18)F-FDG Uptake at PET/CT in Asymptomatic SARS-CoV-2-vaccinated and Nonvaccinated Patients, Radiology,
doi:10.1148/radiol.230743Nallusamy, Mannu, Ravikumar, Angamuthu, Nathan et al., Exploring Phytochemicals of Traditional Medicinal Plants Exhibiting Inhibitory Activity Against Main Protease, Spike Glycoprotein, RNA-dependent RNA Polymerase and Non-Structural Proteins of SARS-CoV-2 Through Virtual Screening, Front. Pharmacol,
doi:10.3389/fphar.2021.667704Negri, Piloto, Morinaga, Jardim, Lamy et al., Heparin Therapy Improving Hypoxia in COVID-19 Patients-A Case Series, Front. Physiol,
doi:10.3389/fphys.2020.573044Nelson, Immune Adherence
Nelson, The Immune-Adherence Phenomenon: An Immunologically Specific Reaction Between Microorganisms and Erythrocytes Leading to Enhanced Phagocytosis, Science,
doi:10.1126/science.118.3077.733Nemkov, Reisz, Xia, Zimring, ; D'alessandro, Red blood cells as an organ? How deep omics characterization of the most abundant cell in the human body highlights other systemic metabolic functions beyond oxygen transport, Expert Rev. Proteom,
doi:10.1080/14789450.2018.1531710Nicosia, Ligresti, Caporarello, Akilesh, Ribatti, COVID-19 Vasculopathy: Mounting Evidence for an Indirect Mechanism of Endothelial Injury, Am. J. Pathol,
doi:10.1016/j.ajpath.2021.05.007Nuovo, Magro, Shaffer, Awad, Suster et al., Endothelial cell damage is the central part of COVID-19 and a mouse model induced by injection of the S1 subunit of the spike protein, Ann. Diagn. Pathol,
doi:10.1016/j.anndiagpath.2020.151682Nycholat, Mcbride, Ekiert, Xu, Rangarajan et al., Recognition of Sialylated Poly-N-acetyllactosamine Chains on N-and O-Linked Glycans by Human and Avian Influenza|A Virus Hemagglutinins, Angew. Chem. Int. Ed,
doi:10.1002/anie.201200596Ogata, Maley, Wu, Gilboa, Norman et al., Ultra-sensitive Serial Profiling of SARS-CoV-2 Antigens and Antibodies in Plasma to Understand Disease Progression in COVID-19 Patients with Severe Disease, Clin. Chem,
doi:10.1093/clinchem/hvaa213Osiaevi, Schulze, Evers, Harmening, Vink et al., Persistent capillary rarefication in long COVID syndrome, Angiogenesis,
doi:10.1007/s10456-022-09850-9Osman, Farouk, Osman, Abdrabou, Longitudinal assessment of chest computerized tomography and oxygen saturation for patients with COVID-19. Egypt, J. Radiol. Nucl. Med,
doi:10.1186/s43055-020-00376-yOverton, Toshner, Mulligan, Vora, Nikkho et al., the PVRI Innovative Drug Development Initiative. Pulmonary thromboembolic events in COVID-19-A systematic literature review, Pulm. Circ,
doi:10.1002/pul2.12113Park, Walls, Wang, Sauer, Li et al., Structures of MERS-CoV spike glycoprotein in complex with sialoside attachment receptors, Nat. Struct. Mol. Biol,
doi:10.1038/s41594-019-0334-7Patterson, Francisco, Yogendra, Long, Pise et al., Persistence of SARS-CoV-2 S1 Protein in CD16+ Monocytes in Post-Acute Sequelae of COVID-19 (PASC) up to 15 Months Post-Infection, Front. Immunol,
doi:10.3389/fimmu.2021.746021Peacock, Brown, Zhou, Thakur, Sukhova et al., The altered entry pathway and antigenic distance of the SARS-CoV-2 Omicron variant map to separate domains of spike protein, bioRxiv,
doi:10.1101/2021.12.31.474653Pellegrini, Kawakami, Guagliumi, Sakamoto, Kawai et al., Microthrombi as a Major Cause of Cardiac Injury in COVID-19: A Pathologic Study, Circulation,
doi:10.1161/CIRCULATIONAHA.120.051828Perico, Morigi, Galbusera, Pezzotta, Gastoldi et al., SARS-CoV-2 Spike Protein 1 Activates Microvascular Endothelial Cells and Complement System Leading to Platelet Aggregation, Front. Immunol,
doi:10.3389/fimmu.2022.827146Petrilli, Jones, Yang, Rajagopalan, O'donnell et al., Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: Prospective cohort study, BMJ,
doi:10.1136/bmj.m1966Poh, Jia Tay, Lin, Chee, Thangavelautham, A review of COVID-19-related thrombosis and anticoagulation strategies specific to the Asian population, Singap. Med. J,
doi:10.11622/smedj.2020174Popel, Johnson, Kameneva, Wild, Capacity for red blood cell aggregation is higher in athletic mammalian species than in sedentary species, J. Appl. Physiol,
doi:10.1152/jappl.1994.77.4.1790Pribush, Zilberman-Kravits, Meyerstein, The mechanism of the dextran-induced red blood cell aggregation, Eur. Biophys. J,
doi:10.1007/s00249-006-0107-1Qing, Hantak, Perlman, Gallagher, Distinct Roles for Sialoside and Protein Receptors in Coronavirus Infection, mBio,
doi:10.1128/mBio.02764-19Quispe-Cholan, Anticona-De-La-Cruz, Cornejo-Cruz, Quispe-Chirinos, Moreno-Lazaro et al., Tomographic findings in patients with COVID-19 according to evolution of the disease. Egypt, J. Radiol. Nucl. Med,
doi:10.1186/s43055-020-00329-5Rajah, Bernier, Buchrieser, Schwartz, The Mechanism and Consequences of SARS-CoV-2 Spike-Mediated Fusion and Syncytia Formation, J. Mol. Biol,
doi:10.1016/j.jmb.2021.167280Rajter, Local Doctor Tries New Coronavirus Drug Treatment, NBC Miami News
Rajter, Sherman, Fatteh, Vogel, Sacks et al., Use of Ivermectin is Associated with Lower Mortality in Hospitalized Patients with COVID-19 (ICON study), Chest,
doi:10.1016/j.chest.2020.10.009Rampling, Meiselman, Neu, Baskurt, Influence of cell-specific factors on red blood cell aggregation, Biorheology
Rapkiewicz, Mai, Carsons, Pittaluga, Kleiner et al., Megakaryocytes and platelet-fibrin thrombi characterize multi-organ thrombosis at autopsy in COVID-19: A case series, eClinicalMedicine,
doi:10.1016/j.eclinm.2020.100434Reinke, Gaehtgens, Johnson, Blood viscosity in small tubes: Effect of shear rate, aggregation, and sedimentation, Am. J. Physiol.-Heart Circ. Physiol,
doi:10.1152/ajpheart.1987.253.3.H540Reis, Moreira Silva, Medeiros Silva, Thabane, Milagres et al., Effect of Early Treatment with Ivermectin among Patients with COVID-19, N. Engl. J. Med,
doi:10.1056/NEJMoa2115869Reis, Moreira Silva, Medeiros Silva, Thabane, Milagres et al., Effect of early treatment with fluvoxamine on risk of emergency care and hospitalisation among patients with COVID-19: The TOGETHER randomised, platform clinical trial, Lancet Glob. Health,
doi:10.1016/S2214-109X(21)00448-4Richardson, Schwartz, Comparison of capillary blood flow in the nailfold circulations of young and elderly men, AGE,
doi:10.1007/BF02432074Rovas, Osiaevi, Buscher, Sackarnd, Tepasse et al., Microvascular dysfunction in COVID-19: The MYSTIC study, Angiogenesis,
doi:10.1007/s10456-020-09753-7Sakariassen, Orning, Turitto, The impact of blood shear rate on arterial thrombus formation, Future Sci. OA,
doi:10.4155/fso.15.28Samocha-Bonet, Ben-Ami, Shapira, Shenkerman, Abu-Abeid et al., Flow-resistant red blood cell aggregation in morbid obesity, Int. J. Obes,
doi:10.1038/sj.ijo.0802791Samocha-Bonet, Lichtenberg, Tomer, Deutsch, Mardi et al., Enhanced erythrocyte adhesiveness/aggregation in obesity corresponds to low-grade inflammation, Obes. Res,
doi:10.1038/oby.2003.54Sano, Kase, Aoyama, Sano, A case of persistent, confluent maculopapular erythema following a COVID-19 mRNA vaccination is possibly associated with the intralesional spike protein expressed by vascular endothelial cells and eccrine glands in the deep dermis, J. Dermatol,
doi:10.1111/1346-8138.16816Santin, Scheim, Mccullough, Yagisawa, Borody, Ivermectin: A multifaceted drug of Nobel prize-honored distinction with indicated efficacy against a new global scourge, COVID-19, New Microbes New Infect,
doi:10.1016/j.nmni.2021.100924Saritas, Yorgun, Gokpinar, Effects of Sinovac-Coronavac and Pfizer-BioNTech mRNA vaccines on choroidal and retinal vascular system, Photodiagn. Photodyn. Ther,
doi:10.1016/j.pdpdt.2023.103702Savastano, Crincoli, Savastano, Younis, Gambini et al., Peripapillary Retinal Vascular Involvement in Early Post-COVID-19 Patients, J. Clin. Med,
doi:10.3390/jcm9092895Saxena, None
Scheim, A Deadly Embrace: Hemagglutination Mediated by SARS-CoV-2 Spike Protein at its 22 N-Glycosylation Sites, Red Blood Cell Surface Sialoglycoproteins, and Antibody, Int. J. Mol. Sci,
doi:10.3390/ijms23052558Scheim, Aldous, Osimani, Fordham, Hoy, When Characteristics of Clinical Trials Require Per-Protocol as Well as Intention-to-Treat Outcomes to Draw Reliable Conclusions: Three Examples, J. Clin. Med,
doi:10.3390/jcm12113625Scheim, From Cold to Killer: How SARS-CoV-2 Evolved without Hemagglutinin Esterase to Agglutinate, Then Clot Blood Cells in Pulmonary and Systemic Microvasculature, OSF Preprints
Scheim, Hibberd, Chamie-Quintero, Protocol Violations in López-Medina et al.: 38 Switched Ivermectin (IVM) and Placebo Doses, Failure of Blinding, Ubiquitous IVM use OTC in Cali, and Nearly Identical AEs for the IVM and Control Groups, OSF Preprints,
doi:10.31219/osf.io/u7ewzSchlick, Lucio, Wallukat, Bartsch, Skornia et al., Post-COVID-19 Syndrome: Retinal Microcirculation as a Potential Marker for Chronic Fatigue, Int. J. Mol. Sci,
doi:10.3390/ijms232213683Schultheiß, Willscher, Paschold, Gottschick, Klee et al., Liquid biomarkers of macrophage dysregulation and circulating spike protein illustrate the biological heterogeneity in patients with post-acute sequelae of COVID-19, J. Med. Virol,
doi:10.1002/jmv.28364Schultze, Cavanagh, Herrler, Neuraminidase treatment of avian infectious bronchitis coronavirus reveals a hemagglutinating activity that is dependent on sialic acid-containing receptors on erythrocytes, Virology,
doi:10.1016/0042-6822(92)90608-RSeet, Quek, Ooi, Sengupta, Lakshminarasappa et al., Positive impact of oral hydroxychloroquine and povidone-iodine throat spray for COVID-19 prophylaxis: An open-label randomized trial, Int. J. Infect. Dis,
doi:10.1016/j.ijid.2021.04.035Seftel, Boulware, Prospective Cohort of Fluvoxamine for Early Treatment of Coronavirus Disease 19, Open Forum Infect. Dis,
doi:10.1093/ofid/ofab050Selickman, Vrettou, Mentzelopoulos, Marini, COVID-19-Related ARDS: Key Mechanistic Features and Treatments, J. Clin. Med,
doi:10.3390/jcm11164896Shafiee, Teymouri Athar, Kohandel Gargari, Jafarabady, Siahvoshi et al., Ivermectin under scrutiny: A systematic review and meta-analysis of efficacy and possible sources of controversies in COVID-19 patients, Virol. J,
doi:10.1186/s12985-022-01829-8Shajahan, Supekar, Gleinich, Azadi, Deducing the N-and O-glycosylation profile of the spike protein of novel coronavirus SARS-CoV-2, Glycobiology,
doi:10.1093/glycob/cwaa042Shilts, Crozier, Greenwood, Lehner, Wright, No evidence for basigin/CD147 as a direct SARS-CoV-2 spike binding receptor, Sci. Rep,
doi:10.1038/s41598-020-80464-1Shoemaker, Brunius, Gelin, Hemodynamic and microcirculatory effects of high and low viscosity dextrans, Surgery
Singh, Satchell, Neal, Mckenzie, Tooke et al., Glomerular endothelial glycocalyx constitutes a barrier to protein permeability, J. Am. Soc. Nephrol,
doi:10.1681/ASN.2007010119Soares, Grosso, Ereño-Orbea, Coelho, Marcelo, Molecular Recognition Insights of Sialic Acid Glycans by Distinct Receptors Unveiled by NMR and Molecular Modeling, Front. Mol. Biosci,
doi:10.3389/fmolb.2021.727847Steinbrook, Kassirer, Angell, Justifying conflicts of interest in medical journals: A very bad idea, BMJ,
doi:10.1136/bmj.h2942Stocker, Ishikawa-Ankerhold, Massberg, Schulz, Small but mighty: Platelets as central effectors of host defense, Thromb. Haemost
Stone, Ndarukwa, Scheim, Dancis, Dancis et al., Changes in SpO2 on Room Air for 34 Severe COVID-19 Patients after Ivermectin-Based Combination Treatment: 62% Normalization within 24 Hours, Biologics,
doi:10.3390/biologics2030015Storz, Zhang, Rott, Comparison of hemagglutinating, receptor-destroying, and acetylesterase activities of avirulent and virulent bovine coronavirus strains, Arch. Virol,
doi:10.1007/BF01309637Sukhatme, Reiersen, Vayttaden, Sukhatme, Fluvoxamine: A Review of Its Mechanism of Action and Its Role in COVID-19, Front. Pharmacol,
doi:10.3389/fphar.2021.652688Swank, Cullen, Circulatory Changes in the Hamster's Cheek Pouch Associated with Alimentary Lipemia, Proc. Soc. Exp. Biol. Med,
doi:10.3181/00379727-82-20123Swank, Senussi, Manickas-Hill, Yu, Li et al., Persistent circulating SARS-CoV-2 spike Is associated With post-acute COVID-19 sequelae, Clin. Infect. Dis,
doi:10.1093/cid/ciac722Tang, Li, Wang, Sun, Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia, J. Thromb. Haemost,
doi:10.1111/jth.14768Thairu, Babalola, Ajayi, Ndanusa, Ogedengbe et al., A comparison of Ivermectin and Non Ivermectin based regimen for COVID-19 in Abuja: Effects on virus clearance, Days-to-Discharge and Mortality, Res. Sq,
doi:10.9734/jpri/2022/v34i44A36328Thillai, Patvardhan, Swietlik, Mclellan, De Backer et al., Functional respiratory imaging identifies redistribution of pulmonary blood flow in patients with COVID-19, Thorax,
doi:10.1136/thoraxjnl-2020-215395Tipre, Cidon, Moats, Imaging Pulmonary Blood Vessels and Ventilation-Perfusion Mismatch in COVID-19, Mol. Imaging Biol,
doi:10.1007/s11307-021-01700-2Tortorici, Walls, Lang, Wang, Li et al., Structural basis for human coronavirus attachment to sialic acid receptors, Nat. Struct. Mol. Biol,
doi:10.1038/s41594-019-0233-yTruong, Dionne, Muniz, Mchugh, Portman et al., Clinically Suspected Myocarditis Temporally Related to COVID-19 Vaccination in Adolescents and Young Adults, Circulation,
doi:10.1161/CIRCULATIONAHA.121.056583Turner, Khan, Putrino, Woodcock, Kell et al., Long COVID: Pathophysiological factors and abnormalities of coagulation, Trends Endocrinol. Metab,
doi:10.1016/j.tem.2023.03.002Unione, Moure, Lenza, Oyenarte, Ereño-Orbea et al., The SARS-CoV-2 Spike Glycoprotein Directly Binds Exogeneous Sialic Acids: A NMR View, Angew. Chem. Int. Ed
Urbina, Pineda, Piñango, Carreira, Lima, 3H]Paroxetine binding to human peripheral lymphocyte membranes of patients with major depression before and after treatment with fluoxetine, Int. J. Immunopharmacol,
doi:10.1016/S0192-0561(99)00035-1Varki, Cummings, Esko, Cold Spring Harbor Laboratory Press
Varki, Schnaar, Schauer, Chapter 15: Sialic Acids and Other Nonulosonic Acids
Vernon Jeffords, Knisely, Concerning the Geometric Shapes of Arteries and Arterioles: A Contribution to the Biophysics of Health, Disease, and Death, Angiology,
doi:10.1177/000331975600700202Vlasak, Luytjes, Spaan, Palese, Human and bovine coronaviruses recognize sialic acid-containing receptors similar to those of influenza C viruses, Proc. Natl. Acad. Sci,
doi:10.1073/pnas.85.12.4526Volger, Schmid-Schönbein, Gosen, Klose, Kline, Microrheology and light transmission of blood, Pflügers Arch,
doi:10.1007/BF00587850Wang, Chen, Zhang, Deng, Lian et al., CD147-spike protein is a novel route for SARS-CoV-2 infection to host cells, Signal Transduct. Target. Ther,
doi:10.1038/s41392-020-00426-xWang, Dong, Hu, Li, Ren et al., Temporal Changes of CT Findings in 90 Patients with COVID-19 Pneumonia: A Longitudinal Study, Radiology,
doi:10.1148/radiol.2020200843Wang, Yu, Ochani, Amella, Tanovic et al., Nicotinic acetylcholine receptor α7 subunit is an essential regulator of inflammation, Nature,
doi:10.1038/nature01339Wautier, Paton, Wautier, Pintigny, Abadie et al., Increased adhesion of erythrocytes to endothelial cells in diabetes mellitus and its relation to vascular complications, N. Engl. J. Med,
doi:10.1056/NEJM198107303050501Weng, Cloutier, Pibarot, Durand, Comparison and simulation of different levels of erythrocyte aggregation with pig, horse, sheep, calf, and normal human blood, Biorheology,
doi:10.3233/BIR-1996-334-506Wernike, Böttcher, Amelung, Albrecht, Gärtner et al., Antibodies against SARS-CoV-2 Suggestive of Single Events of Spillover to Cattle, Germany, Emerg. Infect. Dis. J,
doi:10.3201/eid2809.220125Wichmann, Sperhake, Lütgehetmann, Steurer, Edler et al., Autopsy Findings and Venous Thromboembolism in Patients With COVID-19, Ann. Intern. Med,
doi:10.7326/M20-2003Wielgat, Rogowski, Godlewska, Car, Coronaviruses: Is Sialic Acid a Gate to the Eye of Cytokine Storm? From the Entry to the Effects, Cells,
doi:10.3390/cells9091963Williamson, Walker, Bhaskaran, Bacon, Bates et al., OpenSAFELY: Factors associated with COVID-19 death in 17 million patients, Nature,
doi:10.1038/s41586-020-2521-4Wu, Ai, Cai, Zhang, Qian et al., Predictive Model and Risk Factors for Case Fatality of COVID-19: A Cohort of 21,392 Cases in Hubei, China,
doi:10.1016/j.xinn.2020.100022Yagisawa, Foster, Hanaki, Omura, Global Trends in Clinical Studies of Ivermectin in COVID-19, Jpn. J. Antibiot
Yamakawa, Vanbeselaere, Chang, Yu, Ducrocq et al., Systems glycomics of adult zebrafish identifies organ-specific sialylation and glycosylation patterns, Nat. Commun,
doi:10.1038/s41467-018-06950-3Yamamoto, Kase, Sano, Kamijima, Sano, Persistent varicella zoster virus infection following mRNA COVID-19 vaccination was associated with the presence of encoded spike protein in the lesion, J. Cutan. Immunol. Allergy,
doi:10.1002/cia2.12278Yao, Li, He, Civelek, Li, Likely Common Role of Hypoxia in Driving 18F-FDG Uptake in Cancer, Myocardial Ischemia, Inflammation and Infection, Cancer Biother. Radiopharm
Yoshimoto, The Proteins of Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2 or n-COV19), the Cause of COVID-19, Protein J,
doi:10.1007/s10930-020-09901-4Yu, Armstrong, Tripette, Meiselman, Cloutier et al., A local increase in red blood cell aggregation can trigger deep vein thrombosis: Evidence based on quantitative cellular ultrasound imaging, J. Thromb. Haemost,
doi:10.1182/blood-2017-03-745349Zaki, Van Boheemen, Bestebroer, Osterhaus, Fouchier, Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia, N. Engl. J. Med,
doi:10.1056/NEJMoa1211721Zhen, Guo, Zhang, Liang, Ge et al., Experimental Study on Microthrombi and Myocardial Injuries, Microvasc. Res,
doi:10.1006/mvre.1996.0010Zheng, Zhao, Li, Guo, Sheng et al., SARS-CoV-2 spike protein causes blood coagulation and thrombosis by competitive binding to heparan sulfate, Int. J. Biol. Macromol,
doi:10.1016/j.ijbiomac.2021.10.112Zhou, Yu, Du, Fan, Liu et al., Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study, Lancet,
doi:10.1016/S0140-6736(20)30566-3Zhu, Avsievich, Bykov, Popov, Meglinski, Influence of Pulsed He-Ne Laser Irradiation on the Red Blood Cell Interaction Studied by Optical Tweezers, Micromachines,
doi:10.3390/mi10120853Ziegler, Guerci, Muller, Candiloros, Mejean et al., Increased erythrocyte aggregation in insulin-dependent diabetes mellitus and its relationship to plasma factors: A multivariate analysis, Metabolism,
doi:10.1016/0026-0495(94)90063-9DOI record:
{
"DOI": "10.3390/ijms242317039",
"ISSN": [
"1422-0067"
],
"URL": "http://dx.doi.org/10.3390/ijms242317039",
"abstract": "<jats:p>Consistent with well-established biochemical properties of coronaviruses, sialylated glycan attachments between SARS-CoV-2 spike protein (SP) and host cells are key to the virus’s pathology. SARS-CoV-2 SP attaches to and aggregates red blood cells (RBCs), as shown in many pre-clinical and clinical studies, causing pulmonary and extrapulmonary microthrombi and hypoxia in severe COVID-19 patients. SARS-CoV-2 SP attachments to the heavily sialylated surfaces of platelets (which, like RBCs, have no ACE2) and endothelial cells (having minimal ACE2) compound this vascular damage. Notably, experimentally induced RBC aggregation in vivo causes the same key morbidities as for severe COVID-19, including microvascular occlusion, blood clots, hypoxia and myocarditis. Key risk factors for COVID-19 morbidity, including older age, diabetes and obesity, are all characterized by markedly increased propensity to RBC clumping. For mammalian species, the degree of clinical susceptibility to COVID-19 correlates to RBC aggregability with p = 0.033. Notably, of the five human betacoronaviruses, the two common cold strains express an enzyme that releases glycan attachments, while the deadly SARS, SARS-CoV-2 and MERS do not, although viral loads for COVID-19 and the two common cold infections are similar. These biochemical insights also explain the previously puzzling clinical efficacy of certain generics against COVID-19 and may support the development of future therapeutic strategies for COVID-19 and long COVID patients.</jats:p>",
"alternative-id": [
"ijms242317039"
],
"author": [
{
"ORCID": "http://orcid.org/0000-0001-6841-7054",
"affiliation": [
{
"name": "US Public Health Service, Commissioned Corps, Inactive Reserve, Blacksburg, VA 24060, USA"
}
],
"authenticated-orcid": false,
"family": "Scheim",
"given": "David E.",
"sequence": "first"
},
{
"ORCID": "http://orcid.org/0000-0002-6171-5427",
"affiliation": [
{
"name": "Department of Biomedical Engineering, University of Alberta, Edmonton, AB T6G 1Z2, Canada"
}
],
"authenticated-orcid": false,
"family": "Vottero",
"given": "Paola",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-4724-9738",
"affiliation": [
{
"name": "Department of Obstetrics, Gynecology & Reproductive Sciences, Yale School of Medicine, P.O. Box 208063, New Haven, CT 06520, USA"
}
],
"authenticated-orcid": false,
"family": "Santin",
"given": "Alessandro D.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "CryoBioPhysica Inc., Chevy Chase, MD 20815, USA"
}
],
"family": "Hirsh",
"given": "Allen G.",
"sequence": "additional"
}
],
"container-title": "International Journal of Molecular Sciences",
"container-title-short": "IJMS",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2023,
12,
1
]
],
"date-time": "2023-12-01T13:36:59Z",
"timestamp": 1701437819000
},
"deposited": {
"date-parts": [
[
2023,
12,
1
]
],
"date-time": "2023-12-01T13:51:18Z",
"timestamp": 1701438678000
},
"indexed": {
"date-parts": [
[
2023,
12,
2
]
],
"date-time": "2023-12-02T00:52:36Z",
"timestamp": 1701478356998
},
"is-referenced-by-count": 0,
"issue": "23",
"issued": {
"date-parts": [
[
2023,
12,
1
]
]
},
"journal-issue": {
"issue": "23",
"published-online": {
"date-parts": [
[
2023,
12
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
12,
1
]
],
"date-time": "2023-12-01T00:00:00Z",
"timestamp": 1701388800000
}
}
],
"link": [
{
"URL": "https://www.mdpi.com/1422-0067/24/23/17039/pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "1968",
"original-title": [],
"page": "17039",
"prefix": "10.3390",
"published": {
"date-parts": [
[
2023,
12,
1
]
]
},
"published-online": {
"date-parts": [
[
2023,
12,
1
]
]
},
"publisher": "MDPI AG",
"reference": [
{
"DOI": "10.1183/16000617.0138-2021",
"article-title": "COVID-19 pneumonia: Pathophysiology and management",
"author": "Gattinoni",
"doi-asserted-by": "crossref",
"first-page": "210138",
"journal-title": "Eur. Respir. Rev.",
"key": "ref_1",
"volume": "30",
"year": "2021"
},
{
"DOI": "10.1016/j.chest.2021.06.016",
"article-title": "Pulmonary Thrombosis and Thromboembolism in COVID-19",
"author": "Poor",
"doi-asserted-by": "crossref",
"first-page": "1471",
"journal-title": "Chest",
"key": "ref_2",
"volume": "160",
"year": "2021"
},
{
"DOI": "10.3390/jcm11164896",
"doi-asserted-by": "crossref",
"key": "ref_3",
"unstructured": "Selickman, J., Vrettou, C.S., Mentzelopoulos, S.D., and Marini, J.J. (2022). COVID-19-Related ARDS: Key Mechanistic Features and Treatments. J. Clin. Med., 11."
},
{
"DOI": "10.1038/s41569-021-00640-2",
"article-title": "Potential long-term effects of SARS-CoV-2 infection on the pulmonary vasculature: A global perspective",
"author": "Halawa",
"doi-asserted-by": "crossref",
"first-page": "314",
"journal-title": "Nat. Rev. Cardiol.",
"key": "ref_4",
"volume": "19",
"year": "2022"
},
{
"DOI": "10.1126/science.368.6490.455",
"article-title": "The mystery of the pandemic’s ‘happy hypoxia’",
"doi-asserted-by": "crossref",
"first-page": "455",
"journal-title": "Science",
"key": "ref_5",
"volume": "368",
"year": "2020"
},
{
"DOI": "10.1001/jama.2020.6825",
"article-title": "Management of COVID-19 Respiratory Distress",
"author": "Marini",
"doi-asserted-by": "crossref",
"first-page": "2329",
"journal-title": "JAMA",
"key": "ref_6",
"volume": "323",
"year": "2020"
},
{
"DOI": "10.1371/journal.pcbi.1009892",
"doi-asserted-by": "crossref",
"key": "ref_7",
"unstructured": "Li, H., Deng, Y., Li, Z., Dorken Gallastegi, A., Mantzoros, C.S., Frydman, G.H., and Karniadakis, G.E. (2022). Multiphysics and multiscale modeling of microthrombosis in COVID-19. PLoS Comput. Biol., 18."
},
{
"DOI": "10.1016/j.eclinm.2020.100434",
"article-title": "Megakaryocytes and platelet-fibrin thrombi characterize multi-organ thrombosis at autopsy in COVID-19: A case series",
"author": "Rapkiewicz",
"doi-asserted-by": "crossref",
"first-page": "100434",
"journal-title": "eClinicalMedicine",
"key": "ref_8",
"volume": "24",
"year": "2020"
},
{
"DOI": "10.7326/M20-2003",
"article-title": "Autopsy Findings and Venous Thromboembolism in Patients With COVID-19",
"author": "Wichmann",
"doi-asserted-by": "crossref",
"first-page": "268",
"journal-title": "Ann. Intern. Med.",
"key": "ref_9",
"volume": "173",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2015432",
"article-title": "Pulmonary Vascular Endothelialitis, Thrombosis, and Angiogenesis in COVID-19",
"author": "Ackermann",
"doi-asserted-by": "crossref",
"first-page": "120",
"journal-title": "N. Engl. J. Med.",
"key": "ref_10",
"volume": "383",
"year": "2020"
},
{
"DOI": "10.1016/S2213-2600(20)30243-5",
"article-title": "Pulmonary and cardiac pathology in African American patients with COVID-19: An autopsy series from New Orleans",
"author": "Fox",
"doi-asserted-by": "crossref",
"first-page": "681",
"journal-title": "Lancet Respir. Med.",
"key": "ref_11",
"volume": "8",
"year": "2020"
},
{
"DOI": "10.1002/ajh.25982",
"article-title": "Thrombosis in COVID-19",
"author": "Hanff",
"doi-asserted-by": "crossref",
"first-page": "1578",
"journal-title": "Am. J. Hematol.",
"key": "ref_12",
"volume": "95",
"year": "2020"
},
{
"DOI": "10.1097/CCE.0000000000000427",
"article-title": "Is Microthrombosis the Main Pathology in Coronavirus Disease 2019 Severity?-A Systematic Review of the Postmortem Pathologic Findings",
"author": "Fahmy",
"doi-asserted-by": "crossref",
"first-page": "e0427",
"journal-title": "Crit. Care Explor.",
"key": "ref_13",
"volume": "3",
"year": "2021"
},
{
"DOI": "10.1111/his.14134",
"article-title": "Postmortem examination of COVID-19 patients reveals diffuse alveolar damage with severe capillary congestion and variegated findings in lungs and other organs suggesting vascular dysfunction",
"author": "Menter",
"doi-asserted-by": "crossref",
"first-page": "198",
"journal-title": "Histopathology",
"key": "ref_14",
"volume": "77",
"year": "2020"
},
{
"DOI": "10.1161/CIRCRESAHA.120.317447",
"article-title": "The Emerging Threat of (Micro)Thrombosis in COVID-19 and Its Therapeutic Implications",
"author": "McFadyen",
"doi-asserted-by": "crossref",
"first-page": "571",
"journal-title": "Circ. Res.",
"key": "ref_15",
"volume": "127",
"year": "2020"
},
{
"article-title": "A review of COVID-19-related thrombosis and anticoagulation strategies specific to the Asian population",
"author": "Poh",
"first-page": "350",
"journal-title": "Singap. Med. J.",
"key": "ref_16",
"volume": "63",
"year": "2022"
},
{
"DOI": "10.1016/j.ebiom.2020.103104",
"doi-asserted-by": "crossref",
"key": "ref_17",
"unstructured": "Bussani, R., Schneider, E., Zentilin, L., Collesi, C., Ali, H., Braga, L., Volpe, M.C., Colliva, A., Zanconati, F., and Berlot, G. (2020). Persistence of viral RNA, pneumocyte syncytia and thrombosis are hallmarks of advanced COVID-19 pathology. eBioMedicine, 61."
},
{
"DOI": "10.1002/pul2.12113",
"article-title": "Pulmonary thromboembolic events in COVID-19—A systematic literature review",
"author": "Overton",
"doi-asserted-by": "crossref",
"first-page": "e12113",
"journal-title": "Pulm. Circ.",
"key": "ref_18",
"volume": "12",
"year": "2022"
},
{
"DOI": "10.1111/jth.14768",
"article-title": "Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia",
"author": "Tang",
"doi-asserted-by": "crossref",
"first-page": "844",
"journal-title": "J. Thromb. Haemost.",
"key": "ref_19",
"volume": "18",
"year": "2020"
},
{
"DOI": "10.1161/CIRCULATIONAHA.120.051828",
"article-title": "Microthrombi as a Major Cause of Cardiac Injury in COVID-19: A Pathologic Study",
"author": "Pellegrini",
"doi-asserted-by": "crossref",
"first-page": "1031",
"journal-title": "Circulation",
"key": "ref_20",
"volume": "143",
"year": "2021"
},
{
"DOI": "10.1161/CIRCULATIONAHA.120.050354",
"article-title": "Severe COVID-19 Is a Microvascular Disease",
"author": "Lowenstein",
"doi-asserted-by": "crossref",
"first-page": "1609",
"journal-title": "Circulation",
"key": "ref_21",
"volume": "142",
"year": "2020"
},
{
"DOI": "10.1183/13993003.01608-2020",
"article-title": "Thrombosis and COVID-19 pneumonia: The clot thickens!",
"author": "Price",
"doi-asserted-by": "crossref",
"first-page": "2001608",
"journal-title": "Eur. Respir. J.",
"key": "ref_22",
"volume": "56",
"year": "2020"
},
{
"DOI": "10.1016/j.thromres.2020.04.024",
"article-title": "Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy",
"author": "Lodigiani",
"doi-asserted-by": "crossref",
"first-page": "9",
"journal-title": "Thromb. Res.",
"key": "ref_23",
"volume": "191",
"year": "2020"
},
{
"DOI": "10.1016/j.trsl.2020.04.007",
"article-title": "Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: A report of five cases",
"author": "Magro",
"doi-asserted-by": "crossref",
"first-page": "1",
"journal-title": "Transl. Res.",
"key": "ref_24",
"volume": "220",
"year": "2020"
},
{
"DOI": "10.1016/j.gene.2020.145102",
"article-title": "Angiotensin-converting enzymes (ACE, ACE2) gene variants and COVID-19 outcome",
"author": "Albaiceta",
"doi-asserted-by": "crossref",
"first-page": "145102",
"journal-title": "Gene",
"key": "ref_25",
"volume": "762",
"year": "2020"
},
{
"DOI": "10.1016/j.jinf.2020.11.012",
"article-title": "Angiotensin converting enzyme genotypes and mortality from COVID-19: An ecological study",
"author": "Aung",
"doi-asserted-by": "crossref",
"first-page": "961",
"journal-title": "J. Infect.",
"key": "ref_26",
"volume": "81",
"year": "2020"
},
{
"DOI": "10.3389/fphys.2020.574753",
"article-title": "ACE2, COVID-19 Infection, Inflammation, and Coagulopathy: Missing Pieces in the Puzzle",
"author": "Abassi",
"doi-asserted-by": "crossref",
"first-page": "574753",
"journal-title": "Front. Physiol.",
"key": "ref_27",
"volume": "11",
"year": "2020"
},
{
"DOI": "10.1128/mbio.02308-22",
"doi-asserted-by": "crossref",
"key": "ref_28",
"unstructured": "Kong, W., Montano, M., Corley, M.J., Helmy, E., Kobayashi, H., Kinisu, M., Suryawanshi, R., Luo, X., Royer, L.A., and Roan, N.R. (2022). Neuropilin-1 Mediates SARS-CoV-2 Infection of Astrocytes in Brain Organoids, Inducing Inflammation Leading to Dysfunction and Death of Neurons. mBio, 13."
},
{
"DOI": "10.1126/science.abd2985",
"article-title": "Neuropilin-1 facilitates SARS-CoV-2 cell entry and infectivity",
"author": "Ojha",
"doi-asserted-by": "crossref",
"first-page": "856",
"journal-title": "Science",
"key": "ref_29",
"volume": "370",
"year": "2020"
},
{
"DOI": "10.1016/bs.aivir.2016.08.004",
"article-title": "Chapter Two—Coronavirus Spike Protein and Tropism Changes",
"author": "Ziebuhr",
"doi-asserted-by": "crossref",
"first-page": "29",
"journal-title": "Advances in Virus Research",
"key": "ref_30",
"volume": "Volume 96",
"year": "2016"
},
{
"DOI": "10.3390/ijms23052558",
"doi-asserted-by": "crossref",
"key": "ref_31",
"unstructured": "Scheim, D.E. (2022). A Deadly Embrace: Hemagglutination Mediated by SARS-CoV-2 Spike Protein at its 22 N-Glycosylation Sites, Red Blood Cell Surface Sialoglycoproteins, and Antibody. Int. J. Mol. Sci., 23."
},
{
"DOI": "10.1007/978-3-319-21317-0",
"doi-asserted-by": "crossref",
"key": "ref_32",
"unstructured": "Gerardy-Schahn, R., Delannoy, P., and von Itzstein, M. (2015). SialoGlyco Chemistry and Biology II: Tools and Techniques to Identify and Capture Sialoglycans, Springer International Publishing."
},
{
"DOI": "10.1038/s41594-019-0233-y",
"article-title": "Structural basis for human coronavirus attachment to sialic acid receptors",
"author": "Tortorici",
"doi-asserted-by": "crossref",
"first-page": "481",
"journal-title": "Nat. Struct. Mol. Biol.",
"key": "ref_33",
"volume": "26",
"year": "2019"
},
{
"DOI": "10.1002/anie.202201432",
"article-title": "The SARS-CoV-2 Spike Glycoprotein Directly Binds Exogeneous Sialic Acids: A NMR View",
"author": "Unione",
"doi-asserted-by": "crossref",
"first-page": "e202201432",
"journal-title": "Angew. Chem. Int. Ed.",
"key": "ref_34",
"volume": "61",
"year": "2022"
},
{
"DOI": "10.3181/00379727-139-36105",
"article-title": "Hemadsorption by coronavirus strain OC43",
"author": "Kapikian",
"doi-asserted-by": "crossref",
"first-page": "179",
"journal-title": "Proc. Soc. Exp. Biol. Med.",
"key": "ref_35",
"volume": "139",
"year": "1972"
},
{
"DOI": "10.1023/B:DOBS.0000017131.06970.74",
"article-title": "Primary characterization of SARS coronavirus strain Frankfurt 1",
"author": "Agafonov",
"doi-asserted-by": "crossref",
"first-page": "58",
"journal-title": "Dokl. Biol. Sci.",
"key": "ref_36",
"volume": "394",
"year": "2004"
},
{
"DOI": "10.1073/pnas.85.12.4526",
"article-title": "Human and bovine coronaviruses recognize sialic acid-containing receptors similar to those of influenza C viruses",
"author": "Vlasak",
"doi-asserted-by": "crossref",
"first-page": "4526",
"journal-title": "Proc. Natl. Acad. Sci. USA",
"key": "ref_37",
"volume": "85",
"year": "1988"
},
{
"DOI": "10.1007/BF01309637",
"article-title": "Comparison of hemagglutinating, receptor-destroying, and acetylesterase activities of avirulent and virulent bovine coronavirus strains",
"author": "Storz",
"doi-asserted-by": "crossref",
"first-page": "193",
"journal-title": "Arch. Virol.",
"key": "ref_38",
"volume": "125",
"year": "1992"
},
{
"DOI": "10.1007/978-1-4899-1531-3",
"doi-asserted-by": "crossref",
"key": "ref_39",
"unstructured": "Siddell, S.G. (1995). The Coronaviridae. The Viruses, Springer."
},
{
"DOI": "10.1128/mBio.02764-19",
"article-title": "Distinct Roles for Sialoside and Protein Receptors in Coronavirus Infection",
"author": "Qing",
"doi-asserted-by": "crossref",
"first-page": "e02764-19",
"journal-title": "mBio",
"key": "ref_40",
"volume": "11",
"year": "2020"
},
{
"DOI": "10.1016/0042-6822(92)90608-R",
"article-title": "Neuraminidase treatment of avian infectious bronchitis coronavirus reveals a hemagglutinating activity that is dependent on sialic acid-containing receptors on erythrocytes",
"author": "Schultze",
"doi-asserted-by": "crossref",
"first-page": "792",
"journal-title": "Virology",
"key": "ref_41",
"volume": "189",
"year": "1992"
},
{
"DOI": "10.1073/pnas.1712592114",
"article-title": "Identification of sialic acid-binding function for the Middle East respiratory syndrome coronavirus spike glycoprotein",
"author": "Li",
"doi-asserted-by": "crossref",
"first-page": "E8508",
"journal-title": "Proc. Natl. Acad. Sci. USA",
"key": "ref_42",
"volume": "114",
"year": "2017"
},
{
"DOI": "10.1073/pnas.1809667116",
"article-title": "Human coronaviruses OC43 and HKU1 bind to 9-O-acetylated sialic acids via a conserved receptor-binding site in spike protein domain A",
"author": "Hulswit",
"doi-asserted-by": "crossref",
"first-page": "2681",
"journal-title": "Proc. Natl. Acad. Sci. USA",
"key": "ref_43",
"volume": "116",
"year": "2019"
},
{
"DOI": "10.1016/j.sbi.2011.08.009",
"article-title": "Viruses and sialic acids: Rules of engagement",
"author": "Neu",
"doi-asserted-by": "crossref",
"first-page": "610",
"journal-title": "Curr. Opin. Struct. Biol.",
"key": "ref_44",
"volume": "21",
"year": "2011"
},
{
"DOI": "10.1128/JVI.00854-15",
"article-title": "Human Coronavirus HKU1 Spike Protein Uses O-Acetylated Sialic Acid as an Attachment Receptor Determinant and Employs Hemagglutinin-Esterase Protein as a Receptor-Destroying Enzyme",
"author": "Huang",
"doi-asserted-by": "crossref",
"first-page": "7202",
"journal-title": "J. Virol.",
"key": "ref_45",
"volume": "89",
"year": "2015"
},
{
"DOI": "10.1038/s41594-019-0334-7",
"article-title": "Structures of MERS-CoV spike glycoprotein in complex with sialoside attachment receptors",
"author": "Park",
"doi-asserted-by": "crossref",
"first-page": "1151",
"journal-title": "Nat. Struct. Mol. Biol.",
"key": "ref_46",
"volume": "26",
"year": "2019"
},
{
"DOI": "10.3390/cells9091963",
"doi-asserted-by": "crossref",
"key": "ref_47",
"unstructured": "Wielgat, P., Rogowski, K., Godlewska, K., and Car, H. (2020). Coronaviruses: Is Sialic Acid a Gate to the Eye of Cytokine Storm? From the Entry to the Effects. Cells, 9."
},
{
"DOI": "10.1146/annurev-virology-122019-070025",
"article-title": "Initial Step of Virus Entry: Virion Binding to Cell-Surface Glycans",
"author": "Koehler",
"doi-asserted-by": "crossref",
"first-page": "143",
"journal-title": "Annu. Rev. Virol.",
"key": "ref_48",
"volume": "7",
"year": "2020"
},
{
"DOI": "10.1146/annurev-virology-031413-085417",
"article-title": "Glycan Engagement by Viruses: Receptor Switches and Specificity",
"author": "Stehle",
"doi-asserted-by": "crossref",
"first-page": "285",
"journal-title": "Annu. Rev. Virol.",
"key": "ref_49",
"volume": "1",
"year": "2014"
},
{
"DOI": "10.1080/22221751.2020.1719902",
"article-title": "Genomic characterization of the 2019 novel human-pathogenic coronavirus isolated from a patient with atypical pneumonia after visiting Wuhan",
"author": "Chan",
"doi-asserted-by": "crossref",
"first-page": "221",
"journal-title": "Emerg. Microbes Infect.",
"key": "ref_50",
"volume": "9",
"year": "2020"
},
{
"DOI": "10.1002/jmv.25681",
"article-title": "Emerging coronaviruses: Genome structure, replication, and pathogenesis",
"author": "Chen",
"doi-asserted-by": "crossref",
"first-page": "418",
"journal-title": "J. Med. Virol.",
"key": "ref_51",
"volume": "92",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa1211721",
"article-title": "Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia",
"author": "Zaki",
"doi-asserted-by": "crossref",
"first-page": "1814",
"journal-title": "N. Engl. J. Med.",
"key": "ref_52",
"volume": "367",
"year": "2012"
},
{
"DOI": "10.1007/978-981-15-4814-7",
"doi-asserted-by": "crossref",
"key": "ref_53",
"unstructured": "Saxena, S.K. (2020). Coronavirus Disease 2019 (COVID-19): Epidemiology, Pathogenesis, Diagnosis, and Therapeutics, Springer."
},
{
"DOI": "10.1007/s10930-020-09901-4",
"article-title": "The Proteins of Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2 or n-COV19), the Cause of COVID-19",
"author": "Yoshimoto",
"doi-asserted-by": "crossref",
"first-page": "198",
"journal-title": "Protein J.",
"key": "ref_54",
"volume": "39",
"year": "2020"
},
{
"DOI": "10.1016/j.micinf.2020.08.004",
"article-title": "Viral load of SARS-CoV-2 across patients and compared to other respiratory viruses",
"author": "Jacot",
"doi-asserted-by": "crossref",
"first-page": "617",
"journal-title": "Microbes Infect.",
"key": "ref_55",
"volume": "22",
"year": "2020"
},
{
"DOI": "10.1093/glycob/cwaa042",
"article-title": "Deducing the N- and O-glycosylation profile of the spike protein of novel coronavirus SARS-CoV-2",
"author": "Shajahan",
"doi-asserted-by": "crossref",
"first-page": "981",
"journal-title": "Glycobiology",
"key": "ref_56",
"volume": "30",
"year": "2020"
},
{
"DOI": "10.2147/IJN.S302516",
"article-title": "Glycan Nanostructures of Human Coronaviruses",
"author": "Guo",
"doi-asserted-by": "crossref",
"first-page": "4813",
"journal-title": "Int. J. Nanomed.",
"key": "ref_57",
"volume": "16",
"year": "2021"
},
{
"DOI": "10.1021/acs.jctc.0c01144",
"article-title": "Structure, Dynamics, Receptor Binding, and Antibody Binding of the Fully Glycosylated Full-Length SARS-CoV-2 Spike Protein in a Viral Membrane",
"author": "Choi",
"doi-asserted-by": "crossref",
"first-page": "2479",
"journal-title": "J. Chem. Theory Comput.",
"key": "ref_58",
"volume": "17",
"year": "2021"
},
{
"DOI": "10.1021/acscentsci.0c00855",
"article-title": "The SARS-CoV-2 Spike Protein Binds Sialic Acids and Enables Rapid Detection in a Lateral Flow Point of Care Diagnostic Device",
"author": "Baker",
"doi-asserted-by": "crossref",
"first-page": "2046",
"journal-title": "ACS Cent. Sci.",
"key": "ref_59",
"volume": "6",
"year": "2020"
},
{
"DOI": "10.1016/j.jbc.2021.100375",
"doi-asserted-by": "crossref",
"key": "ref_60",
"unstructured": "Lardone, R.D., Garay, Y.C., Parodi, P., de la Fuente, S., Angeloni, G., Bravo, E.O., Schmider, A.K., and Irazoqui, F.J. (2021). How glycobiology can help us treat and beat the COVID-19 pandemic. J. Biol. Chem., 296."
},
{
"DOI": "10.1101/2020.07.29.227462",
"doi-asserted-by": "crossref",
"key": "ref_61",
"unstructured": "Gao, C., Zeng, J., Jia, N., Stavenhagen, K., Matsumoto, Y., Zhang, H., Li, J., Hume, A.J., Mühlberger, E., and van Die, I. (2020). SARS-CoV-2 Spike Protein Interacts with Multiple Innate Immune Receptors. bioRxiv."
},
{
"key": "ref_62",
"unstructured": "Chen, W., Hui, Z., Ren, X., Luo, Y., Shu, J., Yu, H., and Li, Z. The N-glycosylation sites and Glycan-binding ability of S-protein in SARS-CoV-2 Coronavirus. bioRxiv."
},
{
"DOI": "10.1021/acscentsci.0c01056",
"article-title": "Beyond Shielding: The Roles of Glycans in the SARS-CoV-2 Spike Protein",
"author": "Casalino",
"doi-asserted-by": "crossref",
"first-page": "1722",
"journal-title": "ACS Cent. Sci.",
"key": "ref_63",
"volume": "6",
"year": "2020"
},
{
"DOI": "10.1182/blood-2015-11-680009",
"article-title": "Disturbed sialic acid recognition on endothelial cells and platelets in complement attack causes atypical hemolytic uremic syndrome",
"author": "Meri",
"doi-asserted-by": "crossref",
"first-page": "2701",
"journal-title": "Blood",
"key": "ref_64",
"volume": "127",
"year": "2016"
},
{
"DOI": "10.1046/j.1365-2567.1999.00653.x",
"article-title": "CD147 monoclonal antibodies induce homotypic cell aggregation of monocytic cell line U937 via LFA-1/ICAM-1 pathway",
"author": "Kasinrerk",
"doi-asserted-by": "crossref",
"first-page": "184",
"journal-title": "Immunology",
"key": "ref_65",
"volume": "96",
"year": "1999"
},
{
"DOI": "10.1111/jth.15156",
"article-title": "Is there a role for the ACE2 receptor in SARS-CoV-2 interactions with platelets?",
"author": "Campbell",
"doi-asserted-by": "crossref",
"first-page": "46",
"journal-title": "J. Thromb. Haemost.",
"key": "ref_66",
"volume": "19",
"year": "2021"
},
{
"DOI": "10.3390/app10114053",
"doi-asserted-by": "crossref",
"key": "ref_67",
"unstructured": "Cosic, I., Cosic, D., and Loncarevic, I. (2020). RRM Prediction of Erythrocyte Band3 Protein as Alternative Receptor for SARS-CoV-2 Virus. Appl. Sci., 10."
},
{
"DOI": "10.31219/osf.io/sgdj2",
"doi-asserted-by": "crossref",
"key": "ref_68",
"unstructured": "Scheim, D.E., and From Cold to Killer: How SARS-CoV-2 Evolved without Hemagglutinin Esterase to Agglutinate, Then Clot Blood Cells in Pulmonary and Systemic Microvasculature (2023, October 30). Available online: https://osf.io/sgdj2."
},
{
"DOI": "10.1101/2020.06.23.165324",
"doi-asserted-by": "crossref",
"key": "ref_69",
"unstructured": "Ahmetaj-Shala, B., Vaja, R., Atanur, S., George, P., Kirkby, N., and Mitchell, J. (2020). Systemic analysis of putative SARS-CoV-2 entry and processing genes in cardiovascular tissues identifies a positive correlation of BSG with age in endothelial cells. bioRxiv."
},
{
"DOI": "10.1681/ASN.2007010119",
"article-title": "Glomerular endothelial glycocalyx constitutes a barrier to protein permeability",
"author": "Singh",
"doi-asserted-by": "crossref",
"first-page": "2885",
"journal-title": "J. Am. Soc. Nephrol.",
"key": "ref_70",
"volume": "18",
"year": "2007"
},
{
"DOI": "10.1016/0968-0004(85)90067-2",
"article-title": "The red cell surface revisited",
"author": "Viitala",
"doi-asserted-by": "crossref",
"first-page": "392",
"journal-title": "Trends Biochem. Sci.",
"key": "ref_71",
"volume": "10",
"year": "1985"
},
{
"DOI": "10.1093/oxfordjournals.molbev.a004075",
"article-title": "Natural selection on the erythrocyte surface",
"author": "Baum",
"doi-asserted-by": "crossref",
"first-page": "223",
"journal-title": "Mol. Biol. Evol.",
"key": "ref_72",
"volume": "19",
"year": "2002"
},
{
"DOI": "10.1016/S0006-3495(83)84378-1",
"article-title": "Theory of the electrokinetic behavior of human erythrocytes",
"author": "Levine",
"doi-asserted-by": "crossref",
"first-page": "127",
"journal-title": "Biophys. J.",
"key": "ref_73",
"volume": "42",
"year": "1983"
},
{
"DOI": "10.3390/membranes7040056",
"doi-asserted-by": "crossref",
"key": "ref_74",
"unstructured": "Aoki, T. (2017). A Comprehensive Review of Our Current Understanding of Red Blood Cell (RBC) Glycoproteins. Membranes, 7."
},
{
"DOI": "10.2174/1389203033380304",
"article-title": "Why are glycoproteins modified by poly-N-acetyllactosamine glyco-conjugates?",
"author": "Zhou",
"doi-asserted-by": "crossref",
"first-page": "1",
"journal-title": "Curr. Protein Pept. Sci.",
"key": "ref_75",
"volume": "4",
"year": "2003"
},
{
"DOI": "10.1016/S0021-9258(17)39722-3",
"article-title": "Structure of branched lactosaminoglycan, the carbohydrate moiety of band 3 isolated from adult human erythrocytes",
"author": "Fukuda",
"doi-asserted-by": "crossref",
"first-page": "8260",
"journal-title": "J. Biol. Chem.",
"key": "ref_76",
"volume": "259",
"year": "1984"
},
{
"DOI": "10.20944/preprints202107.0071.v1",
"doi-asserted-by": "crossref",
"key": "ref_77",
"unstructured": "Bua, R.O., Messina, A., Sturiale, L., Barone, R., Garozzo, D., and Palmigiano, A. (2021). N-Glycomics of Human Erythrocytes. Int. J. Mol. Sci., 22."
},
{
"DOI": "10.1016/S0021-9258(19)36803-6",
"article-title": "Identification of N-acetylneuraminyl alpha 2-->3 poly-N-acetyllactosamine glycans as the receptors of sialic acid-binding Streptococcus suis strains",
"author": "Liukkonen",
"doi-asserted-by": "crossref",
"first-page": "21105",
"journal-title": "J. Biol. Chem.",
"key": "ref_78",
"volume": "267",
"year": "1992"
},
{
"DOI": "10.1002/anie.201200596",
"article-title": "Recognition of Sialylated Poly-N-acetyllactosamine Chains on N- and O-Linked Glycans by Human and Avian Influenza|A Virus Hemagglutinins",
"author": "Nycholat",
"doi-asserted-by": "crossref",
"first-page": "4860",
"journal-title": "Angew. Chem. Int. Ed.",
"key": "ref_79",
"volume": "51",
"year": "2012"
},
{
"DOI": "10.1016/0042-6822(87)90351-5",
"article-title": "Glycophorin is the reovirus receptor on human erythrocytes",
"author": "Paul",
"doi-asserted-by": "crossref",
"first-page": "94",
"journal-title": "Virology",
"key": "ref_80",
"volume": "159",
"year": "1987"
},
{
"DOI": "10.1016/0014-5793(88)80307-7",
"article-title": "A N-acetyllactosamine-specific cell-binding activity in a plant pathogen, Erwinia rhapontici",
"author": "Korhonen",
"doi-asserted-by": "crossref",
"first-page": "163",
"journal-title": "FEBS Lett.",
"key": "ref_81",
"volume": "236",
"year": "1988"
},
{
"DOI": "10.1016/S0065-2776(08)60812-3",
"article-title": "Immune Adherence",
"author": "Dixon",
"doi-asserted-by": "crossref",
"first-page": "131",
"journal-title": "Advances in Immunology",
"key": "ref_82",
"volume": "Volume 3",
"year": "1963"
},
{
"DOI": "10.4049/jimmunol.1800565",
"article-title": "The Evolving Erythrocyte: Red Blood Cells as Modulators of Innate Immunity",
"author": "Anderson",
"doi-asserted-by": "crossref",
"first-page": "1343",
"journal-title": "J. Immunol.",
"key": "ref_83",
"volume": "201",
"year": "2018"
},
{
"DOI": "10.1111/j.1749-6632.2012.06517.x",
"article-title": "Multifarious roles of sialic acids in immunity",
"author": "Varki",
"doi-asserted-by": "crossref",
"first-page": "16",
"journal-title": "Ann. N. Y. Acad. Sci.",
"key": "ref_84",
"volume": "1253",
"year": "2012"
},
{
"DOI": "10.1126/science.118.3077.733",
"article-title": "The Immune-Adherence Phenomenon: An Immunologically Specific Reaction Between Microorganisms and Erythrocytes Leading to Enhanced Phagocytosis",
"author": "Nelson",
"doi-asserted-by": "crossref",
"first-page": "733",
"journal-title": "Science",
"key": "ref_85",
"volume": "118",
"year": "1953"
},
{
"DOI": "10.1182/blood.V128.22.1031.1031",
"article-title": "RBC Adhesive Capacity Is Essential for Efficient ‘Immune Adherence Clearance’ and Provide a Generic Target to Deplete Pathogens from Septic Patients",
"author": "Kostova",
"doi-asserted-by": "crossref",
"first-page": "1031",
"journal-title": "Blood",
"key": "ref_86",
"volume": "128",
"year": "2016"
},
{
"DOI": "10.1016/S0140-6736(81)90941-7",
"article-title": "The Red-Cell Immune System",
"author": "Siegel",
"doi-asserted-by": "crossref",
"first-page": "556",
"journal-title": "Lancet",
"key": "ref_87",
"volume": "318",
"year": "1981"
},
{
"DOI": "10.1160/TH16-12-0921",
"article-title": "Small but mighty: Platelets as central effectors of host defense",
"author": "Stocker",
"doi-asserted-by": "crossref",
"first-page": "651",
"journal-title": "Thromb. Haemost.",
"key": "ref_88",
"volume": "117",
"year": "2017"
},
{
"DOI": "10.1097/MOH.0000000000000219",
"article-title": "Red cell receptors as access points for malaria infection",
"author": "Salinas",
"doi-asserted-by": "crossref",
"first-page": "215",
"journal-title": "Curr. Opin. Hematol.",
"key": "ref_89",
"volume": "23",
"year": "2016"
},
{
"DOI": "10.1371/journal.pcbi.1008790",
"doi-asserted-by": "crossref",
"key": "ref_90",
"unstructured": "Sikora, M., von Bülow, S., Blanc, F.E.C., Gecht, M., Covino, R., and Hummer, G. (2021). Computational epitope map of SARS-CoV-2 spike protein. PLoS Comput. Biol., 17."
},
{
"DOI": "10.1101/2022.11.24.517882",
"doi-asserted-by": "crossref",
"key": "ref_91",
"unstructured": "Boschi, C., Scheim, D.E., Bancod, A., Militello, M., Bideau, M.L., Colson, P., Fantini, J., and Scola, B.L. (2022). SARS-CoV-2 Spike Protein Induces Hemagglutination: Implications for COVID-19 Morbidities and Therapeutics and for Vaccine Adverse Effects. Int. J. Mol. Sci., 23."
},
{
"DOI": "10.1053/j.seminhematol.2004.01.001",
"article-title": "Red blood cell blood group antigens: Structure and function",
"author": "Reid",
"doi-asserted-by": "crossref",
"first-page": "93",
"journal-title": "Semin. Hematol.",
"key": "ref_92",
"volume": "41",
"year": "2004"
},
{
"DOI": "10.3389/fphys.2019.00945",
"article-title": "Red Blood Cells: Chasing Interactions",
"author": "Pretini",
"doi-asserted-by": "crossref",
"first-page": "945",
"journal-title": "Front. Physiol.",
"key": "ref_93",
"volume": "10",
"year": "2019"
},
{
"DOI": "10.1186/s13071-019-3575-8",
"article-title": "Erythrocyte glycophorins as receptors for Plasmodium merozoites",
"author": "Jaskiewicz",
"doi-asserted-by": "crossref",
"first-page": "317",
"journal-title": "Parasites Vectors",
"key": "ref_94",
"volume": "12",
"year": "2019"
},
{
"key": "ref_95",
"unstructured": "Varki, A., Cummings, R., and Esko, J. (2017). Essentials of Glycobiology, Cold Spring Harbor Laboratory Press. [3rd ed.]."
},
{
"DOI": "10.3389/fmolb.2021.727847",
"doi-asserted-by": "crossref",
"key": "ref_96",
"unstructured": "Soares, C.O., Grosso, A.S., Ereño-Orbea, J., Coelho, H., and Marcelo, F. (2021). Molecular Recognition Insights of Sialic Acid Glycans by Distinct Receptors Unveiled by NMR and Molecular Modeling. Front. Mol. Biosci., 8."
},
{
"DOI": "10.1016/B978-0-12-800097-7.00003-8",
"article-title": "Chapter Three—Modulation of Glycan Recognition by Clustered Saccharide Patches",
"author": "Jeon",
"doi-asserted-by": "crossref",
"first-page": "75",
"journal-title": "International Review of Cell and Molecular Biology",
"key": "ref_97",
"volume": "Volume 308",
"year": "2014"
},
{
"DOI": "10.1089/omi.2009.0148",
"article-title": "The sialome—Far more than the sum of its parts",
"author": "Cohen",
"doi-asserted-by": "crossref",
"first-page": "455",
"journal-title": "Omics",
"key": "ref_98",
"volume": "14",
"year": "2010"
},
{
"DOI": "10.3390/computation10040051",
"doi-asserted-by": "crossref",
"key": "ref_99",
"unstructured": "Aminpour, M., Cannariato, M., Safaeeardebili, M.E., Preto, J., Moracchiato, A., Doria, D., Donato, F., Zizzi, E.A., Deriu, M.A., and Scheim, D.E. (2022). In Silico Analysis of the Multi-Targeted Mode of Action of Ivermectin and Related Compounds. Computation, 10."
},
{
"DOI": "10.1042/BSR20210611",
"doi-asserted-by": "crossref",
"key": "ref_100",
"unstructured": "Grobbelaar, L.M., Venter, C., Vlok, M., Ngoepe, M., Laubscher, G.J., Lourens, P.J., Steenkamp, J., Kell, D.B., and Pretorius, E. (2021). SARS-CoV-2 spike protein S1 induces fibrin(ogen) resistant to fibrinolysis: Implications for microclot formation in COVID-19. Biosci. Rep., 41."
},
{
"DOI": "10.1038/s41467-018-06950-3",
"article-title": "Systems glycomics of adult zebrafish identifies organ-specific sialylation and glycosylation patterns",
"author": "Yamakawa",
"doi-asserted-by": "crossref",
"first-page": "4647",
"journal-title": "Nat. Commun.",
"key": "ref_101",
"volume": "9",
"year": "2018"
},
{
"DOI": "10.1073/pnas.1524448113",
"article-title": "Clusters of circulating tumor cells traverse capillary-sized vessels",
"author": "Tang",
"doi-asserted-by": "crossref",
"first-page": "4947",
"journal-title": "Proc. Natl. Acad. Sci. USA",
"key": "ref_102",
"volume": "113",
"year": "2016"
},
{
"DOI": "10.1016/j.ijbiomac.2021.10.112",
"article-title": "SARS-CoV-2 spike protein causes blood coagulation and thrombosis by competitive binding to heparan sulfate",
"author": "Zheng",
"doi-asserted-by": "crossref",
"first-page": "1124",
"journal-title": "Int. J. Biol. Macromol.",
"key": "ref_103",
"volume": "193",
"year": "2021"
},
{
"DOI": "10.1016/j.ijbiomac.2021.04.148",
"article-title": "Heparin: A simplistic repurposing to prevent SARS-CoV-2 transmission in light of its in-vitro nanomolar efficacy",
"author": "Gupta",
"doi-asserted-by": "crossref",
"first-page": "203",
"journal-title": "Int. J. Biol. Macromol.",
"key": "ref_104",
"volume": "183",
"year": "2021"
},
{
"article-title": "Coronavirus (SARS-CoV-2) Deactivation via Spike Glycoprotein Shielding by Old Drugs: Molecular Docking Approach",
"author": "Dayer",
"first-page": "31",
"journal-title": "J. Epigenet.",
"key": "ref_105",
"volume": "2",
"year": "2021"
},
{
"DOI": "10.1055/a-1551-9911",
"article-title": "Mild COVID-19 and Impaired Blood Cell–Endothelial Crosstalk: Considering Long-Term Use of Antithrombotics?",
"author": "Melkumyants",
"doi-asserted-by": "crossref",
"first-page": "123",
"journal-title": "Thromb. Haemost.",
"key": "ref_106",
"volume": "122",
"year": "2022"
},
{
"article-title": "Red blood cell morphology in patients with COVID-19-related anaemia",
"author": "Berzuini",
"first-page": "34",
"journal-title": "Blood Transfus.",
"key": "ref_107",
"volume": "19",
"year": "2021"
},
{
"DOI": "10.1093/clinchem/hvaa213",
"article-title": "Ultra-sensitive Serial Profiling of SARS-CoV-2 Antigens and Antibodies in Plasma to Understand Disease Progression in COVID-19 Patients with Severe Disease",
"author": "Ogata",
"doi-asserted-by": "crossref",
"first-page": "1562",
"journal-title": "Clin. Chem.",
"key": "ref_108",
"volume": "66",
"year": "2020"
},
{
"DOI": "10.3389/fimmu.2022.827146",
"article-title": "SARS-CoV-2 Spike Protein 1 Activates Microvascular Endothelial Cells and Complement System Leading to Platelet Aggregation",
"author": "Perico",
"doi-asserted-by": "crossref",
"first-page": "827146",
"journal-title": "Front. Immunol.",
"key": "ref_109",
"volume": "13",
"year": "2022"
},
{
"DOI": "10.1016/j.anndiagpath.2020.151682",
"article-title": "Endothelial cell damage is the central part of COVID-19 and a mouse model induced by injection of the S1 subunit of the spike protein",
"author": "Nuovo",
"doi-asserted-by": "crossref",
"first-page": "151682",
"journal-title": "Ann. Diagn. Pathol.",
"key": "ref_110",
"volume": "51",
"year": "2021"
},
{
"DOI": "10.1093/cid/ciac722",
"article-title": "Persistent circulating SARS-CoV-2 spike Is associated With post-acute COVID-19 sequelae",
"author": "Swank",
"doi-asserted-by": "crossref",
"first-page": "e487",
"journal-title": "Clin. Infect. Dis.",
"key": "ref_111",
"volume": "76",
"year": "2022"
},
{
"DOI": "10.1002/jmv.28568",
"article-title": "Persistent circulation of soluble and extracellular vesicle-linked Spike protein in individuals with postacute sequelae of COVID-19",
"author": "Craddock",
"doi-asserted-by": "crossref",
"first-page": "e28568",
"journal-title": "J. Med. Virol.",
"key": "ref_112",
"volume": "95",
"year": "2023"
},
{
"DOI": "10.1002/jmv.28364",
"article-title": "Liquid biomarkers of macrophage dysregulation and circulating spike protein illustrate the biological heterogeneity in patients with post-acute sequelae of COVID-19",
"author": "Willscher",
"doi-asserted-by": "crossref",
"first-page": "e28364",
"journal-title": "J. Med. Virol.",
"key": "ref_113",
"volume": "95",
"year": "2023"
},
{
"DOI": "10.3389/fimmu.2021.746021",
"article-title": "Persistence of SARS-CoV-2 S1 Protein in CD16+ Monocytes in Post-Acute Sequelae of COVID-19 (PASC) up to 15 Months Post-Infection",
"author": "Patterson",
"doi-asserted-by": "crossref",
"first-page": "5526",
"journal-title": "Front. Immunol.",
"key": "ref_114",
"volume": "12",
"year": "2022"
},
{
"DOI": "10.1016/j.jmb.2021.167280",
"doi-asserted-by": "crossref",
"key": "ref_115",
"unstructured": "Rajah, M.M., Bernier, A., Buchrieser, J., and Schwartz, O. (2021). The Mechanism and Consequences of SARS-CoV-2 Spike-Mediated Fusion and Syncytia Formation. J. Mol. Biol., 434."
},
{
"DOI": "10.1038/s41467-021-25589-1",
"article-title": "Sequences in the cytoplasmic tail of SARS-CoV-2 Spike facilitate expression at the cell surface and syncytia formation",
"author": "Welch",
"doi-asserted-by": "crossref",
"first-page": "5333",
"journal-title": "Nat. Commun.",
"key": "ref_116",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.3389/fimmu.2020.576622",
"article-title": "The SARS-CoV-2 Spike Glycoprotein Biosynthesis, Structure, Function, and Antigenicity: Implications for the Design of Spike-Based Vaccine Immunogens",
"author": "Duan",
"doi-asserted-by": "crossref",
"first-page": "576622",
"journal-title": "Front. Immunol.",
"key": "ref_117",
"volume": "11",
"year": "2020"
},
{
"DOI": "10.1152/ajplung.00231.2021",
"article-title": "Erythrocytes identify complement activation in patients with COVID-19",
"author": "Lam",
"doi-asserted-by": "crossref",
"first-page": "L485",
"journal-title": "Am. J. Physiol. Lung Cell Mol. Physiol.",
"key": "ref_118",
"volume": "321",
"year": "2021"
},
{
"DOI": "10.1038/s41392-020-00426-x",
"article-title": "CD147-spike protein is a novel route for SARS-CoV-2 infection to host cells",
"author": "Wang",
"doi-asserted-by": "crossref",
"first-page": "283",
"journal-title": "Signal Transduct. Target. Ther.",
"key": "ref_119",
"volume": "5",
"year": "2020"
},
{
"DOI": "10.1016/j.scib.2021.01.010",
"article-title": "Binding of the SARS-CoV-2 spike protein to glycans",
"author": "Hao",
"doi-asserted-by": "crossref",
"first-page": "1205",
"journal-title": "Sci. Bull. (Beijing)",
"key": "ref_120",
"volume": "66",
"year": "2021"
},
{
"DOI": "10.1038/s41598-020-80464-1",
"article-title": "No evidence for basigin/CD147 as a direct SARS-CoV-2 spike binding receptor",
"author": "Shilts",
"doi-asserted-by": "crossref",
"first-page": "413",
"journal-title": "Sci. Rep.",
"key": "ref_121",
"volume": "11",
"year": "2021"
},
{
"DOI": "10.31083/j.rcm2204176",
"article-title": "Enoxaparin dose impacts blood cell phenotypes during mild SARS-CoV-2 infection: The observational single-center study",
"author": "Buryachkovskaya",
"doi-asserted-by": "crossref",
"first-page": "1685",
"journal-title": "Rev. Cardiovasc. Med.",
"key": "ref_122",
"volume": "22",
"year": "2021"
},
{
"DOI": "10.3390/jcm11071995",
"doi-asserted-by": "crossref",
"key": "ref_123",
"unstructured": "Melkumyants, A., Buryachkovskaya, L., Lomakin, N., Antonova, O., Docenko, J., Ermishkin, V., and Serebruany, V. (2022). Effect of Sulodexide on Circulating Blood Cells in Patients with Mild COVID-19. J. Clin. Med., 11."
},
{
"DOI": "10.1097/CCM.0000000000004862",
"article-title": "Capillary Leukocytes, Microaggregates, and the Response to Hypoxemia in the Microcirculation of Coronavirus Disease 2019 Patients",
"author": "Favaron",
"doi-asserted-by": "crossref",
"first-page": "661",
"journal-title": "Crit. Care Med.",
"key": "ref_124",
"volume": "49",
"year": "2021"
},
{
"DOI": "10.1002/ajh.26440",
"article-title": "Increased blood viscosity and red blood cell aggregation in patients with COVID-19",
"author": "Nader",
"doi-asserted-by": "crossref",
"first-page": "283",
"journal-title": "Am. J. Hematol.",
"key": "ref_125",
"volume": "97",
"year": "2022"
},
{
"DOI": "10.1136/bmj.m1966",
"article-title": "Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: Prospective cohort study",
"author": "Petrilli",
"doi-asserted-by": "crossref",
"first-page": "m1966",
"journal-title": "BMJ",
"key": "ref_126",
"volume": "369",
"year": "2020"
},
{
"DOI": "10.3233/CH-221554",
"article-title": "COVID-19 hemodynamic and thrombotic effect on the eye microcirculation after hospitalization: A quantitative case-control study",
"author": "Koutsiaris",
"doi-asserted-by": "crossref",
"first-page": "379",
"journal-title": "Clin. Hemorheol. Microcirc.",
"key": "ref_127",
"volume": "82",
"year": "2022"
},
{
"DOI": "10.1111/cup.13866",
"article-title": "Discordant anti-SARS-CoV-2 spike protein and RNA staining in cutaneous perniotic lesions suggests endothelial deposition of cleaved spike protein",
"author": "Ko",
"doi-asserted-by": "crossref",
"first-page": "47",
"journal-title": "J. Cutan. Pathol.",
"key": "ref_128",
"volume": "48",
"year": "2021"
},
{
"DOI": "10.3389/fcimb.2021.701278",
"doi-asserted-by": "crossref",
"key": "ref_129",
"unstructured": "Liu, F., Han, K., Blair, R., Kenst, K., Qin, Z., Upcin, B., Wörsdörfer, P., Midkiff, C.C., Mudd, J., and Belyaeva, E. (2021). SARS-CoV-2 Infects Endothelial Cells In Vivo and In Vitro. Front. Cell. Infect. Microbiol., 11."
},
{
"DOI": "10.1016/j.humpath.2020.10.002",
"article-title": "Docked severe acute respiratory syndrome coronavirus 2 proteins within the cutaneous and subcutaneous microvasculature and their role in the pathogenesis of severe coronavirus disease 2019",
"author": "Magro",
"doi-asserted-by": "crossref",
"first-page": "106",
"journal-title": "Hum. Pathol.",
"key": "ref_130",
"volume": "106",
"year": "2020"
},
{
"DOI": "10.1016/j.tim.2023.06.004",
"doi-asserted-by": "crossref",
"key": "ref_131",
"unstructured": "Perico, L., Benigni, A., and Remuzzi, G. (2023). SARS-CoV-2 and the spike protein in endotheliopathy. Trends Microbiol."
},
{
"DOI": "10.1016/S2352-3026(20)30216-7",
"article-title": "Endotheliopathy in COVID-19-associated coagulopathy: Evidence from a single-centre, cross-sectional study",
"author": "Goshua",
"doi-asserted-by": "crossref",
"first-page": "e575",
"journal-title": "Lancet Haematol.",
"key": "ref_132",
"volume": "7",
"year": "2020"
},
{
"DOI": "10.1183/13993003.01634-2020",
"article-title": "Endothelial cell dysfunction: A major player in SARS-CoV-2 infection (COVID-19)?",
"author": "Huertas",
"doi-asserted-by": "crossref",
"first-page": "2001634",
"journal-title": "Eur. Respir. J.",
"key": "ref_133",
"volume": "56",
"year": "2020"
},
{
"DOI": "10.1016/j.stemcr.2022.03.016",
"article-title": "The SARS-CoV-2 receptor ACE2 is expressed in mouse pericytes but not endothelial cells: Implications for COVID-19 vascular research",
"author": "Muhl",
"doi-asserted-by": "crossref",
"first-page": "1089",
"journal-title": "Stem Cell Rep.",
"key": "ref_134",
"volume": "17",
"year": "2022"
},
{
"DOI": "10.1016/j.ajpath.2021.05.007",
"article-title": "COVID-19 Vasculopathy: Mounting Evidence for an Indirect Mechanism of Endothelial Injury",
"author": "Nicosia",
"doi-asserted-by": "crossref",
"first-page": "1374",
"journal-title": "Am. J. Pathol.",
"key": "ref_135",
"volume": "191",
"year": "2021"
},
{
"DOI": "10.1201/9780203503287",
"doi-asserted-by": "crossref",
"key": "ref_136",
"unstructured": "Blom, J.A. (2003). Monitoring of Respiration and Circulation, CRC Press."
},
{
"DOI": "10.1126/science.142.3597.1319",
"article-title": "Red Blood Cells: Change in Shape in Capillaries",
"author": "Guest",
"doi-asserted-by": "crossref",
"first-page": "1319",
"journal-title": "Science",
"key": "ref_137",
"volume": "142",
"year": "1963"
},
{
"DOI": "10.1161/CIRCRESAHA.117.310939",
"article-title": "Translational Implications of Platelets as Vascular First Responders",
"author": "Becker",
"doi-asserted-by": "crossref",
"first-page": "506",
"journal-title": "Circ. Res.",
"key": "ref_138",
"volume": "122",
"year": "2018"
},
{
"DOI": "10.1016/S2352-3026(20)30215-5",
"article-title": "Endothelial cells orchestrate COVID-19 coagulopathy",
"author": "Gonagle",
"doi-asserted-by": "crossref",
"first-page": "e553",
"journal-title": "Lancet Haematol.",
"key": "ref_139",
"volume": "7",
"year": "2020"
},
{
"DOI": "10.1016/j.tem.2023.03.002",
"article-title": "Long COVID: Pathophysiological factors and abnormalities of coagulation",
"author": "Turner",
"doi-asserted-by": "crossref",
"first-page": "321",
"journal-title": "Trends Endocrinol. Metab.",
"key": "ref_140",
"volume": "34",
"year": "2023"
},
{
"DOI": "10.1007/s00134-020-06062-x",
"article-title": "High risk of thrombosis in patients with severe SARS-CoV-2 infection: A multicenter prospective cohort study",
"author": "Helms",
"doi-asserted-by": "crossref",
"first-page": "1089",
"journal-title": "Intensive Care Med.",
"key": "ref_141",
"volume": "46",
"year": "2020"
},
{
"DOI": "10.1093/jalm/jfab042",
"article-title": "Role of von Willebrand Factor in COVID-19 Associated Coagulopathy",
"author": "Mei",
"doi-asserted-by": "crossref",
"first-page": "1305",
"journal-title": "J. Appl. Lab. Med.",
"key": "ref_142",
"volume": "6",
"year": "2021"
},
{
"article-title": "Hemodynamic and microcirculatory effects of high and low viscosity dextrans",
"author": "Shoemaker",
"first-page": "518",
"journal-title": "Surgery",
"key": "ref_143",
"volume": "58",
"year": "1965"
},
{
"DOI": "10.1002/path.1700930120",
"article-title": "Histological changes produced by experimental erythrocyte aggregation",
"author": "Stalker",
"doi-asserted-by": "crossref",
"first-page": "203",
"journal-title": "J. Pathol. Bacteriol.",
"key": "ref_144",
"volume": "93",
"year": "1967"
},
{
"DOI": "10.1002/path.1700930119",
"article-title": "The microcirculatory effects of dextran",
"author": "Stalker",
"doi-asserted-by": "crossref",
"first-page": "191",
"journal-title": "J. Pathol. Bacteriol.",
"key": "ref_145",
"volume": "93",
"year": "1967"
},
{
"DOI": "10.1111/j.1699-0463.1959.tb00322.x",
"article-title": "Kidney-, liver- and heart-damages from trauma and from induced intravascular aggregation of blood-cells: An experimental study",
"author": "Fajers",
"doi-asserted-by": "crossref",
"first-page": "97",
"journal-title": "Acta Pathol. Microbiol. Scand.",
"key": "ref_146",
"volume": "46",
"year": "1959"
},
{
"DOI": "10.1161/01.RES.13.5.392",
"article-title": "Aggregation of Blood Cells by 5-Hydroxytryptamine (Serotonin)",
"author": "Swank",
"doi-asserted-by": "crossref",
"first-page": "392",
"journal-title": "Circ. Res.",
"key": "ref_147",
"volume": "13",
"year": "1963"
},
{
"DOI": "10.1161/01.CIR.9.3.335",
"article-title": "Intravascular Aggregation and Adhesiveness of the Blood Elements Associated with Alimentary Lipemia and Injections of Large Molecular Substances",
"author": "Cullen",
"doi-asserted-by": "crossref",
"first-page": "335",
"journal-title": "Circulation",
"key": "ref_148",
"volume": "9",
"year": "1954"
},
{
"DOI": "10.1007/s00249-006-0107-1",
"article-title": "The mechanism of the dextran-induced red blood cell aggregation",
"author": "Pribush",
"doi-asserted-by": "crossref",
"first-page": "85",
"journal-title": "Eur. Biophys. J.",
"key": "ref_149",
"volume": "36",
"year": "2007"
},
{
"DOI": "10.3390/mi10120853",
"doi-asserted-by": "crossref",
"key": "ref_150",
"unstructured": "Zhu, R., Avsievich, T., Bykov, A., Popov, A., and Meglinski, I. (2019). Influence of Pulsed He–Ne Laser Irradiation on the Red Blood Cell Interaction Studied by Optical Tweezers. Micromachines, 10."
},
{
"DOI": "10.1364/BOE.9.002755",
"article-title": "Dextran adsorption onto red blood cells revisited: Single cell quantification by laser tweezers combined with microfluidics",
"author": "Lee",
"doi-asserted-by": "crossref",
"first-page": "2755",
"journal-title": "Biomed. Opt. Express",
"key": "ref_151",
"volume": "9",
"year": "2018"
},
{
"DOI": "10.1016/0026-2862(73)90068-X",
"article-title": "Ultrastructural basis of the mechanism of rouleaux formation",
"author": "Chien",
"doi-asserted-by": "crossref",
"first-page": "155",
"journal-title": "Microvasc. Res.",
"key": "ref_152",
"volume": "5",
"year": "1973"
},
{
"DOI": "10.1006/mvre.1996.0010",
"article-title": "Experimental Study on Microthrombi and Myocardial Injuries",
"author": "Zhen",
"doi-asserted-by": "crossref",
"first-page": "99",
"journal-title": "Microvasc. Res.",
"key": "ref_153",
"volume": "51",
"year": "1996"
},
{
"key": "ref_154",
"unstructured": "Bicher, H.I. (1972). Blood Cell Aggregation in Thrombotic Processes, C. C. Thomas."
},
{
"DOI": "10.1177/000331976101200705",
"article-title": "Experimental Investigation of Microcirculation in Bone Marrow",
"doi-asserted-by": "crossref",
"first-page": "293",
"journal-title": "Angiology",
"key": "ref_155",
"volume": "12",
"year": "1961"
},
{
"DOI": "10.1093/cvr/1.4.379",
"article-title": "Effects of Dextran 40 on Red Cell Aggregation in Rabbits",
"author": "Engeset",
"doi-asserted-by": "crossref",
"first-page": "379",
"journal-title": "Cardiovasc. Res.",
"key": "ref_156",
"volume": "1",
"year": "1967"
},
{
"DOI": "10.3181/00379727-82-20123",
"article-title": "Circulatory Changes in the Hamster’s Cheek Pouch Associated with Alimentary Lipemia",
"author": "Swank",
"doi-asserted-by": "crossref",
"first-page": "381",
"journal-title": "Proc. Soc. Exp. Biol. Med.",
"key": "ref_157",
"volume": "82",
"year": "1953"
},
{
"DOI": "10.1097/00000658-196403000-00017",
"article-title": "Correlation between sludge in the bulbar conjunctiva and the mesentery",
"author": "Bjoerk",
"doi-asserted-by": "crossref",
"first-page": "428",
"journal-title": "Ann. Surg.",
"key": "ref_158",
"volume": "159",
"year": "1964"
},
{
"DOI": "10.1152/ajpheart.1987.253.3.H540",
"article-title": "Blood viscosity in small tubes: Effect of shear rate, aggregation, and sedimentation",
"author": "Reinke",
"doi-asserted-by": "crossref",
"first-page": "H540",
"journal-title": "Am. J. Physiol.-Heart Circ. Physiol.",
"key": "ref_159",
"volume": "253",
"year": "1987"
},
{
"DOI": "10.1007/BF00587850",
"article-title": "Microrheology and light transmission of blood",
"author": "Volger",
"doi-asserted-by": "crossref",
"first-page": "319",
"journal-title": "Pflügers Arch.",
"key": "ref_160",
"volume": "354",
"year": "1975"
},
{
"DOI": "10.1038/s41598-022-24166-w",
"article-title": "Relationship between red blood cell aggregation and dextran molecular mass",
"author": "Bosek",
"doi-asserted-by": "crossref",
"first-page": "19751",
"journal-title": "Sci. Rep.",
"key": "ref_161",
"volume": "12",
"year": "2022"
},
{
"DOI": "10.1529/biophysj.108.130328",
"article-title": "Effects of dextran molecular weight on red blood cell aggregation",
"author": "Neu",
"doi-asserted-by": "crossref",
"first-page": "3059",
"journal-title": "Biophys. J.",
"key": "ref_162",
"volume": "95",
"year": "2008"
},
{
"DOI": "10.1093/cvr/1.4.385",
"article-title": "Objective measurement of the dispersing effect of dextran 40 on red cells from man, dog, and rabbit",
"author": "Engeset",
"doi-asserted-by": "crossref",
"first-page": "385",
"journal-title": "Cardiovasc. Res.",
"key": "ref_163",
"volume": "1",
"year": "1967"
},
{
"DOI": "10.3233/CH-2012-1573",
"article-title": "Erythrocyte aggregation: Basic aspects and clinical importance",
"author": "Baskurt",
"doi-asserted-by": "crossref",
"first-page": "23",
"journal-title": "Clin. Hemorheol. Microcirc.",
"key": "ref_164",
"volume": "53",
"year": "2013"
},
{
"DOI": "10.1016/S0006-3495(00)76791-9",
"article-title": "Kinetics of linear rouleaux formation studied by visual monitoring of red cell dynamic organization",
"author": "Barshtein",
"doi-asserted-by": "crossref",
"first-page": "2470",
"journal-title": "Biophys. J.",
"key": "ref_165",
"volume": "78",
"year": "2000"
},
{
"key": "ref_166",
"unstructured": "Fung, Y.-C. (1993). Biomechanics: Mechanical Properties of Living Tissues, Springer."
},
{
"article-title": "Chapter I: Red cell aggregation in thrombotic disease, trauma and shock",
"author": "Bicher",
"first-page": "5",
"journal-title": "Blood Cell Aggregation in Thrombotic Processes",
"key": "ref_167",
"volume": "Volume I",
"year": "1972"
},
{
"article-title": "Chapter III: Mechanism of red cell aggregation",
"author": "Bicher",
"first-page": "47",
"journal-title": "Blood Cell Aggregation in Thrombotic Processes",
"key": "ref_168",
"volume": "Volume III",
"year": "1972"
},
{
"key": "ref_169",
"unstructured": "Chien, S., Dormandy, J., Ernst, E., and Matrai, A. (1987). Clinical Hemorheology: Applications in Cardiovascular and Hematological Disease, Diabetes, Surgery and Gynecology, Springer."
},
{
"key": "ref_170",
"unstructured": "Chien, S., Dormandy, J., Ernst, E., and Matrai, A. (1987). Clinical Hemorheology: Applications in Cardiovascular and Hematological Disease, Diabetes, Surgery and Gynecology, Springer."
},
{
"DOI": "10.1152/ajpheart.2001.280.1.H222",
"article-title": "Effect of erythrocyte aggregation on velocity profiles in venules",
"author": "Bishop",
"doi-asserted-by": "crossref",
"first-page": "H222",
"journal-title": "Am. J. Physiol.-Heart Circ. Physiol.",
"key": "ref_171",
"volume": "280",
"year": "2001"
},
{
"DOI": "10.3233/CH-1987-7108",
"article-title": "Physicochemical basis and clinical implications of red cell aggregation",
"author": "Chien",
"doi-asserted-by": "crossref",
"first-page": "71",
"journal-title": "Clin. Hemorheol. Microcirc.",
"key": "ref_172",
"volume": "7",
"year": "1987"
},
{
"DOI": "10.1007/978-1-4615-9510-6",
"doi-asserted-by": "crossref",
"key": "ref_173",
"unstructured": "Mochizuki, M., Honig, C.R., Koyama, T., Goldstick, T.K., and Bruley, D.F. (1988). Oxygen Transport to Tissue X, Springer US."
},
{
"DOI": "10.4155/fso.15.28",
"article-title": "The impact of blood shear rate on arterial thrombus formation",
"author": "Sakariassen",
"doi-asserted-by": "crossref",
"first-page": "FSO30",
"journal-title": "Future Sci. OA",
"key": "ref_174",
"volume": "1",
"year": "2015"
},
{
"DOI": "10.1126/science.106.2758.431",
"article-title": "Sludged Blood",
"author": "Knisely",
"doi-asserted-by": "crossref",
"first-page": "431",
"journal-title": "Science",
"key": "ref_175",
"volume": "106",
"year": "1947"
},
{
"DOI": "10.1586/14779072.5.4.743",
"article-title": "Role of red blood cell flow behavior in hemodynamics and hemostasis",
"author": "Barshtein",
"doi-asserted-by": "crossref",
"first-page": "743",
"journal-title": "Expert Rev. Cardiovasc. Ther.",
"key": "ref_176",
"volume": "5",
"year": "2007"
},
{
"DOI": "10.1056/NEJM195404082501401",
"article-title": "Morphologic and hemodynamic changes in the smaller blood vessels in diabetes mellitus. II. The degenerative and hemodynamic changes in the bulbar conjunctiva of normotensive diabetic patients",
"author": "Ditzel",
"doi-asserted-by": "crossref",
"first-page": "587",
"journal-title": "N. Engl. J. Med.",
"key": "ref_177",
"volume": "250",
"year": "1954"
},
{
"DOI": "10.1177/000331975600700202",
"article-title": "Concerning the Geometric Shapes of Arteries and Arterioles: A Contribution to the Biophysics of Health, Disease, and Death",
"author": "Knisely",
"doi-asserted-by": "crossref",
"first-page": "105",
"journal-title": "Angiology",
"key": "ref_178",
"volume": "7",
"year": "1956"
},
{
"DOI": "10.1111/j.1538-7836.2010.04164.x",
"article-title": "A local increase in red blood cell aggregation can trigger deep vein thrombosis: Evidence based on quantitative cellular ultrasound imaging",
"author": "Yu",
"doi-asserted-by": "crossref",
"first-page": "481",
"journal-title": "J. Thromb. Haemost.",
"key": "ref_179",
"volume": "9",
"year": "2011"
},
{
"DOI": "10.1182/blood-2017-03-745349",
"article-title": "Red blood cells in thrombosis",
"author": "Byrnes",
"doi-asserted-by": "crossref",
"first-page": "1795",
"journal-title": "Blood",
"key": "ref_180",
"volume": "130",
"year": "2017"
},
{
"DOI": "10.1152/ajpheart.2001.280.5.H1982",
"article-title": "Parameters of red blood cell aggregation as correlates of the inflammatory state",
"author": "Ami",
"doi-asserted-by": "crossref",
"first-page": "H1982",
"journal-title": "Am. J. Physiol.-Heart Circ. Physiol.",
"key": "ref_181",
"volume": "280",
"year": "2001"
},
{
"DOI": "10.1016/j.crhy.2013.04.004",
"article-title": "Aggregation of red blood cells: From rouleaux to clot formation",
"author": "Wagner",
"doi-asserted-by": "crossref",
"first-page": "459",
"journal-title": "C. R. Phys.",
"key": "ref_182",
"volume": "14",
"year": "2013"
},
{
"DOI": "10.3233/BIR-2009-0522",
"article-title": "Red blood cell aggregation: 45 years being curious",
"author": "Meiselman",
"doi-asserted-by": "crossref",
"first-page": "1",
"journal-title": "Biorheology",
"key": "ref_183",
"volume": "46",
"year": "2009"
},
{
"DOI": "10.1016/S0254-6272(17)30034-1",
"article-title": "Microvascular pathological features and changes in related injury factors in a rat acute blood stasis model",
"author": "Junxiu",
"doi-asserted-by": "crossref",
"first-page": "108",
"journal-title": "J. Tradit. Chin. Med.",
"key": "ref_184",
"volume": "37",
"year": "2017"
},
{
"DOI": "10.1152/ajpheart.00690.2004",
"article-title": "Aggregate formation of erythrocytes in postcapillary venules",
"author": "Kim",
"doi-asserted-by": "crossref",
"first-page": "H584",
"journal-title": "Am. J. Physiol.-Heart Circ. Physiol.",
"key": "ref_185",
"volume": "288",
"year": "2005"
},
{
"DOI": "10.1152/jappl.1958.12.1.125",
"article-title": "Suspension Stability of the Blood After Injections of Dextran",
"author": "Swank",
"doi-asserted-by": "crossref",
"first-page": "125",
"journal-title": "J. Appl. Physiol.",
"key": "ref_186",
"volume": "12",
"year": "1958"
},
{
"DOI": "10.1152/jappl.1957.10.1.45",
"article-title": "Effects of Dextran Injections on Blood Viscosity in Dogs",
"author": "Swank",
"doi-asserted-by": "crossref",
"first-page": "45",
"journal-title": "J. Appl. Physiol.",
"key": "ref_187",
"volume": "10",
"year": "1957"
},
{
"DOI": "10.1113/jphysiol.1971.sp009594",
"article-title": "Effect of microcirculation changes on brain tissue oxygenation",
"author": "Bicher",
"doi-asserted-by": "crossref",
"first-page": "689",
"journal-title": "J. Physiol.",
"key": "ref_188",
"volume": "217",
"year": "1971"
},
{
"DOI": "10.1117/1.JBO.17.12.125006",
"doi-asserted-by": "crossref",
"key": "ref_189",
"unstructured": "Hysi, E., Saha, R.K., and Kolios, M.C. (2012). Photoacoustic ultrasound spectroscopy for assessing red blood cell aggregation and oxygenation. J. Biomed. Opt., 17."
},
{
"DOI": "10.1161/01.CIR.14.3.386",
"article-title": "Angioscopic Changes in the Smaller Blood Vessels in Diabetes Mellitus and their Relationship to Aging",
"author": "Ditzel",
"doi-asserted-by": "crossref",
"first-page": "386",
"journal-title": "Circulation",
"key": "ref_190",
"volume": "14",
"year": "1956"
},
{
"DOI": "10.1016/0026-0495(94)90063-9",
"article-title": "Increased erythrocyte aggregation in insulin-dependent diabetes mellitus and its relationship to plasma factors: A multivariate analysis",
"author": "Ziegler",
"doi-asserted-by": "crossref",
"first-page": "1182",
"journal-title": "Metabolism",
"key": "ref_191",
"volume": "43",
"year": "1994"
},
{
"DOI": "10.1177/000331976301400807",
"article-title": "Intravascular Red Cell Aggregation and the Chylomicron Count in Diabetes",
"author": "Chazan",
"doi-asserted-by": "crossref",
"first-page": "426",
"journal-title": "Angiology",
"key": "ref_192",
"volume": "14",
"year": "1963"
},
{
"DOI": "10.1177/193229680800200622",
"article-title": "Hemorheological Disorders in Diabetes Mellitus",
"author": "Cho",
"doi-asserted-by": "crossref",
"first-page": "1130",
"journal-title": "J. Diabetes Sci. Technol.",
"key": "ref_193",
"volume": "2",
"year": "2008"
},
{
"DOI": "10.1056/NEJM198107303050501",
"article-title": "Increased adhesion of erythrocytes to endothelial cells in diabetes mellitus and its relation to vascular complications",
"author": "Wautier",
"doi-asserted-by": "crossref",
"first-page": "237",
"journal-title": "N. Engl. J. Med.",
"key": "ref_194",
"volume": "305",
"year": "1981"
},
{
"DOI": "10.1007/s11606-020-05983-z",
"article-title": "Risk Factors for Mortality in Patients with COVID-19 in New York City",
"author": "Mikami",
"doi-asserted-by": "crossref",
"first-page": "17",
"journal-title": "J. Gen. Intern. Med.",
"key": "ref_195",
"volume": "36",
"year": "2020"
},
{
"DOI": "10.1016/j.thromres.2022.02.016",
"article-title": "COVID-19 and thrombosis: The role of hemodynamics",
"author": "Sastry",
"doi-asserted-by": "crossref",
"first-page": "51",
"journal-title": "Thromb. Res.",
"key": "ref_196",
"volume": "212",
"year": "2022"
},
{
"DOI": "10.1016/j.ajem.2020.09.065",
"article-title": "Thrombotic complications of COVID-19",
"author": "Avila",
"doi-asserted-by": "crossref",
"first-page": "213",
"journal-title": "Am. J. Emerg. Med.",
"key": "ref_197",
"volume": "39",
"year": "2021"
},
{
"DOI": "10.1182/blood.2020006520",
"article-title": "COVID-19 and coagulation: Bleeding and thrombotic manifestations of SARS-CoV-2 infection",
"author": "Dzik",
"doi-asserted-by": "crossref",
"first-page": "489",
"journal-title": "Blood",
"key": "ref_198",
"volume": "136",
"year": "2020"
},
{
"DOI": "10.1111/jth.14844",
"article-title": "Pathological evidence of pulmonary thrombotic phenomena in severe COVID-19",
"author": "Dolhnikoff",
"doi-asserted-by": "crossref",
"first-page": "1517",
"journal-title": "J. Thromb. Haemost.",
"key": "ref_199",
"volume": "18",
"year": "2020"
},
{
"DOI": "10.1016/S0140-6736(20)31209-5",
"article-title": "COVID-19-associated hyperviscosity: A link between inflammation and thrombophilia?",
"author": "Maier",
"doi-asserted-by": "crossref",
"first-page": "1758",
"journal-title": "Lancet",
"key": "ref_200",
"volume": "395",
"year": "2020"
},
{
"DOI": "10.1007/s10456-020-09753-7",
"article-title": "Microvascular dysfunction in COVID-19: The MYSTIC study",
"author": "Rovas",
"doi-asserted-by": "crossref",
"first-page": "145",
"journal-title": "Angiogenesis",
"key": "ref_201",
"volume": "24",
"year": "2021"
},
{
"key": "ref_202",
"unstructured": "US National Institutes of Health (NIH) (2023, October 30). Clinical Spectrum of SARS-CoV-2 Infection, Updated 6 March 2023, Available online: https://www.covid19treatmentguidelines.nih.gov/overview/clinical-spectrum/."
},
{
"DOI": "10.1007/s10456-022-09850-9",
"article-title": "Persistent capillary rarefication in long COVID syndrome",
"author": "Osiaevi",
"doi-asserted-by": "crossref",
"first-page": "53",
"journal-title": "Angiogenesis",
"key": "ref_203",
"volume": "26",
"year": "2023"
},
{
"DOI": "10.1016/j.acra.2020.07.019",
"article-title": "Assessment of Small Pulmonary Blood Vessels in COVID-19 Patients Using HRCT",
"author": "Lins",
"doi-asserted-by": "crossref",
"first-page": "1449",
"journal-title": "Acad. Radiol.",
"key": "ref_204",
"volume": "27",
"year": "2020"
},
{
"DOI": "10.1136/thoraxjnl-2020-215395",
"article-title": "Functional respiratory imaging identifies redistribution of pulmonary blood flow in patients with COVID-19",
"author": "Thillai",
"doi-asserted-by": "crossref",
"first-page": "182",
"journal-title": "Thorax",
"key": "ref_205",
"volume": "76",
"year": "2021"
},
{
"DOI": "10.1183/13993003.04133-2020",
"article-title": "Altered pulmonary blood volume distribution as a biomarker for predicting outcomes in COVID-19 disease",
"author": "Morris",
"doi-asserted-by": "crossref",
"first-page": "2004133",
"journal-title": "Eur. Respir. J.",
"key": "ref_206",
"volume": "58",
"year": "2021"
},
{
"DOI": "10.1152/japplphysiol.00458.2022",
"article-title": "CT-derived measurements of pulmonary blood volume in small vessels and the need for supplemental oxygen in COVID-19 patients",
"author": "Dierckx",
"doi-asserted-by": "crossref",
"first-page": "1295",
"journal-title": "J. Appl. Physiol. (1985)",
"key": "ref_207",
"volume": "133",
"year": "2022"
},
{
"DOI": "10.1007/s11307-021-01700-2",
"article-title": "Imaging Pulmonary Blood Vessels and Ventilation-Perfusion Mismatch in COVID-19",
"author": "Tipre",
"doi-asserted-by": "crossref",
"first-page": "526",
"journal-title": "Mol. Imaging Biol.",
"key": "ref_208",
"volume": "24",
"year": "2022"
},
{
"DOI": "10.1177/25158414221090092",
"doi-asserted-by": "crossref",
"key": "ref_209",
"unstructured": "Atilgan, C.U., Goker, Y.S., Hondur, G., Kosekahya, P., Kocer, A.M., and Citirik, M. (2022). Evaluation of the radial peripapillary capillary density in unilateral branch retinal vein occlusion and the unaffected fellow eyes. Ther. Adv. Ophthalmol., 14."
},
{
"DOI": "10.1016/j.pdpdt.2022.102920",
"article-title": "Retinal microvascular morphology versus COVID-19: What to anticipate?",
"author": "Erogul",
"doi-asserted-by": "crossref",
"first-page": "102920",
"journal-title": "Photodiagn. Photodyn. Ther.",
"key": "ref_210",
"volume": "39",
"year": "2022"
},
{
"DOI": "10.3390/jcm9092895",
"doi-asserted-by": "crossref",
"key": "ref_211",
"unstructured": "Savastano, A., Crincoli, E., Savastano, M.C., Younis, S., Gambini, G., De Vico, U., Cozzupoli, G.M., Culiersi, C., and Rizzo, S. (2020). Peripapillary Retinal Vascular Involvement in Early Post-COVID-19 Patients. J. Clin. Med., 9."
},
{
"DOI": "10.1101/2022.09.23.22280264",
"doi-asserted-by": "crossref",
"key": "ref_212",
"unstructured": "Schlick, S., Lucio, M., Wallukat, G., Bartsch, A., Skornia, A., Hoffmanns, J., Szewczykowski, C., Schröder, T., Raith, F., and Rogge, L. (2022). Post-COVID-19 Syndrome: Retinal Microcirculation as a Potential Marker for Chronic Fatigue. Int. J. Mol. Sci., 23."
},
{
"article-title": "Predictive Model and Risk Factors for Case Fatality of COVID-19: A Cohort of 21,392 Cases in Hubei, China",
"author": "Wu",
"first-page": "100022",
"journal-title": "Innovation",
"key": "ref_213",
"volume": "1",
"year": "2020"
},
{
"DOI": "10.1016/S0140-6736(20)30566-3",
"article-title": "Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study",
"author": "Zhou",
"doi-asserted-by": "crossref",
"first-page": "1054",
"journal-title": "Lancet",
"key": "ref_214",
"volume": "395",
"year": "2020"
},
{
"DOI": "10.1038/s41586-020-2521-4",
"article-title": "OpenSAFELY: Factors associated with COVID-19 death in 17 million patients",
"author": "Williamson",
"doi-asserted-by": "crossref",
"first-page": "430",
"journal-title": "Nature",
"key": "ref_215",
"volume": "584",
"year": "2020"
},
{
"DOI": "10.1016/j.jamda.2020.05.045",
"article-title": "The Effect of Age on Mortality in Patients With COVID-19: A Meta-Analysis With 611,583 Subjects",
"author": "Bonanad",
"doi-asserted-by": "crossref",
"first-page": "915",
"journal-title": "J. Am. Med. Dir. Assoc.",
"key": "ref_216",
"volume": "21",
"year": "2020"
},
{
"article-title": "Partially opposite hemorheological effects of aging and training at middle age",
"author": "Manetta",
"first-page": "239",
"journal-title": "Clin. Hemorheol. Microcirc.",
"key": "ref_217",
"volume": "35",
"year": "2006"
},
{
"DOI": "10.3233/CH-1994-14115",
"article-title": "Determination of red blood cell aggregation in young and elderly subjects evaluated by ultrasound",
"author": "Hammi",
"doi-asserted-by": "crossref",
"first-page": "117",
"journal-title": "Clin. Hemorheol. Microcirc.",
"key": "ref_218",
"volume": "14",
"year": "1994"
},
{
"DOI": "10.1007/BF02432074",
"article-title": "Comparison of capillary blood flow in the nailfold circulations of young and elderly men",
"author": "Richardson",
"doi-asserted-by": "crossref",
"first-page": "70",
"journal-title": "AGE",
"key": "ref_219",
"volume": "8",
"year": "1985"
},
{
"DOI": "10.1016/0026-2862(91)90010-9",
"article-title": "The cutaneous microcirculation of the forearm in young and old subjects",
"author": "Richardson",
"doi-asserted-by": "crossref",
"first-page": "84",
"journal-title": "Microvasc. Res.",
"key": "ref_220",
"volume": "41",
"year": "1991"
},
{
"DOI": "10.1016/0923-1811(93)90764-G",
"article-title": "The effect of aging and arteriosclerosis on human skin blood flow",
"author": "Tsuchida",
"doi-asserted-by": "crossref",
"first-page": "175",
"journal-title": "J. Dermatol. Sci.",
"key": "ref_221",
"volume": "5",
"year": "1993"
},
{
"DOI": "10.1159/000021993",
"article-title": "Hemorheological changes during human aging",
"author": "Ajmani",
"doi-asserted-by": "crossref",
"first-page": "111",
"journal-title": "Gerontology",
"key": "ref_222",
"volume": "44",
"year": "1998"
},
{
"DOI": "10.1161/01.CIR.100.2.164",
"article-title": "Limb Blood Flow and Vascular Conductance Are Reduced With Age in Healthy Humans",
"author": "Dinenno",
"doi-asserted-by": "crossref",
"first-page": "164",
"journal-title": "Circulation",
"key": "ref_223",
"volume": "100",
"year": "1999"
},
{
"DOI": "10.2214/ajr.172.1.9888770",
"article-title": "Transcranial color Doppler sonography of basal cerebral arteries in 182 healthy subjects: Age and sex variability and normal reference values for blood flow parameters",
"author": "Krejza",
"doi-asserted-by": "crossref",
"first-page": "213",
"journal-title": "AJR Am. J. Roentgenol.",
"key": "ref_224",
"volume": "172",
"year": "1999"
},
{
"DOI": "10.1080/01616412.1990.11739941",
"article-title": "Influence of biological factors on changes in mean cerebral blood flow velocity in normal ageing: A transcranial Doppler study",
"author": "Ackerstaff",
"doi-asserted-by": "crossref",
"first-page": "187",
"journal-title": "Neurol. Res.",
"key": "ref_225",
"volume": "12",
"year": "1990"
},
{
"article-title": "Cardiovascular risk factors, cardiovascular disease, and COVID-19: An umbrella review of systematic reviews",
"author": "Harrison",
"first-page": "330",
"journal-title": "Eur. Heart J.-Qual. Care Clin. Outcomes",
"key": "ref_226",
"volume": "7",
"year": "2021"
},
{
"DOI": "10.1038/sj.ijo.0802791",
"article-title": "Flow-resistant red blood cell aggregation in morbid obesity",
"author": "Shapira",
"doi-asserted-by": "crossref",
"first-page": "1528",
"journal-title": "Int. J. Obes.",
"key": "ref_227",
"volume": "28",
"year": "2004"
},
{
"DOI": "10.1038/oby.2003.54",
"article-title": "Enhanced erythrocyte adhesiveness/aggregation in obesity corresponds to low-grade inflammation",
"author": "Lichtenberg",
"doi-asserted-by": "crossref",
"first-page": "403",
"journal-title": "Obes. Res.",
"key": "ref_228",
"volume": "11",
"year": "2003"
},
{
"DOI": "10.3389/fimmu.2023.1120298",
"article-title": "Exploring SARS-CoV-2 and Plasmodium falciparum coinfection in human erythrocytes",
"author": "Irigoyen",
"doi-asserted-by": "crossref",
"first-page": "1120298",
"journal-title": "Front. Immunol.",
"key": "ref_229",
"volume": "14",
"year": "2023"
},
{
"DOI": "10.1016/S0368-1319(67)80019-X",
"article-title": "Induction of ischemic myocardial damage by red blood cell aggregation (sludge) in the rabbit",
"author": "Bicher",
"doi-asserted-by": "crossref",
"first-page": "409",
"journal-title": "J. Atheroscler Res.",
"key": "ref_230",
"volume": "7",
"year": "1967"
},
{
"DOI": "10.1002/cia2.12278",
"article-title": "Persistent varicella zoster virus infection following mRNA COVID-19 vaccination was associated with the presence of encoded spike protein in the lesion",
"author": "Yamamoto",
"doi-asserted-by": "crossref",
"first-page": "18",
"journal-title": "J. Cutan. Immunol. Allergy",
"key": "ref_231",
"volume": "6",
"year": "2023"
},
{
"DOI": "10.1111/1346-8138.16816",
"article-title": "A case of persistent, confluent maculopapular erythema following a COVID-19 mRNA vaccination is possibly associated with the intralesional spike protein expressed by vascular endothelial cells and eccrine glands in the deep dermis",
"author": "Sano",
"doi-asserted-by": "crossref",
"first-page": "1208",
"journal-title": "J. Dermatol.",
"key": "ref_232",
"volume": "50",
"year": "2023"
},
{
"DOI": "10.20944/preprints202206.0308.v1",
"doi-asserted-by": "crossref",
"key": "ref_233",
"unstructured": "Morz, M. (2022). A Case Report: Multifocal Necrotizing Encephalitis and Myocarditis after BNT162b2 mRNA Vaccination against COVID-19. Vaccines, 10."
},
{
"DOI": "10.1093/cid/ciab707",
"article-title": "Intravenous Injection of Coronavirus Disease 2019 (COVID-19) mRNA Vaccine Can Induce Acute Myopericarditis in Mouse Model",
"author": "Li",
"doi-asserted-by": "crossref",
"first-page": "1933",
"journal-title": "Clin. Infect. Dis.",
"key": "ref_234",
"volume": "74",
"year": "2022"
},
{
"article-title": "COVID 19 m-RNA (Pfizer) vaccination impairs cardiac functions in adult male rats",
"author": "Hassan",
"first-page": "211",
"journal-title": "Bull. Egypt. Soc. Physiol. Sci.",
"key": "ref_235",
"volume": "43",
"year": "2023"
},
{
"DOI": "10.1016/j.pdpdt.2022.102742",
"article-title": "The assesment of retina and optic disc vascular structures in people who received CoronaVac vaccine",
"author": "Gedik",
"doi-asserted-by": "crossref",
"first-page": "102742",
"journal-title": "Photodiagn. Photodyn. Ther.",
"key": "ref_236",
"volume": "38",
"year": "2022"
},
{
"DOI": "10.1016/j.pdpdt.2023.103702",
"article-title": "Effects of Sinovac-Coronavac and Pfizer-BioNTech mRNA vaccines on choroidal and retinal vascular system",
"author": "Saritas",
"doi-asserted-by": "crossref",
"first-page": "103702",
"journal-title": "Photodiagn. Photodyn. Ther.",
"key": "ref_237",
"volume": "43",
"year": "2023"
},
{
"DOI": "10.1016/j.mvr.2023.104500",
"article-title": "Evaluation of retinal and optic disc vascular structures in individuals before and after Pfizer-BioNTech vaccination",
"author": "Gedik",
"doi-asserted-by": "crossref",
"first-page": "104500",
"journal-title": "Microvasc. Res.",
"key": "ref_238",
"volume": "147",
"year": "2023"
},
{
"article-title": "Vascular retinal findings after COVID-19 vaccination in 11 cases: A coincidence or consequence?",
"author": "Finamor",
"first-page": "158",
"journal-title": "Arq. Bras. Oftalmol.",
"key": "ref_239",
"volume": "85",
"year": "2022"
},
{
"DOI": "10.3390/vaccines10020342",
"doi-asserted-by": "crossref",
"key": "ref_240",
"unstructured": "Haseeb, A.A., Solyman, O., Abushanab, M.M., Abo Obaia, A.S., and Elhusseiny, A.M. (2022). Ocular Complications Following Vaccination for COVID-19: A One-Year Retrospective. Vaccines, 10."
},
{
"DOI": "10.3390/jcm9072279",
"doi-asserted-by": "crossref",
"key": "ref_241",
"unstructured": "Haider, A., Bengs, S., Schade, K., Wijnen, W.J., Portmann, A., Etter, D., Fröhlich, S., Warnock, G.I., Treyer, V., and Burger, I.A. (2020). Myocardial 18F-FDG Uptake Pattern for Cardiovascular Risk Stratification in Patients Undergoing Oncologic PET/CT. J. Clin. Med., 9."
},
{
"article-title": "Likely Common Role of Hypoxia in Driving 18F-FDG Uptake in Cancer, Myocardial Ischemia, Inflammation and Infection",
"author": "Yao",
"first-page": "624",
"journal-title": "Cancer Biother. Radiopharm.",
"key": "ref_242",
"volume": "36",
"year": "2021"
},
{
"DOI": "10.1148/radiol.230743",
"article-title": "Assessment of Myocardial (18)F-FDG Uptake at PET/CT in Asymptomatic SARS-CoV-2-vaccinated and Nonvaccinated Patients",
"author": "Nakahara",
"doi-asserted-by": "crossref",
"first-page": "e230743",
"journal-title": "Radiology",
"key": "ref_243",
"volume": "308",
"year": "2023"
},
{
"DOI": "10.1161/circ.144.suppl_1.10712",
"article-title": "Abstract 10712: Observational Findings of PULS Cardiac Test Findings for Inflammatory Markers in Patients Receiving mRNA Vaccines",
"author": "Gundry",
"doi-asserted-by": "crossref",
"first-page": "A10712",
"journal-title": "Circulation",
"key": "ref_244",
"volume": "144",
"year": "2021"
},
{
"key": "ref_245",
"unstructured": "U.S. Centers for Disease Control and Prevention, Vaccines and Related Biological Products Advisory Committee (VRBPAC) (2023, October 30). Update on Myocarditis following mRNA COVID-19 Vaccination, Available online: https://www.fda.gov/media/159007/download."
},
{
"DOI": "10.1161/CIRCULATIONAHA.121.056583",
"article-title": "Clinically Suspected Myocarditis Temporally Related to COVID-19 Vaccination in Adolescents and Young Adults",
"author": "Truong",
"doi-asserted-by": "crossref",
"first-page": "345",
"journal-title": "Circulation",
"key": "ref_246",
"volume": "145",
"year": "2022"
},
{
"DOI": "10.1007/s00431-022-04786-0",
"article-title": "Changes of ECG parameters after BNT162b2 vaccine in the senior high school students",
"author": "Chiu",
"doi-asserted-by": "crossref",
"first-page": "1155",
"journal-title": "Eur. J. Pediatr.",
"key": "ref_247",
"volume": "182",
"year": "2023"
},
{
"DOI": "10.20944/preprints202208.0151.v1",
"doi-asserted-by": "crossref",
"key": "ref_248",
"unstructured": "Mansanguan, S., Charunwatthana, P., Piyaphanee, W., Dechkhajorn, W., Poolcharoen, A., and Mansanguan, C. (2022). Cardiovascular Manifestation of the BNT162b2 mRNA COVID-19 Vaccine in Adolescents. Trop. Med. Infect. Dis., 7."
},
{
"DOI": "10.1161/CIRCULATIONAHA.122.061025",
"article-title": "Circulating Spike Protein Detected in Post–COVID-19 mRNA Vaccine Myocarditis",
"author": "Yonker",
"doi-asserted-by": "crossref",
"first-page": "867",
"journal-title": "Circulation",
"key": "ref_249",
"volume": "147",
"year": "2023"
},
{
"DOI": "10.1038/s41541-023-00742-7",
"article-title": "Duration of SARS-CoV-2 mRNA vaccine persistence and factors associated with cardiac involvement in recently vaccinated patients",
"author": "Krauson",
"doi-asserted-by": "crossref",
"first-page": "141",
"journal-title": "NPJ Vaccines",
"key": "ref_250",
"volume": "8",
"year": "2023"
},
{
"DOI": "10.3390/ijms23136940",
"doi-asserted-by": "crossref",
"key": "ref_251",
"unstructured": "Baumeier, C., Aleshcheva, G., Harms, D., Gross, U., Hamm, C., Assmus, B., Westenfeld, R., Kelm, M., Rammos, S., and Wenzel, P. (2022). Intramyocardial Inflammation after COVID-19 Vaccination: An Endomyocardial Biopsy-Proven Case Series. Int. J. Mol. Sci., 23."
},
{
"DOI": "10.3389/fphys.2020.573044",
"article-title": "Heparin Therapy Improving Hypoxia in COVID-19 Patients—A Case Series",
"author": "Negri",
"doi-asserted-by": "crossref",
"first-page": "1341",
"journal-title": "Front. Physiol.",
"key": "ref_252",
"volume": "11",
"year": "2020"
},
{
"DOI": "10.3389/fcvm.2022.866113",
"article-title": "Sulodexide Significantly Improves Endothelial Dysfunction and Alleviates Chest Pain and Palpitations in Patients With Long-COVID-19: Insights From TUN-EndCOV Study",
"author": "Charfeddine",
"doi-asserted-by": "crossref",
"first-page": "866113",
"journal-title": "Front. Cardiovasc. Med.",
"key": "ref_253",
"volume": "9",
"year": "2022"
},
{
"key": "ref_254",
"unstructured": "(2023, October 30). Prominent Researchers Look Deeper into Fluvoxamine and See Potential as COVID-19 Treatment. Available online: https://trialsitenews.com/prominent-researchers-look-deeper-into-fluvoxamine-see-potential-as-covid-19-treatment/."
},
{
"key": "ref_255",
"unstructured": "Johnson, C.K. (2023, October 30). Cheap Antidepressant Shows Promise Treating Early COVID-19. Available online: News.yahoo.com/cheap-antidepressant-shows-promise-treating-223735055.html."
},
{
"DOI": "10.3389/fphar.2021.652688",
"article-title": "Fluvoxamine: A Review of Its Mechanism of Action and Its Role in COVID-19",
"author": "Sukhatme",
"doi-asserted-by": "crossref",
"first-page": "763",
"journal-title": "Front. Pharmacol.",
"key": "ref_256",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.1093/ofid/ofab050",
"article-title": "Prospective Cohort of Fluvoxamine for Early Treatment of Coronavirus Disease 19",
"author": "Seftel",
"doi-asserted-by": "crossref",
"first-page": "ofab050",
"journal-title": "Open Forum Infect. Dis.",
"key": "ref_257",
"volume": "8",
"year": "2021"
},
{
"DOI": "10.1007/s40265-021-01636-5",
"article-title": "Fluvoxamine for the Early Treatment of SARS-CoV-2 Infection: A Review of Current Evidence",
"author": "Facente",
"doi-asserted-by": "crossref",
"first-page": "2081",
"journal-title": "Drugs",
"key": "ref_258",
"volume": "81",
"year": "2021"
},
{
"DOI": "10.1016/S2214-109X(21)00448-4",
"article-title": "Effect of early treatment with fluvoxamine on risk of emergency care and hospitalisation among patients with COVID-19: The TOGETHER randomised, platform clinical trial",
"author": "Reis",
"doi-asserted-by": "crossref",
"first-page": "e42",
"journal-title": "Lancet Glob. Health",
"key": "ref_259",
"volume": "10",
"year": "2022"
},
{
"DOI": "10.1001/jama.2020.22760",
"article-title": "Fluvoxamine vs Placebo and Clinical Deterioration in Outpatients With Symptomatic COVID-19: A Randomized Clinical Trial",
"author": "Lenze",
"doi-asserted-by": "crossref",
"first-page": "2292",
"journal-title": "JAMA",
"key": "ref_260",
"volume": "324",
"year": "2020"
},
{
"DOI": "10.1016/0026-2862(68)90003-4",
"article-title": "Microvascular occlusion by platelet emboli after transfusion and shock",
"author": "Swank",
"doi-asserted-by": "crossref",
"first-page": "15",
"journal-title": "Microvasc. Res.",
"key": "ref_261",
"volume": "1",
"year": "1968"
},
{
"DOI": "10.1007/s002130050924",
"article-title": "Plasma serotonin level after 1 day of fluoxetine treatment: A biological predictor for antidepressant response?",
"author": "Alvarez",
"doi-asserted-by": "crossref",
"first-page": "97",
"journal-title": "Psychopharmacology",
"key": "ref_262",
"volume": "143",
"year": "1999"
},
{
"DOI": "10.1016/0165-0327(92)90082-H",
"article-title": "Effects of acute and chronic treatment with fluvoxamine on extracellular and platelet serotonin in the blood of major depressive patients. Relationship to clinical improvement",
"author": "Celada",
"doi-asserted-by": "crossref",
"first-page": "243",
"journal-title": "J. Affect. Disord.",
"key": "ref_263",
"volume": "25",
"year": "1992"
},
{
"DOI": "10.1182/blood-2012-06-437392",
"article-title": "Platelet serotonin promotes the recruitment of neutrophils to sites of acute inflammation in mice",
"author": "Duerschmied",
"doi-asserted-by": "crossref",
"first-page": "1008",
"journal-title": "Blood",
"key": "ref_264",
"volume": "121",
"year": "2013"
},
{
"DOI": "10.1038/mp.2016.6",
"article-title": "TSPAN5, ERICH3 and selective serotonin reuptake inhibitors in major depressive disorder: Pharmacometabolomics-informed pharmacogenomics",
"author": "Gupta",
"doi-asserted-by": "crossref",
"first-page": "1717",
"journal-title": "Mol. Psychiatry",
"key": "ref_265",
"volume": "21",
"year": "2016"
},
{
"DOI": "10.1016/j.jad.2019.02.063",
"article-title": "Plasma serotonin levels are associated with antidepressant response to SSRIs",
"author": "Holck",
"doi-asserted-by": "crossref",
"first-page": "65",
"journal-title": "J. Affect. Disord.",
"key": "ref_266",
"volume": "250",
"year": "2019"
},
{
"DOI": "10.1002/jbmr.3443",
"article-title": "High Serum Serotonin Predicts Increased Risk for Hip Fracture and Nonvertebral Osteoporotic Fractures: The MrOS Sweden Study",
"author": "Kristjansdottir",
"doi-asserted-by": "crossref",
"first-page": "1560",
"journal-title": "J. Bone Miner. Res.",
"key": "ref_267",
"volume": "33",
"year": "2018"
},
{
"DOI": "10.1016/S0192-0561(99)00035-1",
"article-title": "[3H]Paroxetine binding to human peripheral lymphocyte membranes of patients with major depression before and after treatment with fluoxetine",
"author": "Urbina",
"doi-asserted-by": "crossref",
"first-page": "631",
"journal-title": "Int. J. Immunopharmacol.",
"key": "ref_268",
"volume": "21",
"year": "1999"
},
{
"DOI": "10.1172/JCI33374",
"article-title": "Interactions between integrin alphaIIbbeta3 and the serotonin transporter regulate serotonin transport and platelet aggregation in mice and humans",
"author": "Carneiro",
"doi-asserted-by": "crossref",
"first-page": "1544",
"journal-title": "J. Clin. Investig.",
"key": "ref_269",
"volume": "118",
"year": "2008"
},
{
"DOI": "10.1016/j.trsl.2007.10.004",
"article-title": "Selective serotonin reuptake inhibitors: Measurement of effect on platelet function",
"author": "McCloskey",
"doi-asserted-by": "crossref",
"first-page": "168",
"journal-title": "Transl. Res.",
"key": "ref_270",
"volume": "151",
"year": "2008"
},
{
"DOI": "10.1016/j.ijantimicag.2020.105949",
"article-title": "Hydroxychloroquine and azithromycin as a treatment of COVID-19: Results of an open-label non-randomized clinical trial",
"author": "Gautret",
"doi-asserted-by": "crossref",
"first-page": "105949",
"journal-title": "Int. J. Antimicrob. Agents",
"key": "ref_271",
"volume": "56",
"year": "2020"
},
{
"DOI": "10.3390/molecules25215064",
"doi-asserted-by": "crossref",
"key": "ref_272",
"unstructured": "Gendrot, M., Andreani, J., Jardot, P., Hutter, S., Delandre, O., Boxberger, M., Mosnier, J., Le Bideau, M., Duflot, I., and Fonta, I. (2020). In Vitro Antiviral Activity of Doxycycline against SARS-CoV-2. Molecules, 25."
},
{
"DOI": "10.1101/2023.04.03.23287649",
"doi-asserted-by": "crossref",
"key": "ref_273",
"unstructured": "Million, M., Cortaredona, S., Delorme, L., Colson, P., Levasseur, A., Hervé, T.-D., Karim, B., Salima, L., Bernard La, S., and Laurence, C.-J. (2023). Early Treatment with Hydroxychloroquine and Azithromycin: A ‘Real-Life’ Monocentric Retrospective Cohort Study of 30,423 COVID-19 Patients. medRxiv."
},
{
"DOI": "10.1001/jama.1960.03020150054010",
"article-title": "Use of antimalarial drugs as “desludging” agents in vascular disease processes: Preliminary report",
"author": "Madow",
"doi-asserted-by": "crossref",
"first-page": "1630",
"journal-title": "JAMA",
"key": "ref_274",
"volume": "172",
"year": "1960"
},
{
"article-title": "Desludging Action of Hydroxychloroquine in R.A",
"author": "Cecchi",
"first-page": "214",
"journal-title": "Acta Rheumatol. Scand.",
"key": "ref_275",
"volume": "8",
"year": "1962"
},
{
"DOI": "10.1161/01.CIR.96.12.4380",
"article-title": "Hydroxychloroquine Reverses Thrombogenic Properties of Antiphospholipid Antibodies in Mice",
"author": "Edwards",
"doi-asserted-by": "crossref",
"first-page": "4380",
"journal-title": "Circulation",
"key": "ref_276",
"volume": "96",
"year": "1997"
},
{
"DOI": "10.3389/fphar.2021.667704",
"article-title": "Exploring Phytochemicals of Traditional Medicinal Plants Exhibiting Inhibitory Activity Against Main Protease, Spike Glycoprotein, RNA-dependent RNA Polymerase and Non-Structural Proteins of SARS-CoV-2 Through Virtual Screening",
"author": "Nallusamy",
"doi-asserted-by": "crossref",
"first-page": "667704",
"journal-title": "Front. Pharmacol.",
"key": "ref_277",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.1080/07391102.2020.1824816",
"article-title": "Repurposing of the approved small molecule drugs in order to inhibit SARS-CoV-2 S protein and human ACE2 interaction through virtual screening approaches",
"author": "Kalhor",
"doi-asserted-by": "crossref",
"first-page": "1299",
"journal-title": "J. Biomol. Struct. Dyn.",
"key": "ref_278",
"volume": "40",
"year": "2020"
},
{
"DOI": "10.20944/preprints202005.0439.v1",
"doi-asserted-by": "crossref",
"key": "ref_279",
"unstructured": "Suravajhala, R., Parashar, A., Malik, B., Nagaraj, V.A., Padmanaban, G., Kavi Kishor, P.B., Polavarapu, R., and Suravajhala, P. (2020). Comparative Docking Studies on Curcumin with COVID-19 Proteins. Preprints.Org."
},
{
"article-title": "Global Trends in Clinical Studies of Ivermectin in COVID-19",
"author": "Yagisawa",
"first-page": "44",
"journal-title": "Jpn. J. Antibiot.",
"key": "ref_280",
"volume": "74",
"year": "2021"
},
{
"article-title": "The multitargeted drug ivermectin: From an antiparasitic agent to a repositioned cancer drug",
"author": "Juarez",
"first-page": "317",
"journal-title": "Am. J. Cancer Res.",
"key": "ref_281",
"volume": "8",
"year": "2018"
},
{
"DOI": "10.2174/138920112800399095",
"article-title": "History of avermectin and ivermectin, with notes on the history of other macrocyclic lactone antiparasitic agents",
"author": "Campbell",
"doi-asserted-by": "crossref",
"first-page": "853",
"journal-title": "Curr. Pharm. Biotechnol.",
"key": "ref_282",
"volume": "13",
"year": "2012"
},
{
"DOI": "10.1021/ci700044s",
"article-title": "Analysis of HIV Wild-Type and Mutant Structures via in Silico Docking against Diverse Ligand Libraries",
"author": "Chang",
"doi-asserted-by": "crossref",
"first-page": "1258",
"journal-title": "J. Chem. Inf. Model.",
"key": "ref_283",
"volume": "47",
"year": "2007"
},
{
"DOI": "10.20944/preprints202005.0084.v1",
"doi-asserted-by": "crossref",
"key": "ref_284",
"unstructured": "Dasgupta, J., Sen, U., Bakashi, A., and Dasgupta, A. (2020). Nsp7 and Spike Glycoprotein of SARS-CoV-2 Are Envisaged as Potential Targets of Vitamin D and Ivermectin. Preprints.Org."
},
{
"DOI": "10.1007/s13721-020-00263-6",
"doi-asserted-by": "crossref",
"key": "ref_285",
"unstructured": "Hussien, M.A., and Abdelaziz, A.E.M. (2020). Molecular docking suggests repurposing of brincidofovir as a potential drug targeting SARS-CoV-2 ACE2 receptor and main protease. Netw. Model. Anal. Health Inform. Bioinform., 9."
},
{
"DOI": "10.1007/s43440-020-00195-y",
"article-title": "Ivermectin as a potential drug for treatment of COVID-19: An in-sync review with clinical and computational attributes",
"author": "Kaur",
"doi-asserted-by": "crossref",
"first-page": "736",
"journal-title": "Pharmacol. Rep.",
"key": "ref_286",
"volume": "73",
"year": "2021"
},
{
"DOI": "10.26434/chemrxiv.12630539",
"doi-asserted-by": "crossref",
"key": "ref_287",
"unstructured": "Maurya, D. (2020). A Combination of Ivermectin and Doxycycline Possibly Blocks the Viral Entry and Modulate the Innate Immune Response in COVID-19 Patients. ChemRxiv."
},
{
"DOI": "10.1007/s11224-021-01776-0",
"article-title": "The Binding mechanism of Ivermectin and levosalbutamol with spike protein of SARS-CoV-2",
"author": "Saha",
"doi-asserted-by": "crossref",
"first-page": "1985",
"journal-title": "Struct. Chem.",
"key": "ref_288",
"volume": "32",
"year": "2021"
},
{
"DOI": "10.21873/invivo.12134",
"article-title": "Ivermectin Docks to the SARS-CoV-2 Spike Receptor-binding Domain Attached to ACE2",
"author": "Lehrer",
"doi-asserted-by": "crossref",
"first-page": "3023",
"journal-title": "In Vivo",
"key": "ref_289",
"volume": "34",
"year": "2020"
},
{
"key": "ref_290",
"unstructured": "Rajter, J.J. Personal communication."
},
{
"key": "ref_291",
"unstructured": "(2023, October 30). Local Doctor Tries New Coronavirus Drug Treatment. Available online: https://www.nbcmiami.com/news/local/local-doctor-tries-new-coronavirus-drug-treatment/2219465/."
},
{
"DOI": "10.1016/j.chest.2020.10.009",
"article-title": "Use of Ivermectin is Associated with Lower Mortality in Hospitalized Patients with COVID-19 (ICON study)",
"author": "Rajter",
"doi-asserted-by": "crossref",
"first-page": "85",
"journal-title": "Chest",
"key": "ref_292",
"volume": "159",
"year": "2020"
},
{
"DOI": "10.1016/j.nmni.2021.100924",
"article-title": "Ivermectin: A multifaceted drug of Nobel prize-honored distinction with indicated efficacy against a new global scourge, COVID-19",
"author": "Santin",
"doi-asserted-by": "crossref",
"first-page": "100924",
"journal-title": "New Microbes New Infect.",
"key": "ref_293",
"volume": "43",
"year": "2021"
},
{
"DOI": "10.1056/NEJMe2209017",
"article-title": "Time to Stop Using Ineffective COVID-19 Drugs",
"author": "Devnarain",
"doi-asserted-by": "crossref",
"first-page": "654",
"journal-title": "N. Engl. J. Med.",
"key": "ref_294",
"volume": "387",
"year": "2022"
},
{
"DOI": "10.1186/s12985-022-01829-8",
"article-title": "Ivermectin under scrutiny: A systematic review and meta-analysis of efficacy and possible sources of controversies in COVID-19 patients",
"author": "Shafiee",
"doi-asserted-by": "crossref",
"first-page": "102",
"journal-title": "Virol. J.",
"key": "ref_295",
"volume": "19",
"year": "2022"
},
{
"DOI": "10.1056/NEJMoa2115869",
"article-title": "Effect of Early Treatment with Ivermectin among Patients with COVID-19",
"author": "Reis",
"doi-asserted-by": "crossref",
"first-page": "1721",
"journal-title": "N. Engl. J. Med.",
"key": "ref_296",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.3390/jcm12113625",
"doi-asserted-by": "crossref",
"key": "ref_297",
"unstructured": "Scheim, D.E., Aldous, C., Osimani, B., Fordham, E.J., and Hoy, W.E. (2023). When Characteristics of Clinical Trials Require Per-Protocol as Well as Intention-to-Treat Outcomes to Draw Reliable Conclusions: Three Examples. J. Clin. Med., 12."
},
{
"key": "ref_298",
"unstructured": "U.S. Food & Drug Administration (2023, October 30). Memorandum Explaining Basis for Declining Request for Emergency Use Authorization of Fluvoxamine Maleate, Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2020/EUA%20110%20Fluvoxamine%20Decisional%20Memo_Redacted.pdf."
},
{
"key": "ref_299",
"unstructured": "NIH COVID-19 Treatment Guidelines (2023, October 30). Fluvoxamine: Selected Clinical Data, Limitations and Interpretation. Table 4c, Available online: https://www.covid19treatmentguidelines.nih.gov/tables/fluvoxamine-data/."
},
{
"key": "ref_300",
"unstructured": "TOGETHER Trial DSS and Data Repository Screenshots (2023, October 30). Date-Time Stamped Screenshots from Publications of the TOGETHER trial (NCT04727424). Available online: https://drive.google.com/file/d/1pBZ1GihxW_ROB3Aid6tFMplqAyMYOGDl/preview."
},
{
"key": "ref_301",
"unstructured": "(2023, October 30). Email from Sarah Fullegar, Sent 7 June 2022 to Edmund Fordham, Screenshot, Email Addresses Redacted. Available online: https://drive.google.com/file/d/1lUsSRf1KX-pa9T5EX4HbegdK8mYNQ_Ty/preview."
},
{
"DOI": "10.1001/jama.2021.3071",
"article-title": "Effect of Ivermectin on Time to Resolution of Symptoms Among Adults With Mild COVID-19: A Randomized Clinical Trial",
"author": "Hurtado",
"doi-asserted-by": "crossref",
"first-page": "1426",
"journal-title": "JAMA",
"key": "ref_302",
"volume": "325",
"year": "2021"
},
{
"key": "ref_303",
"unstructured": "Scheim, D.E., Hibberd, J.A., and Chamie-Quintero, J.J. Protocol Violations in López-Medina et al.: 38 Switched Ivermectin (IVM) and Placebo Doses, Failure of Blinding, Ubiquitous IVM use OTC in Cali, and Nearly Identical AEs for the IVM and Control Groups."
},
{
"DOI": "10.5694/j.1326-5377.1990.tb136833.x",
"article-title": "Cure of duodenal ulcer after eradication of Helicobacter pylori",
"author": "George",
"doi-asserted-by": "crossref",
"first-page": "145",
"journal-title": "Med. J. Aust.",
"key": "ref_304",
"volume": "153",
"year": "1990"
},
{
"DOI": "10.1016/S0140-6736(87)91545-5",
"article-title": "Campylobacter pylori and recurrence of duodenal ulcers—A 12-month follow-up study",
"author": "Coghlan",
"doi-asserted-by": "crossref",
"first-page": "1109",
"journal-title": "Lancet",
"key": "ref_305",
"volume": "2",
"year": "1987"
},
{
"DOI": "10.7326/0003-4819-116-9-705",
"article-title": "Effect of treatment of Helicobacter pylori infection on the long-term recurrence of gastric or duodenal ulcer. A randomized, controlled study",
"author": "Graham",
"doi-asserted-by": "crossref",
"first-page": "705",
"journal-title": "Ann. Intern. Med.",
"key": "ref_306",
"volume": "116",
"year": "1992"
},
{
"DOI": "10.1136/bmj.331.7520.795",
"article-title": "Nobel prize is awarded to doctors who discovered H pylori",
"author": "Watts",
"doi-asserted-by": "crossref",
"first-page": "795",
"journal-title": "BMJ",
"key": "ref_307",
"volume": "331",
"year": "2005"
},
{
"article-title": "On the Antibacterial Action of Cultures of a Penicillium, with Special Reference to their Use in the Isolation of B. influenzæ",
"author": "Fleming",
"first-page": "226",
"journal-title": "Br. J. Exp. Pathol.",
"key": "ref_308",
"volume": "10",
"year": "1929"
},
{
"DOI": "10.1016/S0140-6736(01)08728-1",
"article-title": "Penicillin as a chemotherapeutic agent",
"author": "Chain",
"doi-asserted-by": "crossref",
"first-page": "226",
"journal-title": "Lancet",
"key": "ref_309",
"volume": "236",
"year": "1940"
},
{
"article-title": "Penicillin’s Discovery and Antibiotic Resistance: Lessons for the Future?",
"author": "Lobanovska",
"first-page": "135",
"journal-title": "Yale J. Biol. Med.",
"key": "ref_310",
"volume": "90",
"year": "2017"
},
{
"DOI": "10.3390/pathogens10040413",
"doi-asserted-by": "crossref",
"key": "ref_311",
"unstructured": "Annunziata, A., Coppola, A., Carannante, N., Simioli, F., Lanza, M., Di Micco, P., and Fiorentino, G. (2021). Home Management of Patients with Moderate or Severe Respiratory Failure Secondary to COVID-19, Using Remote Monitoring and Oxygen with or without HFNC. Pathogens, 10."
},
{
"DOI": "10.1007/s11604-020-01085-2",
"article-title": "Pulmonary vascular enlargement and lesion extent on computed tomography are correlated with COVID-19 disease severity",
"author": "Aoki",
"doi-asserted-by": "crossref",
"first-page": "451",
"journal-title": "Jpn. J. Radiol.",
"key": "ref_312",
"volume": "39",
"year": "2021"
},
{
"DOI": "10.1016/j.ejrad.2020.109009",
"article-title": "Chest CT findings of COVID-19 pneumonia by duration of symptoms",
"author": "Ding",
"doi-asserted-by": "crossref",
"first-page": "109009",
"journal-title": "Eur. J. Radiol.",
"key": "ref_313",
"volume": "127",
"year": "2020"
},
{
"DOI": "10.1186/s43055-021-00470-9",
"article-title": "Clinical and radiological imaging as prognostic predictors in COVID-19 patients",
"author": "Metwally",
"doi-asserted-by": "crossref",
"first-page": "100",
"journal-title": "Egypt. J. Radiol. Nucl. Med.",
"key": "ref_314",
"volume": "52",
"year": "2021"
},
{
"DOI": "10.1186/s43055-020-00376-y",
"article-title": "Longitudinal assessment of chest computerized tomography and oxygen saturation for patients with COVID-19",
"author": "Osman",
"doi-asserted-by": "crossref",
"first-page": "255",
"journal-title": "Egypt. J. Radiol. Nucl. Med.",
"key": "ref_315",
"volume": "51",
"year": "2020"
},
{
"DOI": "10.1186/s43055-020-00329-5",
"article-title": "Tomographic findings in patients with COVID-19 according to evolution of the disease",
"doi-asserted-by": "crossref",
"first-page": "215",
"journal-title": "Egypt. J. Radiol. Nucl. Med.",
"key": "ref_316",
"volume": "51",
"year": "2020"
},
{
"DOI": "10.1148/radiol.2020200843",
"article-title": "Temporal Changes of CT Findings in 90 Patients with COVID-19 Pneumonia: A Longitudinal Study",
"author": "Wang",
"doi-asserted-by": "crossref",
"first-page": "e55",
"journal-title": "Radiology",
"key": "ref_317",
"volume": "296",
"year": "2020"
},
{
"DOI": "10.3390/biologics2030015",
"article-title": "Changes in SpO2 on Room Air for 34 Severe COVID-19 Patients after Ivermectin-Based Combination Treatment: 62% Normalization within 24 Hours",
"author": "Stone",
"doi-asserted-by": "crossref",
"first-page": "196",
"journal-title": "Biologics",
"key": "ref_318",
"volume": "2",
"year": "2022"
},
{
"DOI": "10.2217/fmb-2022-0014",
"article-title": "Effectiveness of ivermectin-based multidrug therapy in severely hypoxic, ambulatory COVID-19 patients",
"author": "Hazan",
"doi-asserted-by": "crossref",
"first-page": "339",
"journal-title": "Future Microbiol.",
"key": "ref_319",
"volume": "17",
"year": "2022"
},
{
"DOI": "10.23937/2474-3658/1510233",
"article-title": "A Randomized Controlled Trial of Ivermectin Monotherapy Versus HCQ, IVM, and AZ Combination Therapy in COVID-19 Patients in Nigeria",
"author": "Babalola",
"doi-asserted-by": "crossref",
"first-page": "233",
"journal-title": "J. Infect. Dis. Epidemiol.",
"key": "ref_320",
"volume": "7",
"year": "2021"
},
{
"key": "ref_321",
"unstructured": "Babalola, O.E. Personal communication."
},
{
"article-title": "A comparison of Ivermectin and Non Ivermectin based regimen for COVID-19 in Abuja: Effects on virus clearance, Days-to-Discharge and Mortality",
"author": "Thairu",
"first-page": "1",
"journal-title": "Res. Sq.",
"key": "ref_322",
"volume": "34",
"year": "2022"
},
{
"article-title": "COVID-19 Excess Deaths in Peru’s 25 States in 2020: Nationwide Trends, Confounding Factors, and Correlations With the Extent of Ivermectin Treatment by State",
"author": "Chamie",
"first-page": "e43168",
"journal-title": "Cureus",
"key": "ref_323",
"volume": "15",
"year": "2023"
},
{
"DOI": "10.3390/v13101993",
"doi-asserted-by": "crossref",
"key": "ref_324",
"unstructured": "Meekins, D.A., Gaudreault, N.N., and Richt, J.A. (2021). Natural and Experimental SARS-CoV-2 Infection in Domestic and Wild Animals. Viruses, 13."
},
{
"DOI": "10.3233/CH-2012-1576",
"article-title": "Comparative hemorheology",
"author": "Baskurt",
"doi-asserted-by": "crossref",
"first-page": "61",
"journal-title": "Clin. Hemorheol. Microcirc.",
"key": "ref_325",
"volume": "53",
"year": "2013"
},
{
"article-title": "Influence of cell-specific factors on red blood cell aggregation",
"author": "Rampling",
"first-page": "91",
"journal-title": "Biorheology",
"key": "ref_326",
"volume": "41",
"year": "2004"
},
{
"DOI": "10.1152/jappl.1994.77.4.1790",
"article-title": "Capacity for red blood cell aggregation is higher in athletic mammalian species than in sedentary species",
"author": "Popel",
"doi-asserted-by": "crossref",
"first-page": "1790",
"journal-title": "J. Appl. Physiol. (1985)",
"key": "ref_327",
"volume": "77",
"year": "1994"
},
{
"DOI": "10.3233/BIR-1996-334-506",
"article-title": "Comparison and simulation of different levels of erythrocyte aggregation with pig, horse, sheep, calf, and normal human blood",
"author": "Weng",
"doi-asserted-by": "crossref",
"first-page": "365",
"journal-title": "Biorheology",
"key": "ref_328",
"volume": "33",
"year": "1996"
},
{
"DOI": "10.3201/eid2809.220125",
"article-title": "Antibodies against SARS-CoV-2 Suggestive of Single Events of Spillover to Cattle, Germany",
"author": "Wernike",
"doi-asserted-by": "crossref",
"first-page": "1916",
"journal-title": "Emerg. Infect. Dis. J.",
"key": "ref_329",
"volume": "28",
"year": "2022"
},
{
"DOI": "10.1080/22221751.2021.2003724",
"article-title": "Susceptibility of livestock to SARS-CoV-2 infection",
"author": "Walker",
"doi-asserted-by": "crossref",
"first-page": "2199",
"journal-title": "Emerg. Microbes Infect.",
"key": "ref_330",
"volume": "10",
"year": "2021"
},
{
"key": "ref_331",
"unstructured": "(2023, October 30). Wessa.net Free Statistics Software, Office for Research Development and Education, Version 1.2.1. Available online: https://www.wessa.net/."
},
{
"DOI": "10.1080/14789450.2018.1531710",
"article-title": "Red blood cells as an organ? How deep omics characterization of the most abundant cell in the human body highlights other systemic metabolic functions beyond oxygen transport",
"author": "Nemkov",
"doi-asserted-by": "crossref",
"first-page": "855",
"journal-title": "Expert Rev. Proteom.",
"key": "ref_332",
"volume": "15",
"year": "2018"
},
{
"DOI": "10.1101/036103",
"doi-asserted-by": "crossref",
"key": "ref_333",
"unstructured": "Sender, R., Fuchs, S., and Milo, R. (2016). Revised Estimates for the Number of Human and Bacteria Cells in the Body. PLoS Biol., 14."
},
{
"DOI": "10.1016/j.str.2007.01.011",
"article-title": "Glycoprotein structural genomics: Solving the glycosylation problem",
"author": "Chang",
"doi-asserted-by": "crossref",
"first-page": "267",
"journal-title": "Structure",
"key": "ref_334",
"volume": "15",
"year": "2007"
},
{
"DOI": "10.1038/nprot.2009.29",
"article-title": "An efficient platform for screening expression and crystallization of glycoproteins produced in human cells",
"author": "Lee",
"doi-asserted-by": "crossref",
"first-page": "592",
"journal-title": "Nat. Protoc.",
"key": "ref_335",
"volume": "4",
"year": "2009"
},
{
"DOI": "10.1007/BF01025303",
"article-title": "One- and two-dimensional NMR studies of the N-terminal portion of glycophorin A at 11.7 Tesla",
"author": "Dill",
"doi-asserted-by": "crossref",
"first-page": "129",
"journal-title": "J. Protein Chem.",
"key": "ref_336",
"volume": "9",
"year": "1990"
},
{
"DOI": "10.21037/aob-20-51",
"article-title": "In silico molecular dynamics of human glycophorin A (GPA) extracellular structure",
"author": "Ekman",
"doi-asserted-by": "crossref",
"first-page": "1",
"journal-title": "Ann. Blood",
"key": "ref_337",
"volume": "6",
"year": "2021"
},
{
"DOI": "10.3390/ijms232214050",
"doi-asserted-by": "crossref",
"key": "ref_338",
"unstructured": "Trivedi, R., and Nagarajaram, H.A. (2022). Intrinsically disordered proteins: An overview. Int. J. Mol. Sci., 23."
},
{
"DOI": "10.1016/j.bbapap.2010.01.017",
"article-title": "Understanding protein non-folding",
"author": "Uversky",
"doi-asserted-by": "crossref",
"first-page": "1231",
"journal-title": "Biochim. Biophys. Acta (BBA)-Proteins Proteom.",
"key": "ref_339",
"volume": "1804",
"year": "2010"
},
{
"DOI": "10.1002/1097-0134(20001115)41:3<415::AID-PROT130>3.0.CO;2-7",
"article-title": "Why are “natively unfolded” proteins unstructured under physiologic conditions?",
"author": "Uversky",
"doi-asserted-by": "crossref",
"first-page": "415",
"journal-title": "Proteins",
"key": "ref_340",
"volume": "41",
"year": "2000"
},
{
"DOI": "10.1016/j.ijid.2021.04.035",
"article-title": "Positive impact of oral hydroxychloroquine and povidone-iodine throat spray for COVID-19 prophylaxis: An open-label randomized trial",
"author": "Seet",
"doi-asserted-by": "crossref",
"first-page": "314",
"journal-title": "Int. J. Infect. Dis.",
"key": "ref_341",
"volume": "106",
"year": "2021"
},
{
"DOI": "10.1016/S0140-6736(15)60696-1",
"article-title": "Offline: What is medicine’s 5 sigma?",
"author": "Horton",
"doi-asserted-by": "crossref",
"first-page": "1380",
"journal-title": "Lancet",
"key": "ref_342",
"volume": "385",
"year": "2015"
},
{
"DOI": "10.1016/j.jclinepi.2016.02.012",
"article-title": "Evidence-based medicine has been hijacked: A report to David Sackett",
"author": "Ioannidis",
"doi-asserted-by": "crossref",
"first-page": "82",
"journal-title": "J. Clin. Epidemiol.",
"key": "ref_343",
"volume": "73",
"year": "2016"
},
{
"DOI": "10.1136/bmj.h2942",
"article-title": "Justifying conflicts of interest in medical journals: A very bad idea",
"author": "Steinbrook",
"doi-asserted-by": "crossref",
"first-page": "h2942",
"journal-title": "BMJ",
"key": "ref_344",
"volume": "350",
"year": "2015"
},
{
"DOI": "10.1111/anae.15263",
"article-title": "False individual patient data and zombie randomised controlled trials submitted to Anaesthesia",
"author": "Carlisle",
"doi-asserted-by": "crossref",
"first-page": "472",
"journal-title": "Anaesthesia",
"key": "ref_345",
"volume": "76",
"year": "2021"
},
{
"key": "ref_346",
"unstructured": "Gotzsche, P. (2013). Deadly Medicines and Organised Crime: How Big Pharma Has Corrupted Healthcare, CRC Press. [1st ed.]."
},
{
"DOI": "10.1016/j.futures.2021.102860",
"article-title": "Science, the endless frontier of regulatory capture",
"author": "Saltelli",
"doi-asserted-by": "crossref",
"first-page": "102860",
"journal-title": "Futures",
"key": "ref_347",
"volume": "135",
"year": "2022"
},
{
"DOI": "10.1111/hel.12751",
"article-title": "Clinical and economic impact of “triple therapy” for Helicobacter pylori eradication on peptic ulcer disease in Australia",
"author": "Eslick",
"doi-asserted-by": "crossref",
"first-page": "e12751",
"journal-title": "Helicobacter",
"key": "ref_348",
"volume": "25",
"year": "2020"
},
{
"DOI": "10.7208/chicago/9780226239668.001.0001",
"doi-asserted-by": "crossref",
"key": "ref_349",
"unstructured": "Feenstra, R.C., and Shapiro, M.D. (2003). Scanner Data and Price Indexes, University of Chicago Press."
},
{
"DOI": "10.3390/v15010167",
"doi-asserted-by": "crossref",
"key": "ref_350",
"unstructured": "Chatterjee, S., Bhattacharya, M., Nag, S., Dhama, K., and Chakraborty, C. (2023). A Detailed Overview of SARS-CoV-2 Omicron: Its Sub-Variants, Mutations and Pathophysiology, Clinical Characteristics, Immunological Landscape, Immune Escape, and Therapies. Viruses, 15."
},
{
"DOI": "10.1038/s41586-022-04479-6",
"article-title": "SARS-CoV-2 Omicron variant replication in human bronchus and lung ex vivo",
"author": "Hui",
"doi-asserted-by": "crossref",
"first-page": "715",
"journal-title": "Nature",
"key": "ref_351",
"volume": "603",
"year": "2022"
},
{
"DOI": "10.1101/2021.12.31.474653",
"doi-asserted-by": "crossref",
"key": "ref_352",
"unstructured": "Peacock, T.P., Brown, J.C., Zhou, J., Thakur, N., Sukhova, K., Newman, J., Kugathasan, R., Yan, A.W.C., Furnon, W., and De Lorenzo, G. (2022). The altered entry pathway and antigenic distance of the SARS-CoV-2 Omicron variant map to separate domains of spike protein. bioRxiv."
},
{
"DOI": "10.1126/scitranslmed.abj7790",
"article-title": "Lung epithelial and endothelial damage, loss of tissue repair, inhibition of fibrinolysis, and cellular senescence in fatal COVID-19",
"author": "Walters",
"doi-asserted-by": "crossref",
"first-page": "eabj7790",
"journal-title": "Sci. Transl. Med.",
"key": "ref_353",
"volume": "13",
"year": "2021"
},
{
"key": "ref_354",
"unstructured": "Makary, M. (2023, October 30). The Real Data Behind the New COVID Vaccines the White Houseis Pushing. Available online: https://nypost.com/2023/09/14/the-real-data-behind-the-new-covid-vaccines-the-white-house-is-pushing/."
},
{
"DOI": "10.1016/S1473-3099(23)00299-2",
"article-title": "Outpatient treatment of COVID-19 and incidence of post-COVID-19 condition over 10 months (COVID-OUT): A multicentre, randomised, quadruple-blind, parallel-group, phase 3 trial",
"author": "Bramante",
"doi-asserted-by": "crossref",
"first-page": "1119",
"journal-title": "Lancet Infect. Dis.",
"key": "ref_355",
"volume": "23",
"year": "2023"
},
{
"DOI": "10.1001/jama.2022.24100",
"article-title": "Effect of Fluvoxamine vs Placebo on Time to Sustained Recovery in Outpatients With Mild to Moderate COVID-19: A Randomized Clinical Trial",
"author": "McCarthy",
"doi-asserted-by": "crossref",
"first-page": "296",
"journal-title": "JAMA",
"key": "ref_356",
"volume": "329",
"year": "2023"
},
{
"DOI": "10.1038/nature01339",
"article-title": "Nicotinic acetylcholine receptor α7 subunit is an essential regulator of inflammation",
"author": "Wang",
"doi-asserted-by": "crossref",
"first-page": "384",
"journal-title": "Nature",
"key": "ref_357",
"volume": "421",
"year": "2003"
},
{
"DOI": "10.1124/mol.53.2.283",
"article-title": "Ivermectin: A positive allosteric effector of the alpha7 neuronal nicotinic acetylcholine receptor",
"author": "Krause",
"doi-asserted-by": "crossref",
"first-page": "283",
"journal-title": "Mol. Pharmacol.",
"key": "ref_358",
"volume": "53",
"year": "1998"
},
{
"DOI": "10.1056/NEJMra2026131",
"article-title": "Cytokine Storm",
"author": "Fajgenbaum",
"doi-asserted-by": "crossref",
"first-page": "2255",
"journal-title": "N. Engl. J. Med.",
"key": "ref_359",
"volume": "383",
"year": "2020"
}
],
"reference-count": 359,
"references-count": 359,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.mdpi.com/1422-0067/24/23/17039"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Inorganic Chemistry",
"Organic Chemistry",
"Physical and Theoretical Chemistry",
"Computer Science Applications",
"Spectroscopy",
"Molecular Biology",
"General Medicine",
"Catalysis"
],
"subtitle": [],
"title": "Sialylated Glycan Bindings from SARS-CoV-2 Spike Protein to Blood and Endothelial Cells Govern the Severe Morbidities of COVID-19",
"type": "journal-article",
"volume": "24"
}