SARS-CoV-2-associated lymphopenia: possible mechanisms and the role of CD147
Shaimaa Shouman, Nada El-Kholy, Alaa E Hussien, Azza M El-Derby, Shireen Magdy, Ahmed M Abou-Shanab, Ahmed O Elmehrath, Ahmad Abdelwaly, Mohamed Helal, Nagwa El-Badri
Cell Communication and Signaling, doi:10.1186/s12964-024-01718-3
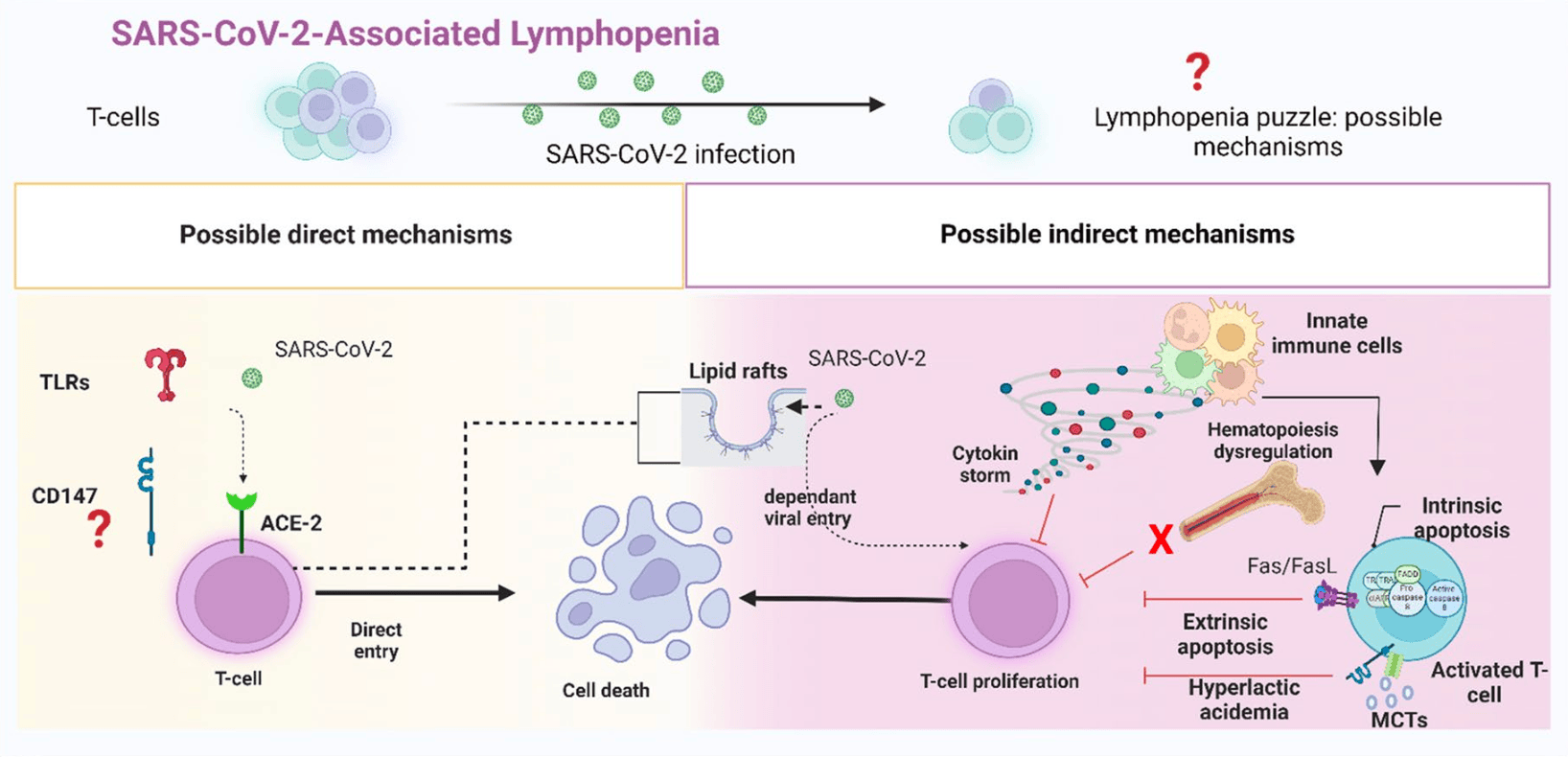
T lymphocytes play a primary role in the adaptive antiviral immunity. Both lymphocytosis and lymphopenia were found to be associated with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). While lymphocytosis indicates an active anti-viral response, lymphopenia is a sign of poor prognosis. T-cells, in essence, rarely express ACE2 receptors, making the cause of cell depletion enigmatic. Moreover, emerging strains posed an immunological challenge, potentially alarming for the next pandemic. Herein, we review how possible indirect and direct key mechanisms could contribute to SARS-CoV-2-associated-lymphopenia. The fundamental mechanism is the inflammatory cytokine storm elicited by viral infection, which alters the host cell metabolism into a more acidic state. This "hyperlactic acidemia" together with the cytokine storm suppresses T-cell proliferation and triggers intrinsic/extrinsic apoptosis. SARS-CoV-2 infection also results in a shift from steady-state hematopoiesis to stress hematopoiesis. Even with low ACE2 expression, the presence of cholesterol-rich lipid rafts on activated T-cells may enhance viral entry and syncytia formation. Finally, direct viral infection of lymphocytes may indicate the participation of other receptors or auxiliary proteins on T-cells, that can work alone or in concert with other mechanisms. Therefore, we address the role of CD147-a novel route-for SARS-CoV-2 and its new variants. CD147 is not only expressed on T-cells, but it also interacts with other co-partners to orchestrate various biological processes. Given these features, CD147 is an appealing candidate for viral pathogenicity. Understanding the molecular and cellular mechanisms behind SARS-CoV-2-associated-lymphopenia will aid in the discovery of potential therapeutic targets to improve the resilience of our immune system against this rapidly evolving virus.
Declarations Ethical approval and consent to participants Not applicable
Consent for publication Not applicable.
Competing interests The authors declare no competing interests.
Publisher's Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Abdullah, Cyclophilin A as a target in the treatment of cytomegalovirus infections
Abers, An immune-based biomarker signature is associated with mortality in COVID-19 patients, JCI Insight
Acharya, Anderson, NRP1 cripples immunological memory
Ajaz, Mitochondrial metabolic manipulation by SARS-CoV-2 in peripheral blood mononuclear cells of patients with COVID-19
Ali, CD147 levels in blood and adipose tissues correlate with vascular dysfunction in obese diabetic adults, Circ Res
Alvarez, TNF-α contributes to caspase-3 independent apoptosis in neuroblastoma cells: role of NFAT, PLoS One
Amraei, CD209L/L-SIGN and CD209/DC-SIGN act as receptors for SARS-CoV-2
André, T cell apoptosis characterizes severe Covid-19 disease
Arora, Extracellular cyclophilins contribute to the regulation of inflammatory responses, J Immunol
Assmann, Finlay, Metabolic Regul Immune Responses: Therapeutic Opportunities
Barathan, Chronic hepatitis C virus infection triggers spontaneous differential expression of biosignatures associated with T cell exhaustion and apoptosis signaling in peripheral blood mononucleocytes, Apoptosis
Behl, CD147-spike protein interaction in COVID-19: get the ball rolling with a novel receptor and therapeutic target, Sci Total Environ
Benlarbi, Identification of a SARS-CoV-2 host metalloproteinasedependent entry pathway differentially used by SARS-CoV-2 and variants of concern alpha, Delta Omicron
Bernard, Pathogenic Neisseria meningitidis utilizes CD147 for vascular colonization
Bestle, TMPRSS2 and furin are both essential for proteolytic activation of SARS-CoV-2 in human airway cells
Bhatt, Glycolytic inhibitor 2-deoxy-d-glucose attenuates SARS-CoV-2 multiplication in host cells and weakens the infective potential of progeny virions
Bian, Meplazumab in hospitalized adults with severe COVID-19 (DEFLECT): a multicenter, seamless phase 2/3, randomized, third-party double-blind clinical trial, Sig Transduct Target Ther
Biasi, Marked T cell activation, senescence, exhaustion and skewing towards TH17 in patients with COVID-19 pneumonia
Biswas, Nugent, Membrane association of collagenase stimulatory factor (s) from B-16 melanoma cells, J Cell Biochem
Biswas, The human tumor cell-derived collagenase stimulatory factor (renamed EMMPRIN) is a member of the immunoglobulin superfamily, Cancer Res
Biswas, Tumor cell stimulation of collagenase production by fibroblasts, Biochem Biophys Res Commun
Bone, Lauder, Cellular immunity, peripheral blood lymphocyte count and pathological staging of tumours in the gastrointestinal tract
Boonnak, Lymphopenia associated with highly virulent H5N1 virus infection due to plasmacytoid dendritic cell-mediated apoptosis of T cells
Brown, London, Structure and function of sphingolipid-and cholesterol-rich membrane rafts, J Biol Chem
Brunetti, SARS-CoV-2 uses CD4 to infect T helper lymphocytes
Bukrinsky, Mukhamedova, Sviridov, lipid rafts and pathogens: the art of deception and exploitation, Thematic Rev Series: Biology Lipid Rafts
Bushman, Population impact of SARS-CoV-2 variants with enhanced transmissibility and/or partial immune escape
Cantuti-Castelvetri, Neuropilin-1 facilitates SARS-CoV-2 cell entry and infectivity
Carpenè, Blood lactate concentration in COVID-19: a systematic literature review
Cautivo, Interferon gamma constrains type 2 lymphocyte niche boundaries during mixed inflammation, Immunity
Cham, Gajewski, Glucose availability regulates IFN-γ production and p70S6 kinase activation in CD8 + effector T cells, J Immunol
Chen, CD147 is required for matrix metalloproteinases-2 production and germ cell migration during spermatogenesis, Mol Hum Reprod
Chen, Clinical and immunological features of severe and moderate coronavirus disease
Chen, Function of HAb18G/CD147 in invasion of host cells by severe acute respiratory syndrome coronavirus
Chen, Human cytomegalovirus encoded miR-US25-1-5p attenuates CD147/EMMPRIN-mediated early antiviral response
Chhetri, A fatal case of COVID-19 due to metabolic acidosis following dysregulate inflammatory response (cytokine storm), Bull Rehabil Med
Choudhury, Mukherjee, In silico studies on the comparative characterization of the interactions of SARS-CoV-2 spike glycoprotein with ACE-2 receptor homologs and human TLRs, J Med Virol
Chu, Middle East respiratory syndrome coronavirus efficiently infects human primary T lymphocytes and activates the extrinsic and intrinsic apoptosis pathways
Chua, Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis
Clausen, SARS-CoV-2 infection depends on cellular heparan sulfate and ACE2
Crosnier, Basigin is a receptor essential for erythrocyte invasion by Plasmodium Falciparum
Dale, Cell-to-cell transfer of HIV-1 via virological synapses leads to endosomal virion maturation that activates viral membrane fusion
Damsker, Bukrinsky, Constant, Preferential chemotaxis of activated human CD4 + T cells by extracellular cyclophilin A, J Leukoc Biol
De Bruin, Voermans, Nolte, The Journal of the American Society of Hematology. Impact interferon-γ Hematopoiesis
De Kleijn, IFN-γ-stimulated neutrophils suppress lymphocyte proliferation through expression of PD-L1, PLoS ONE
De Malefyt, Interleukin 10 (IL-10) and viral IL-10 strongly reduce antigen-specific human T cell proliferation by diminishing the antigen-presenting capacity of monocytes via downregulation of class II major histocompatibility complex expression
Debuc, Smadja, and reports, is COVID-19 a new hematologic disease
Delorey, COVID-19 tissue atlases reveal SARS-CoV-2 pathology and cellular targets, Nature
Diao, Reduction and functional exhaustion of T cells in patients with coronavirus disease 2019 (COVID-19), Front Immunol
Diao, Reduction and functional exhaustion of T cells in patients with coronavirus disease 2019 (COVID-19), Front Immunol
Donnelly, mTORC1-dependent metabolic reprogramming is a prerequisite for NK cell effector function, J Immunol
Doughty, Antigen receptor-mediated changes in glucose metabolism in B lymphocytes: role of phosphatidylinositol 3-kinase signaling in the glycolytic control of growth
Ej, Complex immune dysregulation in COVID-19 patients with severe respiratory failure
Elahi, Sciences, Hematopoietic responses to SARS-CoV-2 infection
Ellis, Beaman, Interferon-γ Activation Polymorphonuclear Neutrophil Function
Ellis, Nabeshima, Cjcr, Monoclon Antib Preparation Purif Tumor cell Collagenase stimulatory Factor, Cancer Res
Farshbafnadi, Zonouzi, Sabahi, Dolatshahi, Aarabi, Aging & COVID-19 susceptibility, disease severity, and clinical outcomes: The role of entangled risk factors, Exp Gerontol,
doi:10.1016/j.exger.2021.111507Fathi, Rezaei, Lymphopenia in COVID-19: therapeutic opportunities, Cell Biology International
Fecchi, Coronavirus interplay with lipid rafts and autophagy unveils promising therapeutic targets, Front Microbiol
Feng, The alteration and clinical significance of Th1/Th2/Th17/ Treg cells in patients with multiple myeloma
Feng, The novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) directly decimates human spleens and lymph nodes
Fenizia, SARS-CoV-2 entry: at the crossroads of CD147 and ACE2
Fischer, Inhibitory effect of tumor cell-derived lactic acid on human T cells, Blood
Floch, CD147 subunit of lactate/H + symporters MCT1 and hypoxia-inducible MCT4 is critical for energetics and growth of glycolytic tumors, Proceed Nat Acad Sci
Foda, Ventilator-induced lung injury upregulates and activates gelatinases and EMMPRIN: attenuation by the synthetic matrix metalloproteinase inhibitor, Prinomastat (AG3340), Am J Respir Cell Mol Biol
Fok, Novel regulators of spermatogenesis, Semin Cell Dev Biol
Frauwirth, Thompson, Regulation of T lymphocyte metabolism, J Immunol
Freemerman, Metabolic reprogramming of macrophages: glucose transporter 1 (GLUT1)-mediated glucose metabolism drives a proinflammatory phenotype
Gaber, Pathophysiological hypoxia affects the redox state and IL-2 signalling of human CD4 + T cells and concomitantly impairs survival and proliferation
Gaber, Strehl, Buttgereit, None, Metabolic Regul Inflamm
Ganesh, Mohanram, Metabolic Reprogramming Immune, Regul Viral Dis
Geng, CD147 antibody specifically and effectively inhibits infection and cytokine storm of SARS-CoV-2 and its variants delta, alpha, beta, and gamma
Geng, Immunological Metabolic Characteristics Omicron Variants, Infect
Grigoriou, Regulatory T-cell transcriptomic reprogramming characterizes adverse events by checkpoint inhibitors in solid tumors
Gu, Interaction network of SARS-CoV-2 with host receptome through spike protein
Gu, Multiple organ infection and the pathogenesis of SARS, J Exp Med
Gu, Receptome profiling identifies KREMEN1 and ASGR1 as alternative functional receptors of SARS-CoV-2
Guan, Clinical characteristics of coronavirus disease 2019 in China, J Emerg Med
Gubser, Rapid effector function of memory CD8 + T cells requires an immediate-early glycolytic switch
Guindolet, Gabison, Role of CD147 (EMMPRIN/Basigin) in tissue remodeling, Anat Rec
Halestrap, Nt ; Kirk Á, CD147 is tightly associated with lactate transporters MCT1 and MCT4 and facilitates their cell surface expression, Biochem J
Hampton, Chtanova, Lymphatic Migration Immune Cells
Hanahan, Weinberg, Hallmarks of cancer: the next generation, Cell
Harvey, SARS-CoV-2 variants, spike mutations and immune escape, Nat Rev Microbiol
Helal, Molecular basis of the potential interaction of SARS-CoV-2 spike protein to CD147 in COVID-19 associated-lymphopenia
Henry, Lymphopenia and neutrophilia at admission predicts severity and mortality in patients with COVID-19: a meta-analysis
Hoffmann, Pöhlmann, Novel sars-Cov-2 receptors: Asgr1 and Kremen1
Hoffmann, SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor
Hu, The JAK/STAT signaling pathway: from bench to clinic, Sig Transduct Target Ther
Huang, Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China, Lancet
Huang, SARS coronavirus, but not human coronavirus NL63, utilizes cathepsin L to infect ACE2-expressing cells
Hudák, Contribution of syndecans to the cellular entry of SARS-CoV-2
Hviid, Kemp, What is the cause of lymphopenia in malaria?, Infect Immun
Igakura, A null mutation in basigin, an immunoglobulin superfamily member, indicates its important roles in peri-implantation development and spermatogenesis, Dev Biol
Igakura, Spread of HTLV-I between lymphocytes by virus-induced polarization of the cytoskeleton
Ikuta, Human endothelial cells: effect of TNF-alpha on peripheral blood mononuclear cell adhesion, Immunology
Janes, The role of lipid rafts in T cell antigen receptor (TCR) signalling, Seminars in immunology
Junqueira, FcγR-mediated SARS-CoV-2 infection of monocytes activates inflammation
Jury, Flores-Borja, Kabouridis, Lipid rafts in T cell signalling and disease
Kabouridis, lipid rafts in T cell receptor signalling, Mol Membr Biol
Karki, Synergism of TNF-α and IFN-γ triggers inflammatory cell death, tissue damage, and mortality in SARS-CoV-2 infection and cytokine shock syndromes, Cell
Kasinrerk, Human leukocyte activation antigen M6, a member of the ig superfamily, is the species homologue of rat OX-47, mouse basigin, and chicken HT7 molecule
Kern, Increased levels of soluble Fas ligand in serum in Plasmodium Falciparum malaria, Infect Immun
Kessenbrock, Plaks, Werb, Matrix Metalloproteinases: Regulators Tumor Microenvironment, Cell
Khanmohammadi, Rezaei, Role of toll-like receptors in the pathogenesis of COVID-19, J Med Virol
Kim, The stimulation of CD147 induces MMP-9 expression through ERK and NF-κB in macrophages: implication for atherosclerosis
Kirkham, Ultrastructural identification of uncoated caveolinindependent early endocytic vehicles, J Cell Biol
Knierman, The human leukocyte antigen class II immunopeptidome of the SARS-CoV-2 spike glycoprotein, Cell Rep
Koch, T cell activation-associated epitopes of CD147 in regulation of the T cell response, and their definition by antibody affinity and antigen density
Kondo, L-SIGN is a receptor on liver sinusoidal endothelial cells for SARS-CoV-2 virus, JCI Insight
Krawczyk, Toll-like receptor-induced changes in glycolytic metabolism regulate dendritic cell activation
Kreuzberger, SARS-CoV-2-neutralising monoclonal antibodies for treatment of COVID-19, Cochrane Database Syst Rev
Krystle, Comorbid illnesses are associated with altered adaptive immune responses to SARS-CoV-2, JCI Insight
Kucia, The ACE2 receptor for COVID-19 entry is expressed on the surface of hematopoietic stem/progenitor cells and endothelial progenitors as well as their precursor cells and becomes activated in nlrp3 inflammasome-dependent manner by virus spike protein-a potential pathway leading to a cytokine storm
Law, The 3a protein of severe acute respiratory syndromeassociated coronavirus induces apoptosis in Vero E6 cells, J Gen Virol
Li, Beg, Induction of necrotic-like cell death by tumor necrosis factor alpha and caspase inhibitors: novel mechanism for killing virus-infected cells, J Virol
Li, Dependence of SARS-CoV-2 infection on cholesterol-rich lipid raft and endosomal acidification, Comput Struct Biotechnol J
Li, Immune dysfunction leads to mortality and organ injury in patients with COVID-19 in China: insights from ERS-COVID-19 study
Li, Jones, Geiger, IL-6 promotes T cell proliferation and expansion under inflammatory conditions in association with low-level RORγt expression, J Immunol
Li, None, Lactate Metabolism Hum Health Disease
Li, Possible Mechanisms of Lymphopenia in Severe Tuberculosis, Microorganisms
Liang, Atorvastatin attenuates plaque vulnerability by downregulation of EMMPRIN expression via COX-2/PGE2 pathway, Exp Ther Med
Liu, Lymphocyte subset (CD4+, CD8+) counts reflect the severity of infection and predict the clinical outcomes in patients with COVID-19, J Infect
Liu, Von Brunn, Zhu, Cyclophilin A and CD147: novel therapeutic targets for the treatment of COVID-19, Med Drug Discov
Long, Principles and practice of pediatric infectious diseases E-Book
Macintyre, The glucose transporter Glut1 is selectively essential for CD4 T cell activation and effector function
Maeda, IL-6 blocks a discrete early step in lymphopoiesis, Blood
Maeda, nterleukin-6 aborts lymphopoiesis and elevates production of myeloid cells in systemic lupus erythematosus-prone B6. Sle1. Yaa animals Blood, J Am Soc Hematol
Maini, Beneficial effects of tumour necrosis factor-alpha (TNF-α) blockade in rheumatoid arthritis (RA), Clin Experimental Immunol
Malesevic, Anti-inflammatory effects of extracellular cyclosporins are exclusively mediated by CD147, J Med Chem
Marconi, Efficacy and safety of baricitinib for the treatment of hospitalised adults with COVID-19 (COV-BARRIER): a randomised, double-blind, parallel-group, placebo-controlled phase 3 trial, Lancet Respir Med
Markov, The evolution of SARS-CoV-2
Marzi, DC-SIGN and DC-SIGNR interact with the glycoprotein of Marburg virus and the S protein of severe acute respiratory syndrome coronavirus
Matsumoto, Malaria infection induces rapid elevation of the soluble Fas ligand level in serum and subsequent T lymphocytopenia: possible factors responsible for the differences in susceptibility of two species of Macaca monkeys to Plasmodium coatneyi infection, Infect Immun
Mcdonnell, Bcl-2-immunoglobulin transgenic mice demonstrate extended B cell survival and follicular lymphoproliferation
Melo, Biomarkers of cytokine storm as red flags for severe and fatal COVID-19 cases: a living systematic review and meta-analysis
Merekoulias, Lymphocyte to monocyte ratio as a screening tool for influenza, PLoS Curr
Merezhinskaya, Presence and localization of three lactic acid transporters (MCT1,-2, and -4) in separated human granulocytes, lymphocytes, and monocytes, J Histochem Cytochemistry
Mobini, Structure-based study of immune receptors as eligible binding targets of coronavirus SARS-CoV-2 spike protein
Mohamad, Mitochondrial Apoptotic Pathways
Moradi, Increased age, neutrophil-to-lymphocyte ratio (NLR) and white blood cells count are associated with higher COVID-19 mortality, Am J Emerg Med
Moreno-Eutimio, Bioinformatic analysis and identification of single-stranded RNA sequences recognized by TLR7/8 in the SARS-CoV-2, SARS-CoV, and MERS-CoV genomes, Microbes Infect
Muramatsu, Miyauchi, Basigin (CD147): a multifunctional transmembrane protein involved in reproduction, neural function, inflammation and tumor invasion, Histol Histopathol
Murray, Role of Rab5 in the recruitment of hVps34/p150 to the early endosome
Ménétrier-Caux, Lymphopenia in cancer patients and its effects on response to immunotherapy: an opportunity for combination with cytokines?
Nakai, Tissue distribution of basigin and monocarboxylate transporter 1 in the adult male mouse: a study using the wild-type and basigin gene knockout mice, Am Assoc Anatomy
Nakaya, Inflammatory T cell responses rely on amino acid transporter ASCT2 facilitation of glutamine uptake and mTORC1 kinase activation
Nechipurenko, The role of acidosis in the pathogenesis of severe forms of COVID-19
Nguyen, And, Kanneganti, PANoptosis Viral Infection: Missing Puzzle Piece cell Death Field
Nichols, Caveosomes Endocytosis Lipid Rafts
Nichols, Niles, Roberts, Human lymphocyte apoptosis after exposure to influenza a virus, J Virol
Nizamudeen, Structural assessment of SARS-CoV2 accessory protein ORF7a predicts LFA-1 and Mac-1 binding potential
O'brien, Zhu, Zhang, The importance of IL-6 in the development of LAT-mediated autoimmunity, J Immunol
Orlandi, Fishman, Filipin-dependent inhibition of cholera toxin: evidence for toxin internalization and activation through caveolae-like domains
Osuna-Ramos, The Role of Host Cholesterol During Flavivirus Infection, Front Cell Infect Microbiol
Ouweneel, Thomas, O.L.R, Sorci-Thomas, the ins and outs of lipid rafts: functions in intracellular cholesterol homeostasis, microparticles, and cell membranes, Thematic Rev Series: Biology Lipid Rafts
Pan, Characteristics of lymphocyte subsets and cytokine profiles of patients with COVID-19
Panfilis, Identification of Fas-L-expressing apoptotic T lymphocytes in normal human peripheral blood: in vivo suicide
Pearce, Fueling Immunity: Insights into Metabolism Lymphocyte Function
Peñaloza, Lee, Ray, Neutrophils and lymphopenia, an unknown axis in severe COVID-19 disease, PLoS Pathog
Philp, Loss of MCT1, MCT3, and MCT4 expression in the retinal pigment epithelium and neural retina of the 5A11/basigin-null mouse, Invest ophthalmol vis sci
Pike, lipid rafts: bringing order to chaos, J Lipid Res
Pistol, Roles of CD147 on T lymphocytes activation and MMP-9 secretion in systemic lupus erythematosus
Pontelli, SARS-CoV-2 productively infects primary human immune system cells in vitro and in COVID-19 patients, J Mol Cell Biol
Poole, Halestrap, Transport of lactate and other monocarboxylates across mammalian plasma membranes, Am J Physiology-Cell Physiol
Post, Wood, Maartens, CD4 and total lymphocyte counts as predictors of HIV disease progression, QJM
Pothlichet, PLA2G1B is involved in CD4 anergy and CD4 lymphopenia in HIV-infected patients, J Clin Invest
Pulliam, Increased risk of SARS-CoV-2 reinfection associated with emergence of Omicron in South Africa
Pushkarsky, CD147 facilitates HIV-1 infection by interacting with virus-associated cyclophilin A, Proc Natl Acad Sci U S A
Radzikowska, Distribution of ACE2, CD147, CD26, and other SARS-CoV-2 associated molecules in tissues and immune cells in health and in asthma, COPD, obesity, hypertension, and COVID-19 risk factors
Ragotte, Human basigin (CD147) does not directly interact with SARS-CoV-2 spike glycoprotein,
doi:10.1128/msphereRatajczak, SARS-CoV-2 entry receptor ACE2 is expressed on very small CD45 -precursors of hematopoietic and endothelial cells and in response to virus spike protein activates the Nlrp3 inflammasome
Redzic, The retinal specific CD147 Ig0 domain: from molecular structure to biological activity, J Mol Biol
Rehermann, The hepatitis B virus persists for decades after patients' recovery from acute viral hepatitis despite active maintenance of a cytotoxic T-lymphocyte response, Nat Med
Ren, COVID-19 immune features revealed by a large-scale singlecell transcriptome atlas
Renno, A role for CD147 in thymic development, J Immunol
Reynolds, And, Dong, toll-like receptor regulation of effector T lymphocyte function
Riesco, Five-year cancer cure: relation to total amount of peripheral lymphocytes and neutrophils
Rodríguez-Espinosa, Metabolic Requirements Neutrophil Extracell Traps Formation
Rodríguez-Prados, Substrate fate in activated macrophages: a comparison between innate, classic, and alternative activation
Roncato, Lipid rafts as viral entry routes and immune platforms: a double-edged sword in SARS-CoV-2 infection?
Ropa, Human hematopoietic stem, progenitor, and immune cells respond ex vivo to SARS-CoV-2 spike protein
Salomão, Involvement of matrix metalloproteinases in COVID-19: molecular targets, mechanisms, and insights for therapeutic interventions
Salvi, SARS-CoV-2-associated ssRNAs activate inflammation and immunity via TLR7/8, JCI Insight
Schürch, Riether, Ochsenbein, Cytotoxic CD8 + T Cells Stimulate Hematopoietic Progenitors Promoting Cytokine Release bone Marrow Mesenchymal Stromal Cells
Sefik, Inflammasome activation in infected macrophages drives COVID-19 pathology
Seita, Weissman, Hematopoietic stem cell: selfrenewal versus Differ
Shahbaz, Erythroid precursors and progenitors suppress adaptive immunity and get invaded by SARS-CoV-2, Stem Cell Reports
Shen, ACE2-independent infection of T lymphocytes by SARS-CoV-2
Shen, ACE2-independent infection of T lymphocytes by SARS-CoV-2
Shilts, No evidence for basigin/CD147 as a direct SARS-CoV-2 spike binding receptor
Simons, Ehehalt, None, Cholesterol Lipid Rafts Disease
Simons, Toomre, None, Lipid Rafts Signal Transduct
Sn, Cholesterol-rich lipid rafts as platforms for SARS-CoV-2 entry
Song, Little to no expression of angiotensin-converting enzyme-2 on most human peripheral blood immune cells but highly expressed on tissue macrophages, Cytometry A
Sorice, Targeting lipid rafts as a strategy against coronavirus, Front Cell Dev Biol
Spees, Neutrophils are a source of gamma interferon during acute Salmonella enterica serovar Typhimurium colitis
Staffler Gn, Selective inhibition of T cell activation via CD147 through novel modulation of lipid rafts, J Immunol
Stefan, Birkenfeld, Schulze, Global pandemics interconnected-obesity, impaired metabolic health and COVID-19, Nat Rev Endocrinol
Stukalov, Multilevel proteomics reveals host perturbations by SARS-CoV-2 and SARS-CoV, Nature
Sturge, TLR-independent neutrophil-derived IFN-γ is important for host resistance to intracellular pathogens, Proc Natl Acad Sci U S A
Supper, Association of CD147 and calcium exporter PMCA4 uncouples IL-2 expression from early TCR signaling
Sviridov, Targeting lipid rafts-a potential therapy for COVID-19
Swanson, Deng, The NLRP3 inflammasome: molecular activation and regulation to therapeutics
Talotta, Robertson ; Shouman, Autoimmunity as the comet tail of COVID-19 pandemic. 2020. 8(17, Cell Communication and Signaling
Tan, Lymphopenia predicts disease severity of COVID-19: a descriptive and predictive study, Signal Transduct Target Ther
Tavakolpour, Lymphopenia during the COVID-19 infection: what it shows and what can be learned, Immunol Lett
Thaker, Ch'ng, Christofk, None, Viral Hijacking Cell Metabolism
Thompson, Metabolic programs define dysfunctional immune responses in severe COVID-19 patients, Cell Rep
Tian, HIF-1α promotes SARS-CoV-2 infection and aggravates inflammatory responses to COVID-19, Signal Transduct Tar Ther
Till, A role for membrane-bound CD147 in NOD2-mediated recognition of bacterial cytoinvasion
Turkmen, The relationship between neutrophil-to-lymphocyte ratio and inflammation in end-stage renal disease patients
Ulich, Mechanisms of Tumor Necrosis factor Alpha-Induced Lymphopenia, Neutropenia, and Biphasic Neutrophilia: a study of lymphocyte recirculation and hematologic interactions of TNFα, Endogenous Mediators Leukoc Trafficking
Vanarsdall, CD147 promotes entry of Pentamer-Expressing Human Cytomegalovirus into Epithelial and endothelial cells, mBio
Vasanthakumar, Beta-adrenergic blockers as a potential treatment for COVID-19 patients, BioEssays
Velazquez-Salinas, The role of interleukin 6 during viral infections, Front Microbiol
Vs, Dipeptidyl peptidase 4 is a functional receptor for the emerging human coronavirus, EMC
Walker, Mcmichael, The T-cell response to HIV, Cold Spring Harbor Perspect Med
Wang, AXL is a candidate receptor for SARS-CoV-2 that promotes infection of pulmonary and bronchial epithelial cells
Wang, CD147-spike protein is a novel route for SARS-CoV-2 infection to host cells
Wang, CD147-spike protein is a novel route for SARS-CoV-2 infection to host cells
Wang, Cholesterol-rich lipid rafts in the cellular membrane play an essential role, Avian Reovirus Replication
Wang, Dysregulated hematopoiesis in bone marrow marks severe COVID-19
Wang, Dysregulated hematopoiesis in bone marrow marks severe COVID-19, Cell Discov
Wang, Glycolysis and oxidative phosphorylation play critical roles in natural killer cell receptor-mediated natural killer cell functions, Front Immunol
Wang, SARS coronavirus entry into host cells through a novel clathrin-and caveolae-independent endocytic pathway
Watanabe, CD147/EMMPRIN acts as a functional entry receptor for measles virus on epithelial cells
Wen, Immune cell profiling of COVID-19 patients in the recovery stage by single-cell sequencing, Cell Discov
Wilson, Basigin (CD147) is the target for organomercurial inhibition of monocarboxylate transporter isoforms 1 and 4: the ancillary protein for the insensitive MCT2 is, EMBIGIN
Wu, CD147 contributes to SARS-CoV-2-induced pulmonary fibrosis
Xiang, SARS-CoV-2 induces lymphocytopenia by promoting inflammation and decimates secondary lymphoid organs
Xiong, Edwards, Iii, J.O.M.S, Zhou, The biological function and clinical utilization of CD147 in human diseases: a review of the current scientific literature
Xiong, Transcriptomic characteristics of bronchoalveolar lavage fluid and peripheral blood mononuclear cells in COVID-19 patients
Xu, Human immunodeficiency viruses pseudotyped with SARS-CoV-2 spike proteins infect a broad spectrum of human cell lines through multiple entry mechanisms
Yan, Structural basis for the recognition of SARS-CoV-2 by fulllength human ACE2
Yang, Bcl-xL inhibits T-cell apoptosis induced by expression of SARS coronavirus E protein in the absence of growth factors
Yang, Cyclophilin A up-regulates MMP-9 expression and adhesion of monocytes/macrophages via CD147 signalling pathway in rheumatoid arthritis
Yang, Cytokine storm promoting T cell exhaustion in severe COVID-19 revealed by single cell sequencing data analysis, Precis Clin Med
Yang, pH-dependent entry of severe acute respiratory syndrome coronavirus is mediated by the spike glycoprotein and enhanced by dendritic cell transfer through DC, SIGN
Yao, Important functional roles of basigin in thymocyte development and T cell activation
Yu, PD-L1 promotes tumor growth and progression by activating WIP and β-catenin signaling pathways and predicts poor prognosis in lung cancer
Yurchenko, Active site residues of cyclophilin A are crucial for its signaling activity via CD147, J Biol Chem
Yurchenko, CD147 is a signaling receptor for cyclophilin B, Biochem Biophys Res Commun
Yurchenko, Constant, Bukrinsky, Dealing with the family: CD147 interactions with cyclophilins, Immunology
Yurchenko, Cyclophilin-CD147 interactions: a new target for anti-inflammatory therapeutics
Zea, Arginase-producing myeloid suppressor cells in renal cell carcinoma patients: a mechanism of tumor evasion
Zeng, SARS-CoV-2 spreads through cell-to-cell transmission
Zhang, Disrupting CD147-RAP2 interaction abrogates erythrocyte invasion by Plasmodium Falciparum
Zhang, Molecular mechanism of interaction between SARS-CoV-2 and host cells and interventional therapy
Zhang, SARS-CoV-2 coronavirus spike protein-induced apoptosis, inflammatory, and oxidative stress responses in THP-1-likemacrophages: potential role of angiotensin-converting enzyme inhibitor (perindopril), Nat Commun
Zhao, The NLRP3 inflammasome and COVID-19: Activation, pathogenesis and therapeutic strategies
Zheng, Elevated exhaustion levels and reduced functional diversity of T cells in peripheral blood may predict severe progression in COVID-19 patients
Zheng, Landscape of SARS-CoV-2 spike protein-interacting cells in human tissues
Zhou, Involvement of CD147 in overexpression of MMP-2 and MMP-9 and enhancement of invasive potential of PMA-differentiated THP-1
Zhou, SARS-CoV-2 pseudovirus enters the host cells through spike protein-CD147 in an Arf6-dependent manner
Zhu, CD147: a novel modulator of inflammatory and immune disorders, Curr Med Chem
Zhu, Single-cell sequencing of peripheral mononuclear cells reveals distinct immune response landscapes of COVID-19 and influenza patients
DOI record:
{
"DOI": "10.1186/s12964-024-01718-3",
"ISSN": [
"1478-811X"
],
"URL": "http://dx.doi.org/10.1186/s12964-024-01718-3",
"abstract": "<jats:title>Abstract</jats:title><jats:p>T lymphocytes play a primary role in the adaptive antiviral immunity. Both lymphocytosis and lymphopenia were found to be associated with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). While lymphocytosis indicates an active anti-viral response, lymphopenia is a sign of poor prognosis. T-cells, in essence, rarely express ACE2 receptors, making the cause of cell depletion enigmatic. Moreover, emerging strains posed an immunological challenge, potentially alarming for the next pandemic. Herein, we review how possible indirect and direct key mechanisms could contribute to SARS-CoV-2-associated-lymphopenia. The fundamental mechanism is the inflammatory cytokine storm elicited by viral infection, which alters the host cell metabolism into a more acidic state. This “hyperlactic acidemia” together with the cytokine storm suppresses T-cell proliferation and triggers intrinsic/extrinsic apoptosis. SARS-CoV-2 infection also results in a shift from steady-state hematopoiesis to stress hematopoiesis. Even with low ACE2 expression, the presence of cholesterol-rich lipid rafts on activated T-cells may enhance viral entry and syncytia formation. Finally, direct viral infection of lymphocytes may indicate the participation of other receptors or auxiliary proteins on T-cells, that can work alone or in concert with other mechanisms. Therefore, we address the role of CD147―a novel route―for SARS-CoV-2 and its new variants. CD147 is not only expressed on T-cells, but it also interacts with other co-partners to orchestrate various biological processes. Given these features, CD147 is an appealing candidate for viral pathogenicity. Understanding the molecular and cellular mechanisms behind SARS-CoV-2-associated-lymphopenia will aid in the discovery of potential therapeutic targets to improve the resilience of our immune system against this rapidly evolving virus.</jats:p>\n <jats:p><jats:bold>Graphical Abstract</jats:bold></jats:p>",
"alternative-id": [
"1718"
],
"article-number": "349",
"assertion": [
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Received",
"name": "received",
"order": 1,
"value": "24 February 2024"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Accepted",
"name": "accepted",
"order": 2,
"value": "15 June 2024"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "First Online",
"name": "first_online",
"order": 3,
"value": "4 July 2024"
},
{
"group": {
"label": "Declarations",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 1
},
{
"group": {
"label": "<!--Emphasis Type='Bold' removed-->Ethical approval and consent to participants",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 2,
"value": "Not applicable"
},
{
"group": {
"label": "Consent for publication",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 3,
"value": "Not applicable."
},
{
"group": {
"label": "Competing interests",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 4,
"value": "The authors declare no competing interests."
}
],
"author": [
{
"affiliation": [],
"family": "Shouman",
"given": "Shaimaa",
"sequence": "first"
},
{
"affiliation": [],
"family": "El-Kholy",
"given": "Nada",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hussien",
"given": "Alaa E.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "El-Derby",
"given": "Azza M.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Magdy",
"given": "Shireen",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Abou-Shanab",
"given": "Ahmed M.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Elmehrath",
"given": "Ahmed O.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Abdelwaly",
"given": "Ahmad",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Helal",
"given": "Mohamed",
"sequence": "additional"
},
{
"affiliation": [],
"family": "El-Badri",
"given": "Nagwa",
"sequence": "additional"
}
],
"container-title": "Cell Communication and Signaling",
"container-title-short": "Cell Commun Signal",
"content-domain": {
"crossmark-restriction": false,
"domain": [
"link.springer.com"
]
},
"created": {
"date-parts": [
[
2024,
7,
4
]
],
"date-time": "2024-07-04T09:08:54Z",
"timestamp": 1720084134000
},
"deposited": {
"date-parts": [
[
2024,
7,
4
]
],
"date-time": "2024-07-04T09:13:38Z",
"timestamp": 1720084418000
},
"funder": [
{
"DOI": "10.13039/501100003009",
"award": [
"46721",
"46721",
"46721",
"46721",
"46721",
"46721",
"46721",
"46721",
"46721",
"46721"
],
"doi-asserted-by": "publisher",
"name": "Science and Technology Development Fund"
},
{
"name": "The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB)."
},
{
"name": "Zewail City of Science & Technology"
}
],
"indexed": {
"date-parts": [
[
2024,
7,
5
]
],
"date-time": "2024-07-05T00:14:54Z",
"timestamp": 1720138494277
},
"is-referenced-by-count": 0,
"issue": "1",
"issued": {
"date-parts": [
[
2024,
7,
4
]
]
},
"journal-issue": {
"issue": "1",
"published-online": {
"date-parts": [
[
2024,
12
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
7,
4
]
],
"date-time": "2024-07-04T00:00:00Z",
"timestamp": 1720051200000
}
},
{
"URL": "https://creativecommons.org/licenses/by/4.0",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
7,
4
]
],
"date-time": "2024-07-04T00:00:00Z",
"timestamp": 1720051200000
}
}
],
"link": [
{
"URL": "https://link.springer.com/content/pdf/10.1186/s12964-024-01718-3.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/article/10.1186/s12964-024-01718-3/fulltext.html",
"content-type": "text/html",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/content/pdf/10.1186/s12964-024-01718-3.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "297",
"original-title": [],
"prefix": "10.1186",
"published": {
"date-parts": [
[
2024,
7,
4
]
]
},
"published-online": {
"date-parts": [
[
2024,
7,
4
]
]
},
"publisher": "Springer Science and Business Media LLC",
"reference": [
{
"DOI": "10.1038/s41579-021-00573-0",
"author": "WT Harvey",
"doi-asserted-by": "publisher",
"first-page": "409",
"issue": "7",
"journal-title": "Nat Rev Microbiol.",
"key": "1718_CR1",
"unstructured": "Harvey WT, et al. SARS-CoV-2 variants, spike mutations and immune escape. Nat Rev Microbiol. 2021;19(7):409–24.",
"volume": "19",
"year": "2021"
},
{
"DOI": "10.1038/s41579-023-00878-2",
"doi-asserted-by": "crossref",
"key": "1718_CR2",
"unstructured": "Markov PV et al. The evolution of SARS-CoV-2. 2023. 21(6): p. 361–79."
},
{
"DOI": "10.1186/s12985-022-01786-2",
"doi-asserted-by": "crossref",
"key": "1718_CR3",
"unstructured": "Pan P et al. Characteristics of lymphocyte subsets and cytokine profiles of patients with COVID-19. 2022. 19(1): p. 57."
},
{
"DOI": "10.1038/s41423-020-0401-3",
"doi-asserted-by": "crossref",
"key": "1718_CR4",
"unstructured": "Zheng H-Y et al. Elevated exhaustion levels and reduced functional diversity of T cells in peripheral blood may predict severe progression in COVID-19 patients. 2020. 17(5): p. 541–3."
},
{
"key": "1718_CR5",
"unstructured": "Shen X-R et al. ACE2-independent infection of T lymphocytes by SARS-CoV-2. 2022. 7(1): p. 83."
},
{
"key": "1718_CR6",
"unstructured": "Shen X-R et al. ACE2-independent infection of T lymphocytes by SARS-CoV-2. 2022. 7(1): p. 1–11."
},
{
"DOI": "10.1016/j.chom.2020.04.009",
"doi-asserted-by": "crossref",
"key": "1718_CR7",
"unstructured": "Giamarellos-Bourboulis EJ et al. Complex immune dysregulation in COVID-19 patients with severe respiratory failure. 2020. 27(6): p. 992–1000. e3."
},
{
"DOI": "10.1152/ajpcell.00426.2020",
"doi-asserted-by": "crossref",
"key": "1718_CR8",
"unstructured": "Ajaz S et al. Mitochondrial metabolic manipulation by SARS-CoV-2 in peripheral blood mononuclear cells of patients with COVID-19. 2021."
},
{
"key": "1718_CR9",
"unstructured": "Li X, et al. Lactate Metabolism Hum Health Disease. 2022;7(1):305."
},
{
"DOI": "10.3390/biology10090852",
"doi-asserted-by": "crossref",
"key": "1718_CR10",
"unstructured": "Nechipurenko YD et al. The role of acidosis in the pathogenesis of severe forms of COVID-19. 2021. 10(9): p. 852."
},
{
"DOI": "10.1515/cclm-2021-1115",
"doi-asserted-by": "crossref",
"key": "1718_CR11",
"unstructured": "Carpenè G et al. Blood lactate concentration in COVID-19: a systematic literature review. 2022. 60(3): p. 332–7."
},
{
"DOI": "10.1038/s41418-022-00936-x",
"doi-asserted-by": "crossref",
"key": "1718_CR12",
"unstructured": "André S et al. T cell apoptosis characterizes severe Covid-19 disease 2022. 29(8): pp. 1486–1499."
},
{
"DOI": "10.1016/j.csbj.2021.04.001",
"author": "X Li",
"doi-asserted-by": "publisher",
"first-page": "1933",
"journal-title": "Comput Struct Biotechnol J.",
"key": "1718_CR13",
"unstructured": "Li X, et al. Dependence of SARS-CoV-2 infection on cholesterol-rich lipid raft and endosomal acidification. Comput Struct Biotechnol J. 2021;19:1933–43.",
"volume": "19",
"year": "2021"
},
{
"author": "PW Janes",
"key": "1718_CR14",
"unstructured": "Janes PW, et al. The role of lipid rafts in T cell antigen receptor (TCR) signalling. Seminars in immunology. Elsevier; 2000.",
"volume-title": "Seminars in immunology",
"year": "2000"
},
{
"DOI": "10.4049/jimmunol.171.4.1707",
"doi-asserted-by": "crossref",
"key": "1718_CR15",
"unstructured": "Staffler Gn, et al. Selective inhibition of T cell activation via CD147 through novel modulation of lipid rafts. J Immunol. 2003;171(4):1707–14."
},
{
"DOI": "10.1007/s00018-022-04220-6",
"doi-asserted-by": "crossref",
"key": "1718_CR16",
"unstructured": "Elahi SJC, Sciences ML. Hematopoietic responses to SARS-CoV-2 infection. 2022. 79(3): p. 187."
},
{
"DOI": "10.1038/s41421-021-00296-9",
"doi-asserted-by": "crossref",
"key": "1718_CR17",
"unstructured": "Wang X et al. Dysregulated hematopoiesis in bone marrow marks severe COVID-19. 2021. 7(1): p. 60."
},
{
"DOI": "10.1101/2020.03.27.20045427",
"doi-asserted-by": "crossref",
"key": "1718_CR18",
"unstructured": "Feng Z et al. The novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) directly decimates human spleens and lymph nodes 2020: p. 2020.03. 27.20045427."
},
{
"DOI": "10.1111/all.14429",
"doi-asserted-by": "crossref",
"key": "1718_CR19",
"unstructured": "Radzikowska U et al. Distribution of ACE2, CD147, CD26, and other SARS-CoV‐2 associated molecules in tissues and immune cells in health and in asthma, COPD, obesity, hypertension, and COVID‐19 risk factors. 2020. 75(11): p. 2829–45."
},
{
"DOI": "10.1016/j.it.2013.06.003",
"doi-asserted-by": "crossref",
"key": "1718_CR20",
"unstructured": "Reynolds JM. And C.J.T.i.i. Dong, toll-like receptor regulation of effector T lymphocyte function. 2013. 34(10): p. 511–9."
},
{
"DOI": "10.1042/BSR20203837",
"doi-asserted-by": "crossref",
"key": "1718_CR21",
"unstructured": "Nizamudeen ZA et al. Structural assessment of SARS-CoV2 accessory protein ORF7a predicts LFA-1 and Mac-1 binding potential. 2021. 41(1): p. BSR20203837."
},
{
"DOI": "10.1126/science.abd2985",
"doi-asserted-by": "crossref",
"key": "1718_CR22",
"unstructured": "Cantuti-Castelvetri L et al. Neuropilin-1 facilitates SARS-CoV-2 cell entry and infectivity. 2020. 370(6518): p. 856–60."
},
{
"DOI": "10.1093/intimm/11.5.777",
"doi-asserted-by": "crossref",
"key": "1718_CR23",
"unstructured": "Koch C et al. T cell activation-associated epitopes of CD147 in regulation of the T cell response, and their definition by antibody affinity and antigen density. 1999. 11(5): p. 777–86."
},
{
"DOI": "10.3390/v13060953",
"doi-asserted-by": "crossref",
"key": "1718_CR24",
"unstructured": "Xu C et al. Human immunodeficiency viruses pseudotyped with SARS-CoV-2 spike proteins infect a broad spectrum of human cell lines through multiple entry mechanisms. 2021. 13(6): p. 953."
},
{
"DOI": "10.1038/s41392-021-00760-8",
"doi-asserted-by": "crossref",
"key": "1718_CR25",
"unstructured": "Geng J et al. CD147 antibody specifically and effectively inhibits infection and cytokine storm of SARS-CoV-2 and its variants delta, alpha, beta, and gamma. 2021. 6(1): p. 347."
},
{
"DOI": "10.1111/j.1365-2249.2010.04115.x",
"doi-asserted-by": "crossref",
"key": "1718_CR26",
"unstructured": "Yurchenko V et al. Cyclophilin–CD147 interactions: a new target for anti-inflammatory therapeutics. 2010. 160(3): p. 305–17."
},
{
"key": "1718_CR27",
"unstructured": "Zhou J et al. Involvement of CD147 in overexpression of MMP-2 and MMP-9 and enhancement of invasive potential of PMA-differentiated THP-1. 2005. 6(1): p. 1–10."
},
{
"DOI": "10.1074/jbc.M411950200",
"doi-asserted-by": "crossref",
"key": "1718_CR28",
"unstructured": "Wilson MC et al. Basigin (CD147) is the target for organomercurial inhibition of monocarboxylate transporter isoforms 1 and 4: the ancillary protein for the insensitive MCT2 is EMBIGIN (gp70). 2005. 280(29): p. 27213–21."
},
{
"DOI": "10.3390/ijms151017411",
"doi-asserted-by": "crossref",
"key": "1718_CR29",
"unstructured": "Xiong L, Edwards CK III. and L.J.I.j.o.m.s. Zhou, The biological function and clinical utilization of CD147 in human diseases: a review of the current scientific literature 2014. 15(10): pp. 17411–17441."
},
{
"DOI": "10.1038/bjc.1974.184",
"doi-asserted-by": "crossref",
"key": "1718_CR30",
"unstructured": "Bone G, I.J.B.J.o C, Lauder. Cellular immunity, peripheral blood lymphocyte count and pathological staging of tumours in the gastrointestinal tract 1974. 30(3): pp. 215–221."
},
{
"DOI": "10.1158/0008-5472.CAN-08-3845",
"doi-asserted-by": "crossref",
"key": "1718_CR31",
"unstructured": "Ray-Coquard I et al. Lymphopenia as a prognostic factor for overall survival in advanced carcinomas, sarcomas, and lymphomas. 2009. 69(13): p. 5383–91."
},
{
"DOI": "10.1002/1097-0142(197001)25:1<135::AID-CNCR2820250120>3.0.CO;2-9",
"doi-asserted-by": "crossref",
"key": "1718_CR32",
"unstructured": "Riesco AJC. Five-year cancer cure: relation to total amount of peripheral lymphocytes and neutrophils. 1970. 25(1): p. 135–40."
},
{
"DOI": "10.3109/0886022X.2011.641514",
"doi-asserted-by": "crossref",
"key": "1718_CR33",
"unstructured": "Turkmen K et al. The relationship between neutrophil-to-lymphocyte ratio and inflammation in end-stage renal disease patients. 2012. 34(2): p. 155–9."
},
{
"DOI": "10.1007/s10753-014-9980-4",
"doi-asserted-by": "crossref",
"key": "1718_CR34",
"unstructured": "Feng P et al. The alteration and clinical significance of Th1/Th2/Th17/Treg cells in patients with multiple myeloma. 2015. 38: p. 705–9."
},
{
"DOI": "10.1038/s41419-020-2701-z",
"doi-asserted-by": "crossref",
"key": "1718_CR35",
"unstructured": "Yu W et al. PD-L1 promotes tumor growth and progression by activating WIP and β-catenin signaling pathways and predicts poor prognosis in lung cancer. 2020. 11(7): p. 506."
},
{
"DOI": "10.1186/s40425-019-0549-5",
"doi-asserted-by": "crossref",
"key": "1718_CR36",
"unstructured": "Ménétrier-Caux C et al. Lymphopenia in cancer patients and its effects on response to immunotherapy: an opportunity for combination with cytokines? 2019. 7(1): p. 1–15."
},
{
"DOI": "10.1158/0008-5472.CAN-04-4505",
"doi-asserted-by": "crossref",
"key": "1718_CR37",
"unstructured": "Zea AH et al. Arginase-producing myeloid suppressor cells in renal cell carcinoma patients: a mechanism of tumor evasion. 2005. 65(8): p. 3044–8."
},
{
"author": "SS Long",
"key": "1718_CR38",
"unstructured": "Long SS, et al. Principles and practice of pediatric infectious diseases E-Book. Elsevier Health Sciences; 2022.",
"volume-title": "Principles and practice of pediatric infectious diseases E-Book",
"year": "2022"
},
{
"DOI": "10.1038/s41392-020-0148-4",
"author": "L Tan",
"doi-asserted-by": "publisher",
"first-page": "33",
"issue": "1",
"journal-title": "Signal Transduct Target Ther.",
"key": "1718_CR39",
"unstructured": "Tan L, et al. Lymphopenia predicts disease severity of COVID-19: a descriptive and predictive study. Signal Transduct Target Ther. 2020;5(1):33.",
"volume": "5",
"year": "2020"
},
{
"DOI": "10.1172/jci.insight.146242",
"doi-asserted-by": "crossref",
"key": "1718_CR40",
"unstructured": "Krystle K et al. Comorbid illnesses are associated with altered adaptive immune responses to SARS-CoV-2. JCI Insight. 2021;22,6(6):e146242."
},
{
"DOI": "10.3389/fimmu.2020.00827",
"author": "B Diao",
"doi-asserted-by": "publisher",
"first-page": "827",
"journal-title": "Front Immunol.",
"key": "1718_CR41",
"unstructured": "Diao B, et al. Reduction and functional exhaustion of T cells in patients with coronavirus disease 2019 (COVID-19). Front Immunol. 2020;11:827.",
"volume": "11",
"year": "2020"
},
{
"DOI": "10.1128/IAI.68.10.6087-6089.2000",
"author": "L Hviid",
"doi-asserted-by": "publisher",
"first-page": "6087",
"issue": "10",
"journal-title": "Infect Immun",
"key": "1718_CR42",
"unstructured": "Hviid L, Kemp K. What is the cause of lymphopenia in malaria? Infect Immun. 2000;68(10):6087–9.",
"volume": "68",
"year": "2000"
},
{
"DOI": "10.3390/microorganisms11112640",
"author": "F Li",
"doi-asserted-by": "publisher",
"first-page": "2640",
"issue": "11",
"journal-title": "Microorganisms.",
"key": "1718_CR43",
"unstructured": "Li F, et al. Possible Mechanisms of Lymphopenia in Severe Tuberculosis. Microorganisms. 2023;11(11):2640.",
"volume": "11",
"year": "2023"
},
{
"DOI": "10.1128/IAI.68.5.3061-3063.2000",
"author": "P Kern",
"doi-asserted-by": "publisher",
"first-page": "3061",
"issue": "5",
"journal-title": "Infect Immun.",
"key": "1718_CR44",
"unstructured": "Kern P, et al. Increased levels of soluble Fas ligand in serum in Plasmodium Falciparum malaria. Infect Immun. 2000;68(5):3061–3.",
"volume": "68",
"year": "2000"
},
{
"DOI": "10.1128/IAI.68.3.1183-1188.2000",
"author": "J Matsumoto",
"doi-asserted-by": "publisher",
"first-page": "1183",
"issue": "3",
"journal-title": "Infect Immun",
"key": "1718_CR45",
"unstructured": "Matsumoto J, et al. Malaria infection induces rapid elevation of the soluble Fas ligand level in serum and subsequent T lymphocytopenia: possible factors responsible for the differences in susceptibility of two species of Macaca monkeys to Plasmodium coatneyi infection. Infect Immun. 2000;68(3):1183–8.",
"volume": "68",
"year": "2000"
},
{
"DOI": "10.1371/currents.RRN1154",
"doi-asserted-by": "crossref",
"key": "1718_CR46",
"unstructured": "Merekoulias G et al. Lymphocyte to monocyte ratio as a screening tool for influenza.PLoS Curr. 2010;2:RRN1154."
},
{
"DOI": "10.1093/qjmed/89.7.505",
"doi-asserted-by": "crossref",
"key": "1718_CR47",
"unstructured": "Post FA, Wood R, Maartens G. CD4 and total lymphocyte counts as predictors of HIV disease progression. QJM.1996;89(7): 505–8."
},
{
"DOI": "10.1038/nm1096-1104",
"author": "B Rehermann",
"doi-asserted-by": "publisher",
"first-page": "1104",
"issue": "10",
"journal-title": "Nat Med.",
"key": "1718_CR48",
"unstructured": "Rehermann B, et al. The hepatitis B virus persists for decades after patients’ recovery from acute viral hepatitis despite active maintenance of a cytotoxic T–lymphocyte response. Nat Med. 1996;2(10):1104–8.",
"volume": "2",
"year": "1996"
},
{
"DOI": "10.1128/JVI.73.13.5921-5929.2001",
"author": "JE Nichols",
"doi-asserted-by": "publisher",
"first-page": "5921",
"issue": "13",
"journal-title": "J Virol",
"key": "1718_CR49",
"unstructured": "Nichols JE, Niles JA, Roberts NJ. Human lymphocyte apoptosis after exposure to influenza a virus. J Virol. 2001;75(13):5921–9.",
"volume": "75",
"year": "2001"
},
{
"DOI": "10.1007/s10495-014-1084-y",
"author": "M Barathan",
"doi-asserted-by": "publisher",
"first-page": "466",
"issue": "4",
"journal-title": "Apoptosis",
"key": "1718_CR50",
"unstructured": "Barathan M, et al. Chronic hepatitis C virus infection triggers spontaneous differential expression of biosignatures associated with T cell exhaustion and apoptosis signaling in peripheral blood mononucleocytes. Apoptosis. 2015;20(4):466–80.",
"volume": "20",
"year": "2015"
},
{
"author": "N Fathi",
"key": "1718_CR51",
"unstructured": "Fathi N, Rezaei N. Lymphopenia in COVID-19: therapeutic opportunities. Cell Biology International; 2020.",
"volume-title": "Lymphopenia in COVID-19: Therapeutic opportunities",
"year": "2020"
},
{
"DOI": "10.1101/cshperspect.a007054",
"author": "B Walker",
"doi-asserted-by": "publisher",
"first-page": "a007054",
"issue": "11",
"journal-title": "Cold Spring Harbor Perspect Med",
"key": "1718_CR52",
"unstructured": "Walker B, McMichael A. The T-cell response to HIV. Cold Spring Harbor Perspect Med. 2012;2(11):a007054.",
"volume": "2",
"year": "2012"
},
{
"DOI": "10.1172/JCI131842",
"author": "J Pothlichet",
"doi-asserted-by": "publisher",
"first-page": "2872",
"issue": "6",
"journal-title": "J Clin Invest.",
"key": "1718_CR53",
"unstructured": "Pothlichet J, et al. PLA2G1B is involved in CD4 anergy and CD4 lymphopenia in HIV-infected patients. J Clin Invest. 2020;130(6):2872–87.",
"volume": "130",
"year": "2020"
},
{
"DOI": "10.1016/S0140-6736(20)30183-5",
"doi-asserted-by": "crossref",
"key": "1718_CR54",
"unstructured": "Huang C, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506."
},
{
"DOI": "10.1016/j.imlet.2020.06.013",
"author": "S Tavakolpour",
"doi-asserted-by": "publisher",
"first-page": "31",
"journal-title": "Immunol Lett",
"key": "1718_CR55",
"unstructured": "Tavakolpour S, et al. Lymphopenia during the COVID-19 infection: what it shows and what can be learned. Immunol Lett. 2020;225:31.",
"volume": "225",
"year": "2020"
},
{
"DOI": "10.3389/fimmu.2020.00827",
"author": "B Diao",
"doi-asserted-by": "publisher",
"first-page": "827",
"journal-title": "Front Immunol",
"key": "1718_CR56",
"unstructured": "Diao B, et al. Reduction and functional exhaustion of T cells in patients with coronavirus disease 2019 (COVID-19). Front Immunol. 2020;11:827.",
"volume": "11",
"year": "2020"
},
{
"author": "Z Liu",
"first-page": "318",
"issue": "2",
"journal-title": "J Infect.",
"key": "1718_CR57",
"unstructured": "Liu Z, et al. Lymphocyte subset (CD4+, CD8+) counts reflect the severity of infection and predict the clinical outcomes in patients with COVID-19. J Infect. 2020;81(2):318–56.",
"volume": "81",
"year": "2020"
},
{
"DOI": "10.1084/jem.20050828",
"doi-asserted-by": "crossref",
"key": "1718_CR58",
"unstructured": "Gu J et al. Multiple organ infection and the pathogenesis of SARS. J Exp Med. 2005;202(3):415–24."
},
{
"DOI": "10.1371/journal.pone.0253894",
"doi-asserted-by": "crossref",
"key": "1718_CR59",
"unstructured": "Melo AKG et al. Biomarkers of cytokine storm as red flags for severe and fatal COVID-19 cases: a living systematic review and meta-analysis. 2021. 16(6): p. e0253894."
},
{
"DOI": "10.1172/JCI137244",
"doi-asserted-by": "crossref",
"key": "1718_CR60",
"unstructured": "Chen G et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. 2020. 130(5): p. 2620–9."
},
{
"author": "W-j Guan",
"first-page": "1708",
"issue": "18",
"journal-title": "J Emerg Med.",
"key": "1718_CR61",
"unstructured": "Guan W-j, et al. Clinical characteristics of coronavirus disease 2019 in China. J Emerg Med. 2020;382(18):1708–20.",
"volume": "382",
"year": "2020"
},
{
"DOI": "10.3389/fmicb.2019.01057",
"author": "L Velazquez-Salinas",
"doi-asserted-by": "publisher",
"first-page": "1057",
"journal-title": "Front Microbiol",
"key": "1718_CR62",
"unstructured": "Velazquez-Salinas L, et al. The role of interleukin 6 during viral infections. Front Microbiol. 2019;10:1057.",
"volume": "10",
"year": "2019"
},
{
"DOI": "10.4049/jimmunol.1800016",
"author": "B Li",
"doi-asserted-by": "publisher",
"first-page": "2934",
"issue": "10",
"journal-title": "J Immunol",
"key": "1718_CR63",
"unstructured": "Li B, Jones LL, Geiger TL. IL-6 promotes T cell proliferation and expansion under inflammatory conditions in association with low-level RORγt expression. J Immunol. 2018;201(10):2934–46.",
"volume": "201",
"year": "2018"
},
{
"DOI": "10.4049/jimmunol.1403187",
"author": "SA O’Brien",
"doi-asserted-by": "publisher",
"first-page": "695",
"issue": "2",
"journal-title": "J Immunol",
"key": "1718_CR64",
"unstructured": "O’Brien SA, Zhu M, Zhang W. The importance of IL-6 in the development of LAT-mediated autoimmunity. J Immunol. 2015;195(2):695–705.",
"volume": "195",
"year": "2015"
},
{
"author": "K Maeda",
"first-page": "4534",
"issue": "19",
"journal-title": "J Am Soc Hematol",
"key": "1718_CR65",
"unstructured": "Maeda K, et al. nterleukin-6 aborts lymphopoiesis and elevates production of myeloid cells in systemic lupus erythematosus–prone B6. Sle1. Yaa animals Blood. J Am Soc Hematol. 2009;113(19):4534–40.",
"volume": "113",
"year": "2009"
},
{
"DOI": "10.1182/blood-2005-02-0456",
"author": "K Maeda",
"doi-asserted-by": "publisher",
"first-page": "879",
"issue": "3",
"journal-title": "Blood",
"key": "1718_CR66",
"unstructured": "Maeda K, et al. IL-6 blocks a discrete early step in lymphopoiesis. Blood. 2005;106(3):879–85.",
"volume": "106",
"year": "2005"
},
{
"key": "1718_CR67",
"unstructured": "Abers MS et al. An immune-based biomarker signature is associated with mortality in COVID-19 patients. JCI Insight. 2021;11;6(1):e144455."
},
{
"DOI": "10.1016/j.cell.2020.11.025",
"doi-asserted-by": "crossref",
"key": "1718_CR68",
"unstructured": "Karki R et al. Synergism of TNF-α and IFN-γ triggers inflammatory cell death, tissue damage, and mortality in SARS-CoV-2 infection and cytokine shock syndromes. Cell. 2021;184(1):149–68.e17."
},
{
"DOI": "10.1038/s41392-021-00791-1",
"author": "X Hu",
"doi-asserted-by": "publisher",
"first-page": "402",
"issue": "1",
"journal-title": "Sig Transduct Target Ther.",
"key": "1718_CR69",
"unstructured": "Hu X, et al. The JAK/STAT signaling pathway: from bench to clinic. Sig Transduct Target Ther. 2021;6(1):402.",
"volume": "6",
"year": "2021"
},
{
"DOI": "10.1111/j.1365-2249.1995.tb08340.x",
"author": "R Maini",
"doi-asserted-by": "publisher",
"first-page": "207",
"issue": "2",
"journal-title": "Clin Experimental Immunol",
"key": "1718_CR70",
"unstructured": "Maini R, et al. Beneficial effects of tumour necrosis factor-alpha (TNF‐α) blockade in rheumatoid arthritis (RA). Clin Experimental Immunol. 1995;101(2):207–12.",
"volume": "101",
"year": "1995"
},
{
"DOI": "10.1371/journal.pone.0016100",
"doi-asserted-by": "crossref",
"key": "1718_CR71",
"unstructured": "Alvarez S et al. TNF-α contributes to caspase-3 independent apoptosis in neuroblastoma cells: role of NFAT. PLoS One. 2011;6(1):e16100."
},
{
"DOI": "10.1128/JVI.74.16.7470-7477.2000",
"doi-asserted-by": "crossref",
"key": "1718_CR72",
"unstructured": "Li M, Beg AA. Induction of necrotic-like cell death by tumor necrosis factor alpha and caspase inhibitors: novel mechanism for killing virus-infected cells. J Virol. 2000;74(16)7470–7."
},
{
"author": "TR Ulich",
"first-page": "155",
"issue": "2",
"journal-title": "Endogenous Mediators Leukoc Trafficking",
"key": "1718_CR73",
"unstructured": "Ulich TR, et al. Mechanisms of Tumor Necrosis factor Alpha-Induced Lymphopenia, Neutropenia, and Biphasic Neutrophilia: a study of lymphocyte recirculation and hematologic interactions of TNFα. Endogenous Mediators Leukoc Trafficking. 1989;45(2):155–67.",
"volume": "45",
"year": "1989"
},
{
"author": "S Ikuta",
"first-page": "71",
"issue": "1",
"journal-title": "Immunology.",
"key": "1718_CR74",
"unstructured": "Ikuta S, et al. Human endothelial cells: effect of TNF-alpha on peripheral blood mononuclear cell adhesion. Immunology. 1991;73(1):71.",
"volume": "73",
"year": "1991"
},
{
"DOI": "10.3389/fimmu.2019.01168",
"doi-asserted-by": "crossref",
"key": "1718_CR75",
"unstructured": "Hampton HR. And T.J.F.i.i. Chtanova. Lymphatic Migration Immune Cells. 2019;10:1168."
},
{
"key": "1718_CR76",
"unstructured": "Henry BM et al. Lymphopenia and neutrophilia at admission predicts severity and mortality in patients with COVID-19: a meta-analysis. 2020:91(3):e2020008."
},
{
"DOI": "10.1111/j.1365-2567.2004.01849.x",
"doi-asserted-by": "crossref",
"key": "1718_CR77",
"unstructured": "Ellis TN, Beaman BLJI. Interferon-γ Activation Polymorphonuclear Neutrophil Function. 2004;112(1):2–12."
},
{
"DOI": "10.1128/IAI.01508-13",
"doi-asserted-by": "crossref",
"key": "1718_CR78",
"unstructured": "Spees AM et al. Neutrophils are a source of gamma interferon during acute Salmonella enterica serovar Typhimurium colitis. 2014. 82(4): p. 1692–7."
},
{
"DOI": "10.1073/pnas.1307868110",
"author": "CR Sturge",
"doi-asserted-by": "publisher",
"first-page": "10711",
"issue": "26",
"journal-title": "Proc Natl Acad Sci U S A.",
"key": "1718_CR79",
"unstructured": "Sturge CR, et al. TLR-independent neutrophil-derived IFN-γ is important for host resistance to intracellular pathogens. Proc Natl Acad Sci U S A. 2013;110(26):10711–6.",
"volume": "110",
"year": "2013"
},
{
"DOI": "10.1371/journal.pone.0072249",
"author": "S de Kleijn",
"doi-asserted-by": "publisher",
"first-page": "e72249",
"issue": "8",
"journal-title": "PLoS ONE",
"key": "1718_CR80",
"unstructured": "de Kleijn S, et al. IFN-γ-stimulated neutrophils suppress lymphocyte proliferation through expression of PD-L1. PLoS ONE. 2013;8(8):e72249.",
"volume": "8",
"year": "2013"
},
{
"DOI": "10.1016/j.immuni.2021.12.014",
"author": "KM Cautivo",
"doi-asserted-by": "publisher",
"first-page": "254",
"issue": "2",
"journal-title": "Immunity",
"key": "1718_CR81",
"unstructured": "Cautivo KM, et al. Interferon gamma constrains type 2 lymphocyte niche boundaries during mixed inflammation. Immunity. 2022;55(2):254–71. e7.",
"volume": "55",
"year": "2022"
},
{
"DOI": "10.1371/journal.ppat.1009850",
"author": "HF Peñaloza",
"doi-asserted-by": "publisher",
"first-page": "e1009850",
"issue": "9",
"journal-title": "PLoS Pathog",
"key": "1718_CR82",
"unstructured": "Peñaloza HF, Lee JS, Ray P. Neutrophils and lymphopenia, an unknown axis in severe COVID-19 disease. PLoS Pathog. 2021;17(9):e1009850.",
"volume": "17",
"year": "2021"
},
{
"DOI": "10.1016/j.ajem.2020.12.003",
"author": "EV Moradi",
"doi-asserted-by": "publisher",
"first-page": "11",
"journal-title": "Am J Emerg Med",
"key": "1718_CR83",
"unstructured": "Moradi EV, et al. Increased age, neutrophil-to-lymphocyte ratio (NLR) and white blood cells count are associated with higher COVID-19 mortality. Am J Emerg Med. 2021;40:11–4.",
"volume": "40",
"year": "2021"
},
{
"DOI": "10.1093/pcmedi/pbac014",
"doi-asserted-by": "crossref",
"key": "1718_CR84",
"unstructured": "Yang M et al. Cytokine storm promoting T cell exhaustion in severe COVID-19 revealed by single cell sequencing data analysis. Precis Clin Med. 2022;5(2):pbac014."
},
{
"DOI": "10.1126/science.1242454",
"doi-asserted-by": "crossref",
"key": "1718_CR85",
"unstructured": "Pearce EL, et al. Fueling Immunity: Insights into Metabolism Lymphocyte Function. 2013;342(6155):1242454."
},
{
"DOI": "10.1038/nrrheum.2017.37",
"doi-asserted-by": "crossref",
"key": "1718_CR86",
"unstructured": "Gaber T, Strehl C, Buttgereit FJNRR. Metabolic Regul Inflamm. 2017;13(5):267–79."
},
{
"DOI": "10.4049/jimmunol.0901698",
"doi-asserted-by": "crossref",
"key": "1718_CR87",
"unstructured": "Rodríguez-Prados J-C et al. Substrate fate in activated macrophages: a comparison between innate, classic, and alternative activation. 2010. 185(1): p. 605–14."
},
{
"key": "1718_CR88",
"unstructured": "Freemerman AJ et al. Metabolic reprogramming of macrophages: glucose transporter 1 (GLUT1)-mediated glucose metabolism drives a proinflammatory phenotype. 2014. 289(11): p. 7884–96."
},
{
"DOI": "10.1182/blood-2009-10-249540",
"doi-asserted-by": "crossref",
"key": "1718_CR89",
"unstructured": "Krawczyk CM et al. Toll-like receptor–induced changes in glycolytic metabolism regulate dendritic cell activation. 2010. 115(23): p. 4742–9."
},
{
"DOI": "10.3389/fimmu.2020.00202",
"author": "Z Wang",
"doi-asserted-by": "publisher",
"first-page": "202",
"journal-title": "Front Immunol.",
"key": "1718_CR90",
"unstructured": "Wang Z, et al. Glycolysis and oxidative phosphorylation play critical roles in natural killer cell receptor-mediated natural killer cell functions. Front Immunol. 2020;11:202.",
"volume": "11",
"year": "2020"
},
{
"author": "O Rodríguez-Espinosa",
"first-page": "213",
"issue": "2",
"journal-title": "Metabolic Requirements Neutrophil Extracell Traps Formation",
"key": "1718_CR91",
"unstructured": "Rodríguez-Espinosa O, et al. Metabolic Requirements Neutrophil Extracell Traps Formation. 2015;145(2):213–24.",
"volume": "145",
"year": "2015"
},
{
"DOI": "10.4049/jimmunol.1401558",
"author": "RP Donnelly",
"doi-asserted-by": "publisher",
"first-page": "4477",
"issue": "9",
"journal-title": "J Immunol.",
"key": "1718_CR92",
"unstructured": "Donnelly RP, et al. mTORC1-dependent metabolic reprogramming is a prerequisite for NK cell effector function. J Immunol. 2014;193(9):4477–84.",
"volume": "193",
"year": "2014"
},
{
"DOI": "10.1182/blood-2005-12-4788",
"doi-asserted-by": "crossref",
"key": "1718_CR93",
"unstructured": "Doughty CA et al. Antigen receptor–mediated changes in glucose metabolism in B lymphocytes: role of phosphatidylinositol 3-kinase signaling in the glycolytic control of growth 2006. 107(11): pp. 4458–4465."
},
{
"DOI": "10.1038/ni.2687",
"doi-asserted-by": "crossref",
"key": "1718_CR94",
"unstructured": "Gubser PM et al. Rapid effector function of memory CD8 + T cells requires an immediate-early glycolytic switch 2013. 14(10): pp. 1064–1072."
},
{
"DOI": "10.1016/j.cmet.2014.05.004",
"doi-asserted-by": "crossref",
"key": "1718_CR95",
"unstructured": "Macintyre AN et al. The glucose transporter Glut1 is selectively essential for CD4 T cell activation and effector function. 2014. 20(1): p. 61–72."
},
{
"DOI": "10.1172/JCI83005",
"doi-asserted-by": "crossref",
"key": "1718_CR96",
"unstructured": "Assmann N. D.K.J.T.J.o.c.i. Finlay. Metabolic Regul Immune Responses: Therapeutic Opportunities. 2016;126(6):2031–9."
},
{
"DOI": "10.1016/j.immuni.2014.04.007",
"doi-asserted-by": "crossref",
"key": "1718_CR97",
"unstructured": "Nakaya M et al. Inflammatory T cell responses rely on amino acid transporter ASCT2 facilitation of glutamine uptake and mTORC1 kinase activation. 2014. 40(5): p. 692–705."
},
{
"DOI": "10.1084/jem.174.4.915",
"doi-asserted-by": "crossref",
"key": "1718_CR98",
"unstructured": "de Malefyt W. R., Interleukin 10 (IL-10) and viral IL-10 strongly reduce antigen-specific human T cell proliferation by diminishing the antigen-presenting capacity of monocytes via downregulation of class II major histocompatibility complex expression. 1991. 174(4): p. 915–24."
},
{
"DOI": "10.1002/eji.201242754",
"doi-asserted-by": "crossref",
"key": "1718_CR99",
"unstructured": "Gaber T et al. Pathophysiological hypoxia affects the redox state and IL-2 signalling of human CD4 + T cells and concomitantly impairs survival and proliferation. 2013. 43(6): p. 1588–97."
},
{
"DOI": "10.1038/s41392-021-00726-w",
"author": "M Tian",
"doi-asserted-by": "publisher",
"first-page": "308",
"issue": "1",
"journal-title": "Signal Transduct Tar Ther.",
"key": "1718_CR100",
"unstructured": "Tian M, et al. HIF-1α promotes SARS-CoV-2 infection and aggravates inflammatory responses to COVID-19. Signal Transduct Tar Ther. 2021;6(1):308.",
"volume": "6",
"year": "2021"
},
{
"DOI": "10.1016/j.exger.2021.111507",
"doi-asserted-by": "publisher",
"key": "1718_CR101",
"unstructured": "Farshbafnadi M, Kamali Zonouzi S, Sabahi M, Dolatshahi M, Aarabi MH. Aging & COVID-19 susceptibility, disease severity, and clinical outcomes: The role of entangled risk factors. Exp Gerontol. 2021;154:111507. https://doi.org/10.1016/j.exger.2021.111507."
},
{
"DOI": "10.1186/s12915-019-0678-9",
"doi-asserted-by": "crossref",
"key": "1718_CR102",
"unstructured": "Thaker SK, Ch’ng J. R.J.B.b. Christofk. Viral Hijacking Cell Metabolism. 2019;17:1–15."
},
{
"DOI": "10.1002/rmv.2268",
"doi-asserted-by": "crossref",
"key": "1718_CR103",
"unstructured": "Ganesh GV. J.R.i.m.v. Mohanram. Metabolic Reprogramming Immune Regul Viral Dis. 2022;32(2):e2268."
},
{
"DOI": "10.1038/s42255-020-0172-2",
"doi-asserted-by": "crossref",
"key": "1718_CR104",
"unstructured": "DeBerardinis RJ. And N.S.J.N.m. Chandel. We need talk about Warburg Effect. 2020;2(2):127–9."
},
{
"DOI": "10.1016/j.idcr.2020.e00829",
"doi-asserted-by": "crossref",
"key": "1718_CR105",
"unstructured": "Chhetri S et al. A fatal case of COVID-19 due to metabolic acidosis following dysregulate inflammatory response (cytokine storm). IDCases. 2020;21:e00829."
},
{
"DOI": "10.38025/2078-1962-2020-97-3-25-30",
"doi-asserted-by": "crossref",
"key": "1718_CR106",
"unstructured": "Lodyagin A et al. Acidosis and toxic hemolysis-goals of pathogenetic treatment of polyorgan pathology in COVID-19. Bull Rehabil Med. 2020;97(3):25–30."
},
{
"DOI": "10.1038/s41467-021-21903-z",
"author": "Y Zhang",
"doi-asserted-by": "publisher",
"first-page": "1676",
"issue": "1",
"journal-title": "Nat Commun.",
"key": "1718_CR107",
"unstructured": "Zhang Y, et al. SARS-CoV-2 hijacks folate and one-carbon metabolism for viral replication. Nat Commun. 2021;12(1):1676.",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.2139/ssrn.3606770",
"doi-asserted-by": "crossref",
"key": "1718_CR108",
"unstructured": "Codo AC et al. Elevated glucose levels favor SARS-CoV-2 infection and monocyte response through a HIF-1α/glycolysis-dependent axis 2020. 32(3): pp. 437–446. e5."
},
{
"DOI": "10.3389/fimmu.2021.728896",
"doi-asserted-by": "crossref",
"key": "1718_CR109",
"unstructured": "Barhoumi T et al. SARS-CoV-2 coronavirus spike protein-induced apoptosis, inflammatory, and oxidative stress responses in THP-1-like-macrophages: potential role of angiotensin-converting enzyme inhibitor (perindopril). 2021. 12: p. 728896."
},
{
"DOI": "10.1016/j.jaci.2022.06.020",
"doi-asserted-by": "crossref",
"key": "1718_CR110",
"unstructured": "Kundura L et al. Angiotensin II induces reactive oxygen species, DNA damage, and T-cell apoptosis in severe COVID-19. 2022. 150(3): p. 594–603. e2."
},
{
"DOI": "10.3389/fimmu.2021.799558",
"doi-asserted-by": "crossref",
"key": "1718_CR111",
"unstructured": "Lage SL et al. Persistent oxidative stress and inflammasome activation in CD14highCD16 – monocytes from COVID-19 patients. 2022. 12: p. 799558."
},
{
"DOI": "10.1038/s41586-022-04702-4",
"doi-asserted-by": "crossref",
"key": "1718_CR112",
"unstructured": "Junqueira C et al. FcγR-mediated SARS-CoV-2 infection of monocytes activates inflammation. 2022. 606(7914): p. 576–84."
},
{
"DOI": "10.1016/j.lfs.2022.120411",
"doi-asserted-by": "crossref",
"key": "1718_CR113",
"unstructured": "Bhatt AN et al. Glycolytic inhibitor 2-deoxy-d-glucose attenuates SARS-CoV-2 multiplication in host cells and weakens the infective potential of progeny virions 2022. 295: p. 120411."
},
{
"DOI": "10.1182/blood-2006-07-035972",
"doi-asserted-by": "crossref",
"key": "1718_CR114",
"unstructured": "Fischer K et al. Inhibitory effect of tumor cell–derived lactic acid on human T cells. Blood. 2007;109(9):3812–9."
},
{
"DOI": "10.1042/bj3430281",
"author": "AP Halestrap",
"doi-asserted-by": "publisher",
"first-page": "281",
"issue": "2",
"journal-title": "Biochem J",
"key": "1718_CR115",
"unstructured": "Halestrap AP, PRICE NT. The proton-linked monocarboxylate transporter (MCT) family: structure, function and regulation. Biochem J. 1999;343(2):281–99.",
"volume": "343",
"year": "1999"
},
{
"DOI": "10.1093/emboj/19.15.3896",
"doi-asserted-by": "crossref",
"key": "1718_CR116",
"unstructured": "Kirk á et al. CD147 is tightly associated with lactate transporters MCT1 and MCT4 and facilitates their cell surface expression. EMBO J. 2000;19(15):3896–904."
},
{
"author": "M Nakai",
"first-page": "527",
"issue": "5",
"journal-title": "Am Assoc Anatomy.",
"key": "1718_CR117",
"unstructured": "Nakai M, et al. Tissue distribution of basigin and monocarboxylate transporter 1 in the adult male mouse: a study using the wild-type and basigin gene knockout mice. Am Assoc Anatomy. 2006;288(5):527–35.",
"volume": "288",
"year": "2006"
},
{
"DOI": "10.1093/emboj/19.15.3896",
"author": "á Kirk",
"doi-asserted-by": "publisher",
"first-page": "3896",
"issue": "15",
"journal-title": "EMBO J",
"key": "1718_CR118",
"unstructured": "Kirk á, et al. CD147 is tightly associated with lactate transporters MCT1 and MCT4 and facilitates their cell surface expression. EMBO J. 2000;19(15):3896–904.",
"volume": "19",
"year": "2000"
},
{
"DOI": "10.1167/iovs.02-0552",
"author": "NJ Philp",
"doi-asserted-by": "publisher",
"first-page": "1305",
"issue": "3",
"journal-title": "Invest ophthalmol vis sci",
"key": "1718_CR119",
"unstructured": "Philp NJ, et al. Loss of MCT1, MCT3, and MCT4 expression in the retinal pigment epithelium and neural retina of the 5A11/basigin-null mouse. Invest ophthalmol vis sci. 2003;44(3):1305–11.",
"volume": "44",
"year": "2003"
},
{
"DOI": "10.1073/pnas.1106123108",
"author": "R Le Floch",
"doi-asserted-by": "publisher",
"first-page": "16663",
"issue": "40",
"journal-title": "Proceed Nat Acad Sci",
"key": "1718_CR120",
"unstructured": "Le Floch R, et al. CD147 subunit of lactate/H + symporters MCT1 and hypoxia-inducible MCT4 is critical for energetics and growth of glycolytic tumors. Proceed Nat Acad Sci. 2011;108(40):16663–8.",
"volume": "108",
"year": "2011"
},
{
"DOI": "10.1152/ajpcell.1993.264.4.C761",
"author": "RC Poole",
"doi-asserted-by": "publisher",
"first-page": "C761",
"issue": "4",
"journal-title": "Am J Physiology-Cell Physiol",
"key": "1718_CR121",
"unstructured": "Poole RC, Halestrap AP. Transport of lactate and other monocarboxylates across mammalian plasma membranes. Am J Physiology-Cell Physiol. 1993;264(4):C761–82.",
"volume": "264",
"year": "1993"
},
{
"DOI": "10.1016/j.cell.2011.02.013",
"author": "D Hanahan",
"doi-asserted-by": "publisher",
"first-page": "646",
"issue": "5",
"journal-title": "Cell",
"key": "1718_CR122",
"unstructured": "Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–74.",
"volume": "144",
"year": "2011"
},
{
"DOI": "10.4049/jimmunol.174.8.4670",
"doi-asserted-by": "crossref",
"key": "1718_CR123",
"unstructured": "Cham CM, T.F.J.T.J.o I, Gajewski. Glucose availability regulates IFN-γ production and p70S6 kinase activation in CD8 + effector T cells. J Immunol. 2005;174(8):4670–7."
},
{
"DOI": "10.1016/j.celrep.2021.108863",
"doi-asserted-by": "crossref",
"key": "1718_CR124",
"unstructured": "Thompson EA et al. Metabolic programs define dysfunctional immune responses in severe COVID-19 patients. Cell Rep. 2021;34(11)."
},
{
"DOI": "10.4049/jimmunol.172.8.4661",
"author": "KA Frauwirth",
"doi-asserted-by": "publisher",
"first-page": "4661",
"issue": "8",
"journal-title": "J Immunol",
"key": "1718_CR125",
"unstructured": "Frauwirth KA, Thompson CB. Regulation of T lymphocyte metabolism. J Immunol. 2004;172(8):4661–5.",
"volume": "172",
"year": "2004"
},
{
"DOI": "10.1369/jhc.4A6306.2004",
"author": "N Merezhinskaya",
"doi-asserted-by": "publisher",
"first-page": "1483",
"issue": "11",
"journal-title": "J Histochem Cytochemistry",
"key": "1718_CR126",
"unstructured": "Merezhinskaya N, et al. Presence and localization of three lactic acid transporters (MCT1,– 2, and – 4) in separated human granulocytes, lymphocytes, and monocytes. J Histochem Cytochemistry. 2004;52(11):1483–93.",
"volume": "52",
"year": "2004"
},
{
"DOI": "10.1099/vir.0.80813-0",
"author": "PT Law",
"doi-asserted-by": "publisher",
"first-page": "1921",
"issue": "7",
"journal-title": "J Gen Virol.",
"key": "1718_CR127",
"unstructured": "Law PT, et al. The 3a protein of severe acute respiratory syndrome-associated coronavirus induces apoptosis in Vero E6 cells. J Gen Virol. 2005;86(7):1921–30.",
"volume": "86",
"year": "2005"
},
{
"DOI": "10.32604/biocell.2005.29.149",
"doi-asserted-by": "crossref",
"key": "1718_CR128",
"unstructured": "Mohamad N, et al. Mitochondrial Apoptotic Pathways. 2005;29(2):149–61."
},
{
"DOI": "10.1016/0092-8674(89)90174-8",
"doi-asserted-by": "crossref",
"key": "1718_CR129",
"unstructured": "McDonnell TJ et al. Bcl-2-immunoglobulin transgenic mice demonstrate extended B cell survival and follicular lymphoproliferation. 1989. 57(1): p. 79–88."
},
{
"DOI": "10.1042/BJ20050698",
"doi-asserted-by": "crossref",
"key": "1718_CR130",
"unstructured": "Yang Y et al. Bcl-xL inhibits T-cell apoptosis induced by expression of SARS coronavirus E protein in the absence of growth factors. 2005. 392(1): p. 135–43."
},
{
"DOI": "10.4049/jimmunol.1302992",
"doi-asserted-by": "crossref",
"key": "1718_CR131",
"unstructured": "Boonnak K et al. Lymphopenia associated with highly virulent H5N1 virus infection due to plasmacytoid dendritic cell–mediated apoptosis of T cells. 2014. 192(12): p. 5906–12."
},
{
"DOI": "10.1111/j.8755-8920.2000.430402.x",
"doi-asserted-by": "crossref",
"key": "1718_CR132",
"unstructured": "DARMOCHWAL-KOLARZ D et al. Fas antigen expression on the decidual lymphocytes of pre‐eclamptic patients. 2000. 43(4): p. 197–201."
},
{
"DOI": "10.1016/S0092-8674(00)81874-7",
"doi-asserted-by": "crossref",
"key": "1718_CR133",
"unstructured": "Nagata SJc. Apoptosis Death Factor. 1997;88(3):355–65."
},
{
"DOI": "10.1016/S0002-9440(10)63981-8",
"doi-asserted-by": "crossref",
"key": "1718_CR134",
"unstructured": "De Panfilis G et al. Identification of Fas-L-expressing apoptotic T lymphocytes in normal human peripheral blood: in vivo suicide. 2001. 158(2): p. 387–91."
},
{
"DOI": "10.1093/infdis/jiv380",
"doi-asserted-by": "crossref",
"key": "1718_CR135",
"unstructured": "Chu H et al. Middle East respiratory syndrome coronavirus efficiently infects human primary T lymphocytes and activates the extrinsic and intrinsic apoptosis pathways 2016. 213(6): pp. 904–914."
},
{
"DOI": "10.1080/22221751.2020.1747363",
"doi-asserted-by": "crossref",
"key": "1718_CR136",
"unstructured": "Xiong Y et al. Transcriptomic characteristics of bronchoalveolar lavage fluid and peripheral blood mononuclear cells in COVID-19 patients. 2020. 9(1): p. 761–70."
},
{
"DOI": "10.1016/j.immuni.2020.07.009",
"doi-asserted-by": "crossref",
"key": "1718_CR137",
"unstructured": "Zhu L et al. Single-cell sequencing of peripheral mononuclear cells reveals distinct immune response landscapes of COVID-19 and influenza patients. 2020. 53(3): p. 685–96. e3."
},
{
"key": "1718_CR138",
"unstructured": "Pontelli MC et al. SARS-CoV-2 productively infects primary human immune system cells in vitro and in COVID-19 patients. J Mol Cell Biol. 2022;14(4):mjac021."
},
{
"author": "LN Nguyen",
"first-page": "167249",
"issue": "4",
"journal-title": "PANoptosis Viral Infection: Missing Puzzle Piece cell Death Field",
"key": "1718_CR139",
"unstructured": "Nguyen LN. And T.-D.J.J.o.m.b. Kanneganti. PANoptosis Viral Infection: Missing Puzzle Piece cell Death Field. 2022;434(4):167249.",
"volume": "434",
"year": "2022"
},
{
"DOI": "10.1038/s41421-020-0168-9",
"author": "W Wen",
"doi-asserted-by": "publisher",
"first-page": "31",
"issue": "1",
"journal-title": "Cell Discov.",
"key": "1718_CR140",
"unstructured": "Wen W, et al. Immune cell profiling of COVID-19 patients in the recovery stage by single-cell sequencing. Cell Discov. 2020;6(1):31.",
"volume": "6",
"year": "2020"
},
{
"DOI": "10.1002/wsbm.86",
"doi-asserted-by": "crossref",
"key": "1718_CR141",
"unstructured": "Seita J, I.L.J.W.I.R.S.B., Weissman, Medicine. Hematopoietic stem cell: self-renewal versus Differ. 2010;2(6):640–53."
},
{
"DOI": "10.1016/j.intimp.2021.107567",
"doi-asserted-by": "crossref",
"key": "1718_CR142",
"unstructured": "Zheng B et al. Landscape of SARS-CoV-2 spike protein-interacting cells in human tissues. 2021. 95: p. 107567."
},
{
"DOI": "10.1021/acscentsci.0c01537",
"doi-asserted-by": "crossref",
"key": "1718_CR143",
"unstructured": "Amraei R et al. CD209L/L-SIGN and CD209/DC-SIGN act as receptors for SARS-CoV-2. 2021. 7(7): p. 1156–65."
},
{
"DOI": "10.1007/s12015-020-10056-z",
"doi-asserted-by": "crossref",
"key": "1718_CR144",
"unstructured": "Ropa J et al. Human hematopoietic stem, progenitor, and immune cells respond ex vivo to SARS-CoV-2 spike protein. 2021. 17(1): p. 253–65."
},
{
"DOI": "10.1007/s12015-020-10010-z",
"doi-asserted-by": "crossref",
"key": "1718_CR145",
"unstructured": "Ratajczak MZ et al. SARS-CoV-2 entry receptor ACE2 is expressed on very small CD45 – precursors of hematopoietic and endothelial cells and in response to virus spike protein activates the Nlrp3 inflammasome. 2021. 17(1): p. 266–77."
},
{
"DOI": "10.1182/blood-2020-137083",
"doi-asserted-by": "crossref",
"key": "1718_CR146",
"unstructured": "Kucia M et al. The ACE2 receptor for COVID-19 entry is expressed on the surface of hematopoietic stem/progenitor cells and endothelial progenitors as well as their precursor cells and becomes activated in nlrp3 inflammasome-dependent manner by virus spike protein-a potential pathway leading to a cytokine storm 2020. 136: p. 8."
},
{
"DOI": "10.1016/j.cytogfr.2021.06.002",
"doi-asserted-by": "crossref",
"key": "1718_CR147",
"unstructured": "Zhao N et al. The NLRP3 inflammasome and COVID-19: Activation, pathogenesis and therapeutic strategies 2021. 61: pp. 2–15."
},
{
"DOI": "10.1038/s41421-021-00296-9",
"author": "X Wang",
"doi-asserted-by": "publisher",
"first-page": "1",
"issue": "1",
"journal-title": "Cell Discov.",
"key": "1718_CR148",
"unstructured": "Wang X, et al. Dysregulated hematopoiesis in bone marrow marks severe COVID-19. Cell Discov. 2021;7(1):1–18.",
"volume": "7",
"year": "2021"
},
{
"author": "CM Schürch",
"first-page": "460",
"issue": "4",
"journal-title": "Cytotoxic CD8 + T Cells Stimulate Hematopoietic Progenitors Promoting Cytokine Release bone Marrow Mesenchymal Stromal Cells",
"key": "1718_CR149",
"unstructured": "Schürch CM, Riether C. F.J.C.s.c. Ochsenbein. Cytotoxic CD8 + T Cells Stimulate Hematopoietic Progenitors Promoting Cytokine Release bone Marrow Mesenchymal Stromal Cells. 2014;14(4):460–72.",
"volume": "14",
"year": "2014"
},
{
"author": "AM de Bruin",
"first-page": "2479",
"issue": "16",
"journal-title": "Impact interferon-γ Hematopoiesis",
"key": "1718_CR150",
"unstructured": "de Bruin AM, Voermans C, Nolte MAJB. The Journal of the American Society of Hematology. Impact interferon-γ Hematopoiesis. 2014;124(16):2479–86.",
"volume": "124",
"year": "2014"
},
{
"DOI": "10.1016/j.stemcr.2021.04.001",
"author": "S Shahbaz",
"doi-asserted-by": "publisher",
"first-page": "1165",
"issue": "5",
"journal-title": "Stem Cell Reports.",
"key": "1718_CR151",
"unstructured": "Shahbaz S, et al. Erythroid precursors and progenitors suppress adaptive immunity and get invaded by SARS-CoV-2. Stem Cell Reports. 2021;16(5):1165–81.",
"volume": "16",
"year": "2021"
},
{
"DOI": "10.3389/fimmu.2021.661052",
"doi-asserted-by": "crossref",
"key": "1718_CR152",
"unstructured": "Xiang Q et al. SARS-CoV-2 induces lymphocytopenia by promoting inflammation and decimates secondary lymphoid organs 2021. 12: p. 661052."
},
{
"DOI": "10.1038/s41586-022-04802-1",
"doi-asserted-by": "crossref",
"key": "1718_CR153",
"unstructured": "Sefik E et al. Inflammasome activation in infected macrophages drives COVID-19 pathology. 2022. 606(7914): p. 585–93."
},
{
"DOI": "10.1038/s41392-020-0163-5",
"doi-asserted-by": "crossref",
"key": "1718_CR154",
"unstructured": "Li D et al. Immune dysfunction leads to mortality and organ injury in patients with COVID-19 in China: insights from ERS-COVID-19 study. 2020. 5(1): p. 62."
},
{
"DOI": "10.1016/j.clinpr.2020.100042",
"doi-asserted-by": "crossref",
"key": "1718_CR155",
"unstructured": "Shaukat I et al. Atraumatic splenic rupture due to covid-19 infection 2021. 10: p. 100042."
},
{
"DOI": "10.1038/s41577-019-0165-0",
"doi-asserted-by": "crossref",
"key": "1718_CR156",
"unstructured": "Swanson KV, Deng M, P.-Y.J.N.R J. The NLRP3 inflammasome: molecular activation and regulation to therapeutics 2019. 19(8): pp. 477–489."
},
{
"DOI": "10.1038/35036052",
"doi-asserted-by": "crossref",
"key": "1718_CR157",
"unstructured": "Simons K. D.J.N.r.M.c.b. Toomre. Lipid Rafts Signal Transduct. 2000;1(1):31–9."
},
{
"DOI": "10.1080/09687860500453673",
"doi-asserted-by": "crossref",
"key": "1718_CR158",
"unstructured": "Kabouridis PS. J.M.m.b., lipid rafts in T cell receptor signalling. Mol Membr Biol. 2006;23(1):49–57."
},
{
"author": "K Simons",
"first-page": "597",
"issue": "5",
"journal-title": "Cholesterol Lipid Rafts Disease",
"key": "1718_CR159",
"unstructured": "Simons K. R.J.T.J.o.c.i. Ehehalt. Cholesterol Lipid Rafts Disease. 2002;110(5):597–603.",
"volume": "110",
"year": "2002"
},
{
"DOI": "10.1083/jcb.200407078",
"author": "M Kirkham",
"doi-asserted-by": "publisher",
"first-page": "465",
"issue": "3",
"journal-title": "J Cell Biol.",
"key": "1718_CR160",
"unstructured": "Kirkham M, et al. Ultrastructural identification of uncoated caveolin-independent early endocytic vehicles. J Cell Biol. 2005;168(3):465–76.",
"volume": "168",
"year": "2005"
},
{
"DOI": "10.1083/jcb.141.4.905",
"doi-asserted-by": "crossref",
"key": "1718_CR161",
"unstructured": "Orlandi PA. and P.H.J.T.J.o.c.b. Fishman, Filipin-dependent inhibition of cholera toxin: evidence for toxin internalization and activation through caveolae-like domains. 1998. 141(4): p. 905–15."
},
{
"DOI": "10.1074/jbc.R000005200",
"author": "DA Brown",
"doi-asserted-by": "publisher",
"first-page": "7221",
"issue": "23",
"journal-title": "J Biol Chem.",
"key": "1718_CR162",
"unstructured": "Brown DA, London E. Structure and function of sphingolipid-and cholesterol-rich membrane rafts. J Biol Chem. 2000;275(23):7221–4.",
"volume": "275",
"year": "2000"
},
{
"author": "BJ Nichols",
"first-page": "4707",
"issue": "23",
"journal-title": "Caveosomes Endocytosis Lipid Rafts",
"key": "1718_CR163",
"unstructured": "Nichols BJ. J.o.c.s. Caveosomes Endocytosis Lipid Rafts. 2003;116(23):4707–14.",
"volume": "116",
"year": "2003"
},
{
"DOI": "10.1194/jlr.R200021-JLR200",
"author": "LJ Pike",
"doi-asserted-by": "publisher",
"first-page": "655",
"issue": "4",
"journal-title": "J Lipid Res.",
"key": "1718_CR164",
"unstructured": "Pike LJ. lipid rafts: bringing order to chaos. J Lipid Res. 2003;44(4):655–67.",
"volume": "44",
"year": "2003"
},
{
"author": "AB Ouweneel",
"first-page": "676",
"issue": "5",
"journal-title": "Thematic Rev Series: Biology Lipid Rafts",
"key": "1718_CR165",
"unstructured": "Ouweneel AB, Thomas MJ. J.J.o.l.r. Sorci-Thomas, the ins and outs of lipid rafts: functions in intracellular cholesterol homeostasis, microparticles, and cell membranes. Thematic Rev Series: Biology Lipid Rafts. 2020;61(5):676–86.",
"volume": "61",
"year": "2020"
},
{
"author": "EC Jury",
"key": "1718_CR166",
"unstructured": "Jury EC, Flores-Borja F, Kabouridis PS. Lipid rafts in T cell signalling and disease. In seminars in cell & developmental biology. Elsevier; 2007.",
"volume-title": "Lipid rafts in T cell signalling and disease. in Seminars in cell & developmental biology",
"year": "2007"
},
{
"DOI": "10.3389/fcell.2020.618296",
"doi-asserted-by": "crossref",
"key": "1718_CR167",
"unstructured": "Sorice M, et al. Targeting lipid rafts as a strategy against coronavirus. Front Cell Dev Biol. 2021;8:618296."
},
{
"author": "MI Bukrinsky",
"first-page": "601",
"issue": "5",
"journal-title": "Thematic Rev Series: Biology Lipid Rafts",
"key": "1718_CR168",
"unstructured": "Bukrinsky MI, Mukhamedova N. J.J.o.l.r. Sviridov, lipid rafts and pathogens: the art of deception and exploitation. Thematic Rev Series: Biology Lipid Rafts. 2020;61(5):601–10.",
"volume": "61",
"year": "2020"
},
{
"DOI": "10.3389/fcimb.2018.00388",
"doi-asserted-by": "crossref",
"key": "1718_CR169",
"unstructured": "Osuna-Ramos JF, et al. The Role of Host Cholesterol During Flavivirus Infection.Front Cell Infect Microbiol. 2018;8:388."
},
{
"DOI": "10.3389/fmicb.2020.597794",
"doi-asserted-by": "crossref",
"key": "1718_CR170",
"unstructured": "Wang Y, et al. Cholesterol-rich lipid rafts in the cellular membrane play an essential role. Avian Reovirus Replication. 2020;11:597794."
},
{
"DOI": "10.3389/fmicb.2020.01821",
"author": "K Fecchi",
"doi-asserted-by": "publisher",
"first-page": "1821",
"journal-title": "Front Microbiol.",
"key": "1718_CR171",
"unstructured": "Fecchi K, et al. Coronavirus interplay with lipid rafts and autophagy unveils promising therapeutic targets. Front Microbiol. 2020;11:1821.",
"volume": "11",
"year": "2020"
},
{
"DOI": "10.1016/j.chemphyslip.2018.08.003",
"doi-asserted-by": "crossref",
"key": "1718_CR172",
"unstructured": "Bieberich EJC. and p.o. lipids, Sphingolipids and lipid rafts: Novel concepts and methods of analysis 2018. 216: pp. 114–131."
},
{
"DOI": "10.3389/fimmu.2020.574508",
"doi-asserted-by": "crossref",
"key": "1718_CR173",
"unstructured": "Sviridov D et al. Targeting lipid rafts—a potential therapy for COVID-19 2020. 11: p. 574508."
},
{
"DOI": "10.1038/cr.2008.15",
"doi-asserted-by": "crossref",
"key": "1718_CR174",
"unstructured": "Wang H et al. SARS coronavirus entry into host cells through a novel clathrin-and caveolae-independent endocytic pathway. 2008. 18(2): p. 290–301."
},
{
"DOI": "10.1128/JVI.78.11.5642-5650.2004",
"doi-asserted-by": "crossref",
"key": "1718_CR175",
"unstructured": "Yang Z-Y et al. pH-dependent entry of severe acute respiratory syndrome coronavirus is mediated by the spike glycoprotein and enhanced by dendritic cell transfer through DC-SIGN. 2004. 78(11): p. 5642–50."
},
{
"DOI": "10.1074/jbc.M508381200",
"doi-asserted-by": "crossref",
"key": "1718_CR176",
"unstructured": "Huang I-C et al. SARS coronavirus, but not human coronavirus NL63, utilizes cathepsin L to infect ACE2-expressing cells. 2006. 281(6): p. 3198–203."
},
{
"DOI": "10.3390/ijms22105336",
"doi-asserted-by": "crossref",
"key": "1718_CR177",
"unstructured": "Hudák A et al. Contribution of syndecans to the cellular entry of SARS-CoV-2. 2021. 22(10): p. 5336."
},
{
"DOI": "10.1016/j.cell.2020.09.033",
"doi-asserted-by": "crossref",
"key": "1718_CR178",
"unstructured": "Clausen TM et al. SARS-CoV-2 infection depends on cellular heparan sulfate and ACE2. 2020. 183(4): p. 1043–57. e15."
},
{
"DOI": "10.7150/ijbs.6818",
"doi-asserted-by": "crossref",
"key": "1718_CR179",
"unstructured": "Yao H et al. Important functional roles of basigin in thymocyte development and T cell activation. 2014. 10(1): p. 43."
},
{
"DOI": "10.1083/jcb.201208172",
"doi-asserted-by": "crossref",
"key": "1718_CR180",
"unstructured": "Maldonado-Báez L et al. Microtubule-dependent endosomal sorting of clathrin-independent cargo by Hook1 2013. 201(2): pp. 233–247."
},
{
"key": "1718_CR181",
"unstructured": "Wang K et al. CD147-spike protein is a novel route for SARS-CoV-2 infection to host cells. 2020. 5(1): p. 283."
},
{
"DOI": "10.1034/j.1600-0854.2002.30605.x",
"doi-asserted-by": "crossref",
"key": "1718_CR182",
"unstructured": "Murray JT et al. Role of Rab5 in the recruitment of hVps34/p150 to the early endosome. 2002. 3(6): p. 416–27."
},
{
"DOI": "10.1080/22221751.2022.2059403",
"doi-asserted-by": "crossref",
"key": "1718_CR183",
"unstructured": "Zhou Y-Q et al. SARS-CoV-2 pseudovirus enters the host cells through spike protein-CD147 in an Arf6-dependent manner 2022. 11(1): pp. 1135–1144."
},
{
"DOI": "10.1002/jmv.25987",
"author": "A Choudhury",
"doi-asserted-by": "publisher",
"first-page": "2105",
"issue": "10",
"journal-title": "J Med Virol.",
"key": "1718_CR184",
"unstructured": "Choudhury A, Mukherjee S. In silico studies on the comparative characterization of the interactions of SARS-CoV-2 spike glycoprotein with ACE-2 receptor homologs and human TLRs. J Med Virol. 2020;92(10):2105–13.",
"volume": "92",
"year": "2020"
},
{
"DOI": "10.1172/jci.insight.148999",
"doi-asserted-by": "crossref",
"key": "1718_CR185",
"unstructured": "Kondo Y, et al. L-SIGN is a receptor on liver sinusoidal endothelial cells for SARS-CoV-2 virus. JCI Insight. 2021;6(14):e148999."
},
{
"DOI": "10.1002/jmv.26826",
"author": "S Khanmohammadi",
"doi-asserted-by": "publisher",
"first-page": "2735",
"issue": "5",
"journal-title": "J Med Virol.",
"key": "1718_CR186",
"unstructured": "Khanmohammadi S, Rezaei N. Role of toll-like receptors in the pathogenesis of COVID-19. J Med Virol. 2021;93(5):2735–9.",
"volume": "93",
"year": "2021"
},
{
"DOI": "10.1016/j.bbalip.2022.159140",
"doi-asserted-by": "crossref",
"key": "1718_CR187",
"unstructured": "Roncato R et al. Lipid rafts as viral entry routes and immune platforms: a double-edged sword in SARS-CoV-2 infection? 2022. 1867(6): p. 159140."
},
{
"DOI": "10.3389/fimmu.2021.796855",
"doi-asserted-by": "crossref",
"key": "1718_CR188",
"unstructured": "Palacios-Rápalo SN et al. Cholesterol-rich lipid rafts as platforms for SARS-CoV-2 entry 2021. 12: p. 796855."
},
{
"DOI": "10.4049/jimmunol.149.3.847",
"doi-asserted-by": "crossref",
"key": "1718_CR189",
"unstructured": "Kasinrerk W et al. Human leukocyte activation antigen M6, a member of the ig superfamily, is the species homologue of rat OX-47, mouse basigin, and chicken HT7 molecule. 1992. 149(3): p. 847–54."
},
{
"DOI": "10.1016/j.chom.2011.10.015",
"doi-asserted-by": "crossref",
"key": "1718_CR190",
"unstructured": "Dale BM et al. Cell-to-cell transfer of HIV-1 via virological synapses leads to endosomal virion maturation that activates viral membrane fusion. 2011. 10(6): p. 551–62."
},
{
"DOI": "10.1073/pnas.2111400119",
"doi-asserted-by": "crossref",
"key": "1718_CR191",
"unstructured": "Zeng C et al. SARS-CoV-2 spreads through cell-to-cell transmission 2022. 119(1): p. e2111400119."
},
{
"DOI": "10.1126/science.1080115",
"doi-asserted-by": "crossref",
"key": "1718_CR192",
"unstructured": "Igakura T et al. Spread of HTLV-I between lymphocytes by virus-induced polarization of the cytoskeleton 2003. 299(5613): pp. 1713–1716."
},
{
"DOI": "10.1038/s41392-021-00653-w",
"doi-asserted-by": "crossref",
"key": "1718_CR193",
"unstructured": "Zhang Q et al. Molecular mechanism of interaction between SARS-CoV-2 and host cells and interventional therapy. 2021. 6(1): p. 1–19."
},
{
"DOI": "10.3389/fimmu.2020.574508",
"doi-asserted-by": "crossref",
"key": "1718_CR194",
"unstructured": "Sviridov D et al. Targeting lipid rafts—a potential therapy for COVID-19 2020. 11: p. 2361."
},
{
"DOI": "10.1016/j.celrep.2020.108454",
"doi-asserted-by": "crossref",
"key": "1718_CR195",
"unstructured": "Knierman MD, et al. The human leukocyte antigen class II immunopeptidome of the SARS-CoV-2 spike glycoprotein. Cell Rep. 2020;33(9):108454."
},
{
"DOI": "10.1038/s41586-021-03570-8",
"author": "TM Delorey",
"doi-asserted-by": "publisher",
"first-page": "107",
"issue": "7865",
"journal-title": "Nature.",
"key": "1718_CR196",
"unstructured": "Delorey TM, et al. COVID-19 tissue atlases reveal SARS-CoV-2 pathology and cellular targets. Nature. 2021;595(7865):107–13.",
"volume": "595",
"year": "2021"
},
{
"key": "1718_CR197",
"unstructured": "Ren X et al. COVID-19 immune features revealed by a large-scale single-cell transcriptome atlas 2021. 184(7): pp. 1895–1913. e19."
},
{
"DOI": "10.1038/s41467-020-17292-4",
"doi-asserted-by": "crossref",
"key": "1718_CR198",
"unstructured": "De Biasi S et al. Marked T cell activation, senescence, exhaustion and skewing towards TH17 in patients with COVID-19 pneumonia. 2020. 11(1): p. 3434."
},
{
"DOI": "10.1038/s41587-020-0602-4",
"doi-asserted-by": "crossref",
"key": "1718_CR199",
"unstructured": "Chua RL et al. COVID-19 severity correlates with airway epithelium–immune cell interactions identified by single-cell analysis. 2020. 38(8): p. 970–9."
},
{
"DOI": "10.1002/path.1570",
"doi-asserted-by": "crossref",
"key": "1718_CR200",
"unstructured": "Hamming I et al. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. 2004. 203(2): p. 631–7."
},
{
"DOI": "10.1016/j.jmgm.2021.107997",
"doi-asserted-by": "crossref",
"key": "1718_CR201",
"unstructured": "Mobini S et al. Structure-based study of immune receptors as eligible binding targets of coronavirus SARS-CoV-2 spike protein. 2021. 108: p. 107997."
},
{
"DOI": "10.1126/science.abb2762",
"doi-asserted-by": "crossref",
"key": "1718_CR202",
"unstructured": "Yan R et al. Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. 2020. 367(6485): p. 1444–8."
},
{
"DOI": "10.1002/cyto.a.24285",
"author": "X Song",
"doi-asserted-by": "publisher",
"first-page": "136",
"issue": "2",
"journal-title": "Cytometry A.",
"key": "1718_CR203",
"unstructured": "Song X, et al. Little to no expression of angiotensin-converting enzyme-2 on most human peripheral blood immune cells but highly expressed on tissue macrophages. Cytometry A. 2023;103(2):136–45.",
"volume": "103",
"year": "2023"
},
{
"DOI": "10.1172/jci.insight.150542",
"doi-asserted-by": "crossref",
"key": "1718_CR204",
"unstructured": "Salvi V, et al. SARS-CoV-2–associated ssRNAs activate inflammation and immunity via TLR7/8. JCI Insight. 2021;6(18):e150542."
},
{
"DOI": "10.1016/j.micinf.2020.04.009",
"author": "MA Moreno-Eutimio",
"doi-asserted-by": "publisher",
"first-page": "226",
"issue": "4–5",
"journal-title": "Microbes Infect.",
"key": "1718_CR205",
"unstructured": "Moreno-Eutimio MA, et al. Bioinformatic analysis and identification of single-stranded RNA sequences recognized by TLR7/8 in the SARS-CoV-2, SARS-CoV, and MERS-CoV genomes. Microbes Infect. 2020;22(4–5):226–9.",
"volume": "22",
"year": "2020"
},
{
"DOI": "10.1080/07391102.2020.1822208",
"doi-asserted-by": "crossref",
"key": "1718_CR206",
"unstructured": "Helal MA et al. Molecular basis of the potential interaction of SARS-CoV-2 spike protein to CD147 in COVID-19 associated-lymphopenia. 2022. 40(3): p. 1109–19."
},
{
"key": "1718_CR207",
"unstructured": "Wang K et al. CD147-spike protein is a novel route for SARS-CoV-2 infection to host cells. 2020. 5(1): p. 1–10."
},
{
"DOI": "10.1038/s41590-020-0759-5",
"doi-asserted-by": "crossref",
"key": "1718_CR208",
"unstructured": "Acharya N, A.C.J.N.I., Anderson. NRP1 cripples immunological memory 2020. 21(9): pp. 972–973."
},
{
"DOI": "10.1038/s41422-020-00460-y",
"doi-asserted-by": "crossref",
"key": "1718_CR209",
"unstructured": "Wang S et al. AXL is a candidate receptor for SARS-CoV-2 that promotes infection of pulmonary and bronchial epithelial cells. 2021. 31(2): p. 126–40."
},
{
"DOI": "10.1128/JVI.78.21.12090-12095.2004",
"doi-asserted-by": "crossref",
"key": "1718_CR210",
"unstructured": "Marzi A et al. DC-SIGN and DC-SIGNR interact with the glycoprotein of Marburg virus and the S protein of severe acute respiratory syndrome coronavirus. 2004. 78(21): p. 12090–5."
},
{
"DOI": "10.1038/nature12005",
"doi-asserted-by": "crossref",
"key": "1718_CR211",
"unstructured": "Raj VS et al. Dipeptidyl peptidase 4 is a functional receptor for the emerging human coronavirus-EMC. 2013. 495(7440): p. 251–4."
},
{
"DOI": "10.1038/s41422-021-00595-6",
"doi-asserted-by": "crossref",
"key": "1718_CR212",
"unstructured": "Gu Y et al. Receptome profiling identifies KREMEN1 and ASGR1 as alternative functional receptors of SARS-CoV-2. 2022. 32(1): p. 24–37."
},
{
"DOI": "10.1038/s41422-021-00603-9",
"doi-asserted-by": "crossref",
"key": "1718_CR213",
"unstructured": "Hoffmann M, Pöhlmann SJCR. Novel sars-Cov-2 receptors: Asgr1 and Kremen1 2022. 32(1): pp. 1–2."
},
{
"DOI": "10.1101/2020.09.09.287508",
"doi-asserted-by": "crossref",
"key": "1718_CR214",
"unstructured": "Gu Y et al. Interaction network of SARS-CoV-2 with host receptome through spike protein 2020: p. 2020.09. 09.287508."
},
{
"DOI": "10.1158/2326-6066.CIR-20-0969",
"doi-asserted-by": "crossref",
"key": "1718_CR215",
"unstructured": "Grigoriou M et al. Regulatory T-cell transcriptomic reprogramming characterizes adverse events by checkpoint inhibitors in solid tumors 2021. 9(7): pp. 726–734."
},
{
"DOI": "10.1016/j.jmb.2011.04.060",
"author": "JS Redzic",
"doi-asserted-by": "publisher",
"first-page": "68",
"issue": "1",
"journal-title": "J Mol Biol",
"key": "1718_CR216",
"unstructured": "Redzic JS, et al. The retinal specific CD147 Ig0 domain: from molecular structure to biological activity. J Mol Biol. 2011;411(1):68–82.",
"volume": "411",
"year": "2011"
},
{
"DOI": "10.1016/0006-291X(82)92042-3",
"author": "C Biswas",
"doi-asserted-by": "publisher",
"first-page": "1026",
"issue": "3",
"journal-title": "Biochem Biophys Res Commun",
"key": "1718_CR217",
"unstructured": "Biswas C. Tumor cell stimulation of collagenase production by fibroblasts. Biochem Biophys Res Commun. 1982;109(3):1026–34.",
"volume": "109",
"year": "1982"
},
{
"author": "C Biswas",
"first-page": "434",
"issue": "2",
"journal-title": "Cancer Res",
"key": "1718_CR218",
"unstructured": "Biswas C, et al. The human tumor cell-derived collagenase stimulatory factor (renamed EMMPRIN) is a member of the immunoglobulin superfamily. Cancer Res. 1995;55(2):434–9.",
"volume": "55",
"year": "1995"
},
{
"DOI": "10.1002/jcb.240350307",
"author": "C Biswas",
"doi-asserted-by": "publisher",
"first-page": "247",
"issue": "3",
"journal-title": "J Cell Biochem.",
"key": "1718_CR219",
"unstructured": "Biswas C, Nugent MA. Membrane association of collagenase stimulatory factor (s) from B-16 melanoma cells. J Cell Biochem. 1987;35(3):247–58.",
"volume": "35",
"year": "1987"
},
{
"key": "1718_CR220",
"unstructured": "Ellis SM, Nabeshima K, Biswas CJCr. Monoclon Antib Preparation Purif Tumor cell Collagenase stimulatory Factor. Cancer Res. 1989;49(12):3385–91."
},
{
"DOI": "10.1006/dbio.1997.8819",
"author": "T Igakura",
"doi-asserted-by": "publisher",
"first-page": "152",
"issue": "2",
"journal-title": "Dev Biol",
"key": "1718_CR221",
"unstructured": "Igakura T, et al. A null mutation in basigin, an immunoglobulin superfamily member, indicates its important roles in peri-implantation development and spermatogenesis. Dev Biol. 1998;194(2):152–65.",
"volume": "194",
"year": "1998"
},
{
"DOI": "10.1093/molehr/gar013",
"author": "H Chen",
"doi-asserted-by": "publisher",
"first-page": "405",
"issue": "7",
"journal-title": "Mol Hum Reprod",
"key": "1718_CR222",
"unstructured": "Chen H, et al. CD147 is required for matrix metalloproteinases-2 production and germ cell migration during spermatogenesis. Mol Hum Reprod. 2011;17(7):405–14.",
"volume": "17",
"year": "2011"
},
{
"DOI": "10.1016/j.semcdb.2014.02.008",
"author": "KL Fok",
"doi-asserted-by": "publisher",
"first-page": "31",
"journal-title": "Semin Cell Dev Biol",
"key": "1718_CR223",
"unstructured": "Fok KL, et al. Novel regulators of spermatogenesis. Semin Cell Dev Biol. 2014;29:31–42.",
"volume": "29",
"year": "2014"
},
{
"DOI": "10.1002/ar.24089",
"author": "D Guindolet",
"doi-asserted-by": "publisher",
"first-page": "1584",
"issue": "6",
"journal-title": "Anat Rec (Hoboken)",
"key": "1718_CR224",
"unstructured": "Guindolet D, Gabison EE. Role of CD147 (EMMPRIN/Basigin) in tissue remodeling. Anat Rec (Hoboken). 2020;303(6):1584–9.",
"volume": "303",
"year": "2020"
},
{
"DOI": "10.1016/j.cell.2010.03.015",
"author": "K Kessenbrock",
"doi-asserted-by": "publisher",
"first-page": "52",
"issue": "1",
"journal-title": "Cell.",
"key": "1718_CR225",
"unstructured": "Kessenbrock K, Plaks V, Werb Z. Matrix Metalloproteinases: Regulators Tumor Microenvironment. Cell. 2010;141(1):52–67.",
"volume": "141",
"year": "2010"
},
{
"author": "T Muramatsu",
"first-page": "981",
"issue": "3",
"journal-title": "Histol Histopathol",
"key": "1718_CR226",
"unstructured": "Muramatsu T, Miyauchi T. Basigin (CD147): a multifunctional transmembrane protein involved in reproduction, neural function, inflammation and tumor invasion. Histol Histopathol. 2003;18(3):981–7.",
"volume": "18",
"year": "2003"
},
{
"DOI": "10.2174/0929867321666131227163352",
"author": "X Zhu",
"doi-asserted-by": "publisher",
"first-page": "2138",
"issue": "19",
"journal-title": "Curr Med Chem",
"key": "1718_CR227",
"unstructured": "Zhu X, et al. CD147: a novel modulator of inflammatory and immune disorders. Curr Med Chem. 2014;21(19):2138–45.",
"volume": "21",
"year": "2014"
},
{
"DOI": "10.1165/ajrcmb.25.6.4558f",
"author": "HD Foda",
"doi-asserted-by": "publisher",
"first-page": "717",
"issue": "6",
"journal-title": "Am J Respir Cell Mol Biol.",
"key": "1718_CR228",
"unstructured": "Foda HD, et al. Ventilator-induced lung injury upregulates and activates gelatinases and EMMPRIN: attenuation by the synthetic matrix metalloproteinase inhibitor, Prinomastat (AG3340). Am J Respir Cell Mol Biol. 2001;25(6):717–24.",
"volume": "25",
"year": "2001"
},
{
"DOI": "10.1093/rheumatology/ken225",
"doi-asserted-by": "crossref",
"key": "1718_CR229",
"unstructured": "Yang Y et al. Cyclophilin A up-regulates MMP-9 expression and adhesion of monocytes/macrophages via CD147 signalling pathway in rheumatoid arthritis. 2008. 47(9): p. 1299–310."
},
{
"DOI": "10.1111/j.1582-4934.2007.00022.x",
"doi-asserted-by": "crossref",
"key": "1718_CR230",
"unstructured": "Pistol G et al. Roles of CD147 on T lymphocytes activation and MMP-9 secretion in systemic lupus erythematosus. 2007. 11(2): p. 339–48."
},
{
"DOI": "10.1161/STROKEAHA.117.018839",
"doi-asserted-by": "crossref",
"key": "1718_CR231",
"unstructured": "Jin R et al. Inhibition of CD147 (cluster of differentiation 147) ameliorates acute ischemic stroke in mice by reducing thromboinflammation. 2017. 48(12): p. 3356–65."
},
{
"DOI": "10.4110/in.2009.9.3.90",
"doi-asserted-by": "crossref",
"key": "1718_CR232",
"unstructured": "Kim J-Y et al. The stimulation of CD147 induces MMP-9 expression through ERK and NF-κB in macrophages: implication for atherosclerosis. 2009. 9(3): p. 90."
},
{
"DOI": "10.4049/jimmunol.168.10.4946",
"author": "T Renno",
"doi-asserted-by": "publisher",
"first-page": "4946",
"issue": "10",
"journal-title": "J Immunol.",
"key": "1718_CR233",
"unstructured": "Renno T, et al. A role for CD147 in thymic development. J Immunol. 2002;168(10):4946–50.",
"volume": "168",
"year": "2002"
},
{
"DOI": "10.1189/jlb.0506317",
"author": "JM Damsker",
"doi-asserted-by": "publisher",
"first-page": "613",
"issue": "3",
"journal-title": "J Leukoc Biol",
"key": "1718_CR234",
"unstructured": "Damsker JM, Bukrinsky MI, Constant SL. Preferential chemotaxis of activated human CD4 + T cells by extracellular cyclophilin A. J Leukoc Biol. 2007;82(3):613–8.",
"volume": "82",
"year": "2007"
},
{
"DOI": "10.1006/bbrc.2001.5847",
"author": "V Yurchenko",
"doi-asserted-by": "publisher",
"first-page": "786",
"issue": "4",
"journal-title": "Biochem Biophys Res Commun",
"key": "1718_CR235",
"unstructured": "Yurchenko V, et al. CD147 is a signaling receptor for cyclophilin B. Biochem Biophys Res Commun. 2001;288(4):786–8.",
"volume": "288",
"year": "2001"
},
{
"DOI": "10.1074/jbc.M201593200",
"author": "V Yurchenko",
"doi-asserted-by": "publisher",
"first-page": "22959",
"issue": "25",
"journal-title": "J Biol Chem",
"key": "1718_CR236",
"unstructured": "Yurchenko V, et al. Active site residues of cyclophilin A are crucial for its signaling activity via CD147. J Biol Chem. 2002;277(25):22959–65.",
"volume": "277",
"year": "2002"
},
{
"DOI": "10.1111/j.1365-2567.2005.02316.x",
"author": "V Yurchenko",
"doi-asserted-by": "publisher",
"first-page": "301",
"issue": "3",
"journal-title": "Immunology.",
"key": "1718_CR237",
"unstructured": "Yurchenko V, Constant S, Bukrinsky MJI. Dealing with the family: CD147 interactions with cyclophilins. Immunology. 2006;117(3):301–9.",
"volume": "117",
"year": "2006"
},
{
"DOI": "10.4049/jimmunol.1501889",
"doi-asserted-by": "crossref",
"key": "1718_CR238",
"unstructured": "Supper V et al. Association of CD147 and calcium exporter PMCA4 uncouples IL-2 expression from early TCR signaling. 2016. 196(3): p. 1387–99."
},
{
"DOI": "10.1016/j.medidd.2020.100056",
"doi-asserted-by": "crossref",
"key": "1718_CR239",
"unstructured": "Liu C, von Brunn A, Zhu D. Cyclophilin A and CD147: novel therapeutic targets for the treatment of COVID-19. Med Drug Discov. 2020;7:100056."
},
{
"DOI": "10.1021/jm4007577",
"author": "M Malesevic",
"doi-asserted-by": "publisher",
"first-page": "7302",
"issue": "18",
"journal-title": "J Med Chem",
"key": "1718_CR240",
"unstructured": "Malesevic M, et al. Anti-inflammatory effects of extracellular cyclosporins are exclusively mediated by CD147. J Med Chem. 2013;56(18):7302–11.",
"volume": "56",
"year": "2013"
},
{
"DOI": "10.4049/jimmunol.175.1.517",
"author": "K Arora",
"doi-asserted-by": "publisher",
"first-page": "517",
"issue": "1",
"journal-title": "J Immunol",
"key": "1718_CR241",
"unstructured": "Arora K, et al. Extracellular cyclophilins contribute to the regulation of inflammatory responses. J Immunol. 2005;175(1):517–22.",
"volume": "175",
"year": "2005"
},
{
"DOI": "10.1038/s41574-020-00462-1",
"author": "N Stefan",
"doi-asserted-by": "publisher",
"first-page": "135",
"issue": "3",
"journal-title": "Nat Rev Endocrinol.",
"key": "1718_CR242",
"unstructured": "Stefan N, Birkenfeld AL, Schulze MB. Global pandemics interconnected—obesity, impaired metabolic health and COVID-19. Nat Rev Endocrinol. 2021;17(3):135–49.",
"volume": "17",
"year": "2021"
},
{
"DOI": "10.3390/jcdd9010007",
"doi-asserted-by": "crossref",
"key": "1718_CR243",
"unstructured": "Ali MM et al. CD147 levels in blood and adipose tissues correlate with vascular dysfunction in obese diabetic adults. 2021. 9(1): p. 7."
},
{
"DOI": "10.1161/CIRCRESAHA.123.323223",
"author": "J-J Lv",
"doi-asserted-by": "publisher",
"first-page": "165",
"issue": "2",
"journal-title": "Circ Res.",
"key": "1718_CR244",
"unstructured": "Lv J-J, et al. CD147 Sparks Atherosclerosis by Driving M1 Phenotype and Impairing Efferocytosis. Circ Res. 2024;134(2):165–85.",
"volume": "134",
"year": "2024"
},
{
"DOI": "10.1073/pnas.111583198",
"author": "T Pushkarsky",
"doi-asserted-by": "publisher",
"first-page": "6360",
"issue": "11",
"journal-title": "Proc Natl Acad Sci U S A",
"key": "1718_CR245",
"unstructured": "Pushkarsky T, et al. CD147 facilitates HIV-1 infection by interacting with virus-associated cyclophilin A. Proc Natl Acad Sci U S A. 2001;98(11):6360–5.",
"volume": "98",
"year": "2001"
},
{
"DOI": "10.1128/mBio.00781-18",
"doi-asserted-by": "crossref",
"key": "1718_CR246",
"unstructured": "Vanarsdall AL et al. CD147 promotes entry of Pentamer-Expressing Human Cytomegalovirus into Epithelial and endothelial cells. mBio. 2018.8;9(3):e00781-18"
},
{
"DOI": "10.1128/JVI.02168-09",
"doi-asserted-by": "crossref",
"key": "1718_CR247",
"unstructured": "Watanabe A et al. CD147/EMMPRIN acts as a functional entry receptor for measles virus on epithelial cells. 2010. 84(9): p. 4183–93."
},
{
"DOI": "10.1038/nature10606",
"doi-asserted-by": "crossref",
"key": "1718_CR248",
"unstructured": "Crosnier C et al. Basigin is a receptor essential for erythrocyte invasion by Plasmodium Falciparum. 2011. 480(7378): p. 534–7."
},
{
"DOI": "10.1182/blood-2017-08-802918",
"doi-asserted-by": "crossref",
"key": "1718_CR249",
"unstructured": "Zhang M-Y et al. Disrupting CD147-RAP2 interaction abrogates erythrocyte invasion by Plasmodium Falciparum. 2018. 131(10): p. 1111–21."
},
{
"DOI": "10.1007/s12015-020-09987-4",
"doi-asserted-by": "crossref",
"key": "1718_CR250",
"unstructured": "Debuc B. D.M.J.S.c.r. Smadja, and reports, is COVID-19 a new hematologic disease? 2021. 17(1): p. 4–8."
},
{
"DOI": "10.1038/nm.3563",
"doi-asserted-by": "crossref",
"key": "1718_CR251",
"unstructured": "Bernard SC et al. Pathogenic Neisseria meningitidis utilizes CD147 for vascular colonization. 2014. 20(7): p. 725–31."
},
{
"DOI": "10.1177/2040206618811413",
"doi-asserted-by": "crossref",
"key": "1718_CR252",
"unstructured": "Abdullah A. A., Cyclophilin A as a target in the treatment of cytomegalovirus infections. 2018. 26: p. 2040206618811413."
},
{
"DOI": "10.3390/v9120365",
"doi-asserted-by": "crossref",
"key": "1718_CR253",
"unstructured": "Chen J et al. Human cytomegalovirus encoded miR-US25-1-5p attenuates CD147/EMMPRIN-mediated early antiviral response. 2017. 9(12): p. 365."
},
{
"DOI": "10.1242/jcs.016980",
"doi-asserted-by": "crossref",
"key": "1718_CR254",
"unstructured": "Till A et al. A role for membrane-bound CD147 in NOD2-mediated recognition of bacterial cytoinvasion. 2008. 121(4): p. 487–95."
},
{
"DOI": "10.1086/427811",
"doi-asserted-by": "crossref",
"key": "1718_CR255",
"unstructured": "Chen Z et al. Function of HAb18G/CD147 in invasion of host cells by severe acute respiratory syndrome coronavirus. 2005. 191(5): p. 755–60."
},
{
"DOI": "10.1134/S0006297922060086",
"doi-asserted-by": "crossref",
"key": "1718_CR256",
"unstructured": "Kuklina EMJB. T lymphocytes as targets for SARS-CoV-2. 2022. 87(6): p. 566–76."
},
{
"DOI": "10.12998/wjcc.v8.i17.3621",
"doi-asserted-by": "crossref",
"key": "1718_CR257",
"unstructured": "Talotta R, E.J.W.J.o.C C, Robertson. Autoimmunity as the comet tail of COVID-19 pandemic. 2020. 8(17): p. 3621."
},
{
"DOI": "10.1016/j.cell.2020.02.052",
"doi-asserted-by": "crossref",
"key": "1718_CR258",
"unstructured": "Hoffmann M et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. 2020. 181(2): p. 271–80. e8."
},
{
"DOI": "10.26508/lsa.202000786",
"doi-asserted-by": "crossref",
"key": "1718_CR259",
"unstructured": "Bestle D et al. TMPRSS2 and furin are both essential for proteolytic activation of SARS-CoV-2 in human airway cells. 2020. 3(9)."
},
{
"DOI": "10.1101/2022.02.19.481107",
"doi-asserted-by": "crossref",
"key": "1718_CR260",
"unstructured": "Benlarbi M et al. Identification of a SARS-CoV-2 host metalloproteinase-dependent entry pathway differentially used by SARS-CoV-2 and variants of concern alpha. Delta Omicron 2022: p. 2022.02. 19.481107."
},
{
"DOI": "10.3390/biology12060843",
"doi-asserted-by": "crossref",
"key": "1718_CR261",
"unstructured": "Salomão R et al. Involvement of matrix metalloproteinases in COVID-19: molecular targets, mechanisms, and insights for therapeutic interventions. 2023. 12(6): p. 843."
},
{
"DOI": "10.1128/msphere",
"doi-asserted-by": "publisher",
"key": "1718_CR262",
"unstructured": "Ragotte RJ et al. Human basigin (CD147) does not directly interact with SARS-CoV-2 spike glycoprotein. 2021. 6(4): p. https://doi.org/10.1128/msphere. 00647 – 21."
},
{
"DOI": "10.1038/s41598-020-80464-1",
"doi-asserted-by": "crossref",
"key": "1718_CR263",
"unstructured": "Shilts J et al. No evidence for basigin/CD147 as a direct SARS-CoV-2 spike binding receptor. 2021. 11(1): p. 413."
},
{
"DOI": "10.3390/cells10061434",
"doi-asserted-by": "crossref",
"key": "1718_CR264",
"unstructured": "Fenizia C et al. SARS-CoV-2 entry: at the crossroads of CD147 and ACE2. 2021. 10(6): p. 1434."
},
{
"key": "1718_CR265",
"unstructured": "Brunetti NS et al. SARS-CoV-2 uses CD4 to infect T helper lymphocytes 2023. 12: p. e84790."
},
{
"DOI": "10.1038/s41392-022-01230-5",
"doi-asserted-by": "crossref",
"key": "1718_CR266",
"unstructured": "Wu J et al. CD147 contributes to SARS-CoV-2-induced pulmonary fibrosis. 2022. 7(1): p. 382."
},
{
"DOI": "10.1016/j.cell.2021.11.026",
"doi-asserted-by": "crossref",
"key": "1718_CR267",
"unstructured": "Bushman M et al. Population impact of SARS-CoV-2 variants with enhanced transmissibility and/or partial immune escape. 2021. 184(26): p. 6229–42. e18."
},
{
"DOI": "10.1126/science.abn4947",
"doi-asserted-by": "crossref",
"key": "1718_CR268",
"unstructured": "Pulliam JR et al. Increased risk of SARS-CoV-2 reinfection associated with emergence of Omicron in South Africa. 2022. 376(6593): p. eabn4947."
},
{
"DOI": "10.1038/s41579-022-00841-7",
"doi-asserted-by": "crossref",
"key": "1718_CR269",
"unstructured": "Carabelli AM et al. SARS-CoV-2 variant biology: immune escape, transmission and fitness 2023. 21(3): pp. 162–177."
},
{
"key": "1718_CR270",
"unstructured": "Geng J, et al. Immunological Metabolic Characteristics Omicron Variants Infect. 2023;8(1):42."
},
{
"DOI": "10.1038/s41392-023-01323-9",
"author": "H Bian",
"doi-asserted-by": "publisher",
"first-page": "46",
"issue": "1",
"journal-title": "Sig Transduct Target Ther.",
"key": "1718_CR271",
"unstructured": "Bian H, et al. Meplazumab in hospitalized adults with severe COVID-19 (DEFLECT): a multicenter, seamless phase 2/3, randomized, third-party double-blind clinical trial. Sig Transduct Target Ther. 2023;8(1):46.",
"volume": "8",
"year": "2023"
},
{
"author": "N Kreuzberger",
"first-page": "CD013825",
"issue": "9",
"journal-title": "Cochrane Database Syst Rev",
"key": "1718_CR272",
"unstructured": "Kreuzberger N, et al. SARS-CoV-2-neutralising monoclonal antibodies for treatment of COVID-19. Cochrane Database Syst Rev. 2021;9(9):CD013825.",
"volume": "9",
"year": "2021"
},
{
"DOI": "10.1016/j.scitotenv.2021.152072",
"author": "T Behl",
"doi-asserted-by": "publisher",
"first-page": "152072",
"journal-title": "Sci Total Environ",
"key": "1718_CR273",
"unstructured": "Behl T, et al. CD147-spike protein interaction in COVID-19: get the ball rolling with a novel receptor and therapeutic target. Sci Total Environ. 2022;808:152072.",
"volume": "808",
"year": "2022"
},
{
"DOI": "10.1002/bies.202000094",
"author": "N Vasanthakumar",
"doi-asserted-by": "publisher",
"first-page": "e2000094",
"issue": "11",
"journal-title": "BioEssays",
"key": "1718_CR274",
"unstructured": "Vasanthakumar N. Beta-adrenergic blockers as a potential treatment for COVID-19 patients. BioEssays. 2020;42(11):e2000094.",
"volume": "42",
"year": "2020"
},
{
"DOI": "10.3892/etm.2017.4062",
"author": "X Liang",
"doi-asserted-by": "publisher",
"first-page": "835",
"issue": "3",
"journal-title": "Exp Ther Med",
"key": "1718_CR275",
"unstructured": "Liang X, et al. Atorvastatin attenuates plaque vulnerability by downregulation of EMMPRIN expression via COX-2/PGE2 pathway. Exp Ther Med. 2017;13(3):835–44.",
"volume": "13",
"year": "2017"
},
{
"DOI": "10.1038/s41586-021-03493-4",
"author": "A Stukalov",
"doi-asserted-by": "publisher",
"first-page": "246",
"issue": "7862",
"journal-title": "Nature.",
"key": "1718_CR276",
"unstructured": "Stukalov A, et al. Multilevel proteomics reveals host perturbations by SARS-CoV-2 and SARS-CoV. Nature. 2021;594(7862):246–52.",
"volume": "594",
"year": "2021"
},
{
"DOI": "10.1016/S2213-2600(21)00331-3",
"author": "VC Marconi",
"doi-asserted-by": "publisher",
"first-page": "1407",
"issue": "12",
"journal-title": "Lancet Respir Med.",
"key": "1718_CR277",
"unstructured": "Marconi VC, et al. Efficacy and safety of baricitinib for the treatment of hospitalised adults with COVID-19 (COV-BARRIER): a randomised, double-blind, parallel-group, placebo-controlled phase 3 trial. Lancet Respir Med. 2021;9(12):1407–18.",
"volume": "9",
"year": "2021"
}
],
"reference-count": 277,
"references-count": 277,
"relation": {},
"resource": {
"primary": {
"URL": "https://biosignaling.biomedcentral.com/articles/10.1186/s12964-024-01718-3"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "SARS-CoV-2-associated lymphopenia: possible mechanisms and the role of CD147",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1007/springer_crossmark_policy",
"volume": "22"
}