COVID-19 Excess Deaths in Peru’s 25 States in 2020: Nationwide Trends, Confounding Factors, and Correlations With the Extent of Ivermectin Treatment by State
Juan J Chamie, Jennifer A Hibberd, David E Scheim
Cureus, doi:10.7759/cureus.43168
Introduction In 2020, nations hastened to contain an emerging COVID-19 pandemic by deploying diverse public health approaches, but conclusive appraisals of the efficacy of these approaches are elusive in most cases. One of the medicines deployed, ivermectin (IVM), a macrocyclic lactone having biochemical activity against SARS-CoV-2 through competitive binding to its spike protein, has yielded mixed results in randomized clinical trials (RCTs) for COVID-19 treatments. In Peru, an opportunity to track the efficacy of IVM with a close consideration of confounding factors was provided through data for excess deaths as correlated with IVM use in 2020, under semi-autonomous policies in its 25 states.
Methods To evaluate possible IVM treatment effects, excess deaths as determined from Peruvian national health data were analyzed by state for ages ≥60 in Peru's 25 states. These data were compared with monthly summary data for excess deaths in Peru for the period 2020-2021 as published by the WHO in 2022. To identify potential confounding factors, Google mobility data, population densities, SARS-CoV-2 genetic variations, and seropositivity rates were also examined.
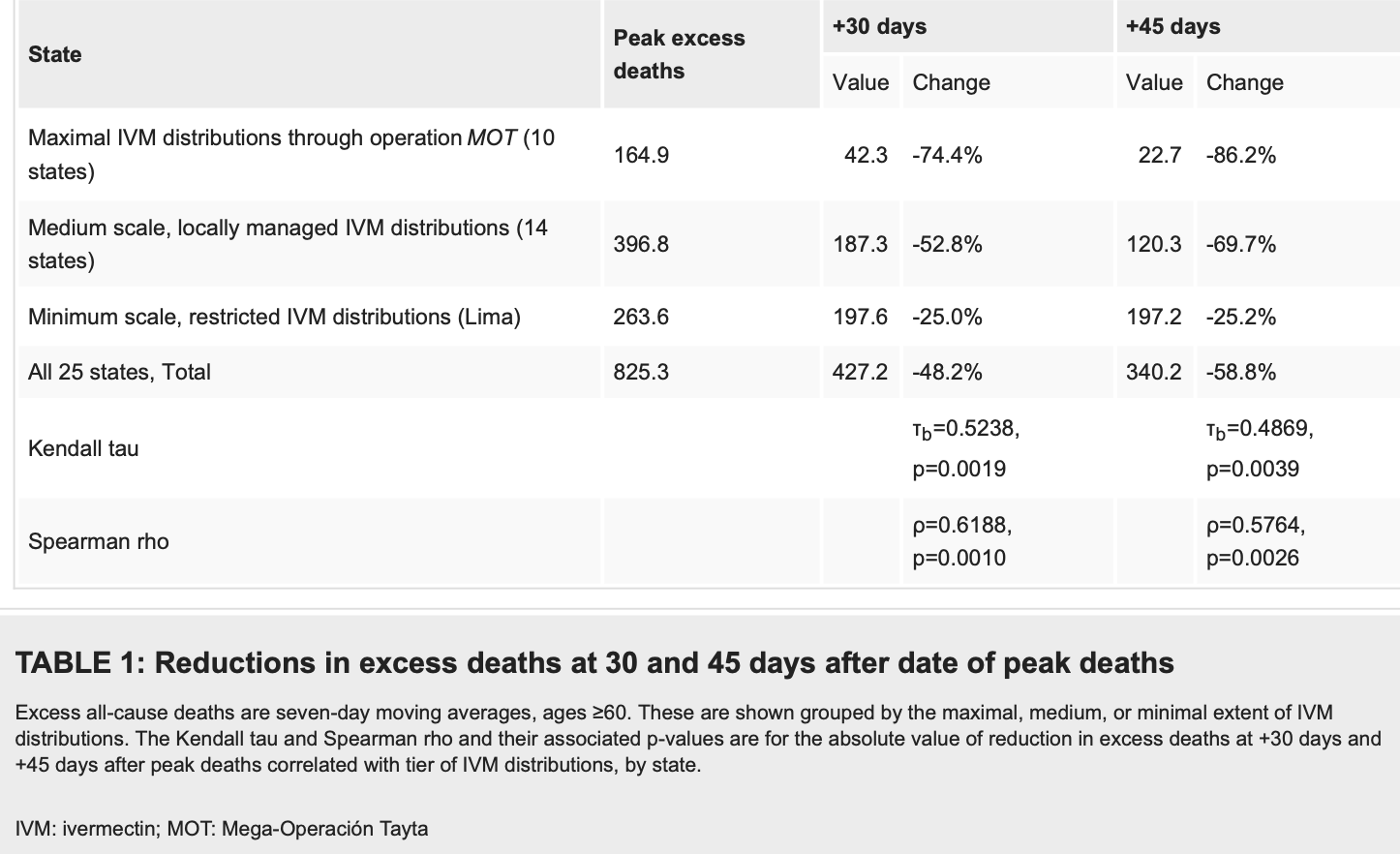
Results Reductions in excess deaths over a period of 30 days after peak deaths averaged 74% in the 10 states with the most intensive IVM use. As determined across all 25 states, these reductions in excess deaths correlated closely with the extent of IVM use (p<0.002). During four months of IVM use in 2020, before a new president of Peru restricted its use, there was a 14-fold reduction in nationwide excess deaths and then a 13-fold increase in the two months following the restriction of IVM use. Notably, these trends in nationwide excess deaths align with WHO summary data for the same period in Peru.
Conclusions The natural experiment that was put into motion with the authorization of IVM use for COVID-19 in Peru in May 2020, as analyzed using data on excess deaths by locality and by state from Peruvian national health sources, resulted in strong evidence for the drug's effectiveness. Several potential confounding factors, including effects of a social isolation mandate imposed in May 2020, variations in the genetic makeup of the SARS-CoV-2 virus, and differences in seropositivity rates and population densities across the 25 states, were considered but did not appear to have significantly influenced these outcomes.
Additional Information Disclosures Human subjects: All authors have confirmed that this study did not involve human participants or tissue.
References
Aminpour, Cannariato, Safaeeardebili, In silico analysis of the multi-targeted mode of action of ivermectin and related compounds, Computation,
doi:10.3390/computation10040051Babalola, Ndanusa, Adesuyi, Ogedengbe, Thairu et al., A randomized controlled trial of ivermectin monotherapy versus HCQ, IVM, and AZ combination therapy in Covid-19 patients in Nigeria, J Infect Dis Epidemiol,
doi:10.23937/2474-3658/1510233Badr, Du, Marshall, Dong, Squire et al., Association between mobility patterns and COVID-19 transmission in the USA: a mathematical modelling study, Lancet Infect Dis,
doi:10.1016/S1473-3099(20)30553-3Bermúdez, If a doctor evaluates a person and prescribes ivermectin, they can use it
Boretti, Zinc augments the antiviral potential of HCQ/CQ and ivermectin to reduce the risks of more serious outcomes from COVID-19 infection, J Trace Elem Med Biol,
doi:10.1016/j.jtemb.2022.126954Bramante, Huling, Tignanelli, Randomized trial of metformin, ivermectin, and fluvoxamine for Covid-19, N Engl J Med,
doi:10.1056/NEJMoa2201662Breakwater, IDL Reporters
Buonfrate, Chesini, Martini, High-dose ivermectin for early treatment of COVID-19 (COVER study): a randomised, double-blind, multicentre, phase II, dose-finding, proof-of-concept clinical trial, Int J Antimicrob Agents,
doi:10.1016/j.ijantimicag.2021.106516Cabezas, Fiestas, García-Mendoza, Palomino, Mamani et al., Dengue in Peru: a quarter of a century after its reemergence, Revista Peruana de Medicina Experimental y Salud Publica
Campbell, History of avermectin and ivermectin, with notes on the history of other macrocyclic lactone antiparasitic agents, Curr Pharm Biotechnol,
doi:10.2174/138920112800399095Chaccour, Lines, Whitty, Effect of ivermectin on Anopheles gambiae mosquitoes fed on humans: the potential of oral insecticides in malaria control, J Infect Dis,
doi:10.1086/653208Chahla, Ruiz, Ortega, Intensive treatment with ivermectin and iota-carrageenan as pre-exposure prophylaxis for COVID-19 in health care workers from Tucuman, Argentina, Am J Ther,
doi:10.1097/MJT.0000000000001433Chamie-Quintero, Hibberd, Scheim, Ivermectin for COVID-19 in Peru: 14-fold reduction in nationwide excess deaths, p<0.002 for effect by state, then 13-fold increase after ivermectin use restricted
Comercio, A trip to the black market of COVID-19
Crump, Ōmura, Ivermectin, wonder drug' from Japan: the human use perspective, Proc Jpn Acad Ser B Phys Biol Sci,
doi:10.2183/pjab.87.13De Castro, Jr, Gregianin, Burger, Continuous high-dose ivermectin appears to be safe in patients with acute myelogenous leukemia and could inform clinical repurposing for COVID-19 infection, Leuk Lymphoma,
doi:10.1080/10428194.2020.1786559Desort-Henin, Kostova, Babiker, Caramel, Malamut, The SAIVE Trial, post-exposure use of ivermectin in COVID-19 prevention: efficacy and safety results
Guzzo, Furtek, Porras, Safety, tolerability, and pharmacokinetics of escalating high doses of ivermectin in healthy adult subjects, J Clin Pharmacol,
doi:10.1177/009127002401382731Hazan, Gunaratne, Dolai, Clancy, Mccullough et al., Effectiveness of ivermectin-based multidrug therapy in severely hypoxic, ambulatory COVID-19 patients, Future Microbiol,
doi:10.2217/fmb-2022-0014Hibberd, Scheim, Google community mobility trends, seropositivity rates, comparisons of SINADEF data with who summary data, and other data items as useful in analysis of excess deaths during the COVID-19 pandemic in Peru
Hibberd, Scheim, Sharp reductions in COVID-19 case fatalities and excess deaths in Peru in close time conjunction, state-bystate
Juarez, Schcolnik-Cabrera, Dueñas-Gonzalez, The multitargeted drug ivermectin: from an antiparasitic agent to a repositioned cancer drug, Am J Cancer Res
Juscamayta-López, Carhuaricra, Tarazona, Valdivia, Rojas et al., Phylogenomics reveals multiple introductions and early spread of SARS-CoV-2 into Peru, J Med Virol,
doi:10.1002/jmv.27167Kerr, Baldi, Lobo, Regular use of ivermectin as prophylaxis for COVID-19 led up to a 92% reduction in COVID-19 mortality rate in a dose-response manner: results of a prospective observational study of a strictly controlled population of 88,
doi:10.7759/cureus.28624Kory, Meduri, Varon, Iglesias, Marik, Review of the emerging evidence demonstrating the efficacy of ivermectin in the prophylaxis and treatment of COVID-19, Am J Ther,
doi:10.1097/MJT.0000000000001377Krolewiecki, Lifschitz, Moragas, Antiviral effect of high-dose ivermectin in adults with COVID-19: A proof-of-concept randomized trial, EClinicalMedicine,
doi:10.1016/j.eclinm.2021.100959Lehrer, Rheinstein, Ivermectin docks to the SARS-CoV-2 spike receptor-binding domain attached to ACE2,
doi:10.21873/invivo.12134Lim, Hor, Tay, Efficacy of ivermectin treatment on disease progression among adults with mild to moderate covid-19 and comorbidities: the I-TECH randomized clinical trial, JAMA Intern Med,
doi:10.1001/jamainternmed.2022.0189Lima, None
López-Medina, 38 switched ivermectin (IVM) and placebo doses, failure of blinding, ubiquitous IVM use OTC in Cali, and nearly identical AEs for the IVM and control groups
López-Medina, López, Hurtado, Effect of ivermectin on time to resolution of symptoms among adults with mild COVID-19: a randomized clinical trial, JAMA,
doi:10.1001/jama.2021.3071Naggie, Boulware, Lindsell, Effect of higher-dose ivermectin for 6 days vs placebo on time to sustained recovery in outpatients with COVID-19: a randomized clinical trial, JAMA,
doi:10.1001/jama.2023.1650Naggie, Boulware, Lindsell, Effect of ivermectin vs placebo on time to sustained recovery in outpatients with mild to moderate COVID-19: a randomized clinical trial, JAMA,
doi:10.1001/jama.2022.18590Navarro, Camprubí, Requena-Méndez, Safety of high-dose ivermectin: a systematic review and meta-analysis, J Antimicrob Chemother,
doi:10.1093/jac/dkz524Nicolelis, Raimundo, Peixoto, Andreazzi, The impact of super-spreader cities, highways, and intensive care availability in the early stages of the COVID-19 epidemic in Brazil, Sci Rep,
doi:10.1038/s41598-021-92263-3Nih Covid-, Fluvoxamine: selected clinical data, limitations and interpretation
Oh, Lee, Khuong, Mobility restrictions were associated with reductions in COVID-19 incidence early in the pandemic: evidence from a real-time evaluation in 34 countries, Sci Rep,
doi:10.1038/s41598-021-92766-zPadilla-Rojas, Vega-Chozo, Galarza-Perez, Genomic analysis reveals local transmission of SARS-CoV-2 in early pandemic phase in Peru, bioRxiv,
doi:10.1101/2020.09.05.284604Puno, Starting tomorrow they will implement the Tayta operation led by the Peruvian Army
Reis, Silva, Silva, Effect of early treatment with ivermectin among patients with COVID-19, N Engl J Med,
doi:10.1056/NEJMoa2115869Santin, Scheim, Mccullough, Yagisawa, Borody, Ivermectin: a multifaceted drug of Nobel prize-honoured distinction with indicated efficacy against a new global scourge, COVID-19, New Microbes New Infect,
doi:10.1016/j.nmni.2021.100924Scheim, A deadly embrace: hemagglutination mediated by SARS-COV-2 spike protein at its 22 nglycosylation sites, red blood cell surface sialoglycoproteins, and antibody, Int J Mol Sci,
doi:10.3390/ijms23052558Scheim, Aldous, Osimani, Fordham, Hoy, When characteristics of clinical trials require perprotocol as well as intention-to-treat outcomes to draw reliable conclusions: three examples, J Clin Med. 2023,
doi:10.3390/jcm12113625Seet, Quek, Ooi, Positive impact of oral hydroxychloroquine and povidone-iodine throat spray for COVID-19 prophylaxis: An open-label randomized trial, Int J Infect Dis,
doi:10.1016/j.ijid.2021.04.035Sempé, Lloyd-Sherlock, Martínez, Ebrahim, Mckee et al., Estimation of all-cause excess mortality by age-specific mortality patterns for countries with incomplete vital statistics: a populationbased study of the case of Peru during the first wave of the COVID-19 pandemic, Lancet Reg Health Am,
doi:10.1016/j.lana.2021.100039Shafiee, Athar, Gargari, Jafarabady, Siahvoshi et al., Ivermectin under scrutiny: a systematic review and meta-analysis of efficacy and possible sources of controversies in COVID-19 patients, Virol J,
doi:10.1186/s12985-022-01829-8Shouman, Hegazy, Nafae, Use of ivermectin as a prophylactic option in asymptomatic family close contacts with patients of COVID-19 (NCT number: 04422561), J Clin Diagnostic Res
Stokes, Turner, Anselmi, Morciano, Hone, The relative effects of non-pharmaceutical interventions on wave one Covid-19 mortality: natural experiment in 130 countries,
doi:10.1186/s12889-022-13546-6Stone, Ndarukwa, Scheim, Changes in SpO2 on room air for 34 severe COVID-19 patients after ivermectin-based combination treatment: 62% normalization within 24 hours, Biologics,
doi:10.3390/biologics2030015Vallejos, Zoni, Bangher, Ivermectin to prevent hospitalizations in patients with COVID-19 (IVERCOR-COVID19) a randomized, double-blind, placebo-controlled trial, BMC Infect Dis,
doi:10.1186/s12879-021-06348-5Wessa, net Free Statistics Software, Office for Research Development and Education, version 1.2.1
Yagisawa, Foster, Hanaki, Omura, Global trends in clinical studies of ivermectin in COVID-19, Jpn J Antibiot
DOI record:
{
"DOI": "10.7759/cureus.43168",
"ISSN": [
"2168-8184"
],
"URL": "http://dx.doi.org/10.7759/cureus.43168",
"author": [
{
"affiliation": [],
"family": "Chamie",
"given": "Juan J",
"sequence": "first"
},
{
"affiliation": [],
"family": "Hibberd",
"given": "Jennifer A",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Scheim",
"given": "David E",
"sequence": "additional"
}
],
"container-title": "Cureus",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2023,
8,
8
]
],
"date-time": "2023-08-08T20:51:03Z",
"timestamp": 1691527863000
},
"deposited": {
"date-parts": [
[
2023,
8,
8
]
],
"date-time": "2023-08-08T20:51:29Z",
"timestamp": 1691527889000
},
"indexed": {
"date-parts": [
[
2023,
8,
9
]
],
"date-time": "2023-08-09T04:31:45Z",
"timestamp": 1691555505186
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2023,
8,
8
]
]
},
"language": "en",
"link": [
{
"URL": "https://www.cureus.com/articles/172991-covid-19-excess-deaths-in-perus-25-states-in-2020-nationwide-trends-confounding-factors-and-correlations-with-the-extent-of-ivermectin-treatment-by-state",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "297",
"original-title": [],
"prefix": "10.7759",
"published": {
"date-parts": [
[
2023,
8,
8
]
]
},
"published-print": {
"date-parts": [
[
2023,
8,
8
]
]
},
"publisher": "Springer Science and Business Media LLC",
"reference": [
{
"key": "ref1",
"unstructured": "First case of coronavirus in Peru. the history of contagion in the pilot [Article in Spanish]. (March 6, 2020). Accessed: July 3, 2023: https://gestion.pe/peru/primer-caso-de-coronavirus-en-peru-los-detalles-del-contagio-del-piloto-noticia/."
},
{
"key": "ref2",
"unstructured": "Inequality and corruption. why Peru is losing its COVID-19 battle. (July 1, 2020). Accessed: July 3, 2023: https://www.devex.com/news/inequality-and-corruption-why-peru-is-losing-its-covid-19-battle-97604."
},
{
"article-title": "",
"author": "Ministry of Health",
"key": "ref3",
"unstructured": "Ministry of Health. Ministerial Resolution #270-2020 [Article in Spanish]. Lima, Peru; May 8, 2020. https://cdn.www.gob.pe/uploads/document/file/694719/RM_270-2020-MINSA.PDF."
},
{
"article-title": "Global trends in clinical studies of ivermectin in COVID-19",
"author": "Yagisawa M",
"journal-title": "Jpn J Antibiot",
"key": "ref4",
"unstructured": "Yagisawa M, Foster PJ, Hanaki H, Omura S. Global trends in clinical studies of ivermectin in COVID-19. Jpn J Antibiot. 2021, 74:44-95.",
"volume": "74",
"year": "2021"
},
{
"article-title": "The multitargeted drug ivermectin: from an antiparasitic agent to a repositioned cancer drug",
"author": "Juarez M",
"journal-title": "Am J Cancer Res",
"key": "ref5",
"unstructured": "Juarez M, Schcolnik-Cabrera A, Dueñas-Gonzalez A. The multitargeted drug ivermectin: from an antiparasitic agent to a repositioned cancer drug. Am J Cancer Res. 2018, 8:317-31.",
"volume": "8",
"year": "2018"
},
{
"DOI": "10.2174/138920112800399095",
"article-title": "History of avermectin and ivermectin, with notes on the history of other macrocyclic lactone antiparasitic agents",
"author": "Campbell WC",
"doi-asserted-by": "publisher",
"journal-title": "Curr Pharm Biotechnol",
"key": "ref6",
"unstructured": "Campbell WC. History of avermectin and ivermectin, with notes on the history of other macrocyclic lactone antiparasitic agents. Curr Pharm Biotechnol. 2012, 13:853-65. 10.2174/138920112800399095",
"volume": "13",
"year": "2012"
},
{
"key": "ref7",
"unstructured": "New York Times. Peru chooses 3rd president in a week amid street protests. (November 16, 2020). Accessed: July 3, 2023: https://www.nytimes.com/2020/11/16/world/americas/Peru-president-Francisco-Sagasti.html."
},
{
"key": "ref8",
"unstructured": "Mazzetti on ivermectin. Minsa cannot freely deliver drugs without international accreditation [Article in Spanish]. (January 29, 2021). Accessed: July 3, 2023: https://elcomercio.pe/lima/sucesos/pilar-mazzetti-sobre-ivermectina-minsa-no-puede-entregar-farmacos-sin-acreditacion...."
},
{
"key": "ref9",
"unstructured": "Violeta Bermúdez. \"If a doctor evaluates a person and prescribes ivermectin, they can use it\" [Article in Spanish]. (January 22, 2021). Accessed: July 3, 2023: https://rpp.pe/peru/actualidad/violeta-bermudez-si-un-medico-evalua-a-una-persona-y-le-receta-ivermectina-puede-utili...."
},
{
"key": "ref10",
"unstructured": "COVID-19. Vizcarra recommended the use of ivermectin to treat disease [Article in Spanish]. (January 9, 2021). Accessed: July 3, 2023: https://canaln.pe/actualidad/covid-19-martin-vizcarra-recomendo-uso-ivermectina-tratamiento-enfermedad-n429808."
},
{
"key": "ref11",
"unstructured": "Breakwater. IDL Reporters [Article in Spanish]. (January 20, 2021). Accessed. July 3, 2023: https://www.idl-reporteros.pe/el-rompeolas/."
},
{
"key": "ref12",
"unstructured": "From the scarf to ivermectin [Article in Spanish]. (February 3, 2021). Accessed. July 3, 2023: https://www.idl-reporteros.pe/del-panuelazo-a-la-ivermectina/."
},
{
"key": "ref13",
"unstructured": "Dryad data repository; frozen data snapshots from the Peruvian national SINADEF death information system from December 13, 2020 and February 23, 2021 and associated data documentation. Dryad Data Repository. (2021). (2022). Accessed. July 3, 2023: https://datadryad.org/stash/dataset/doi:10.5061/dryad.dv41ns1xr."
},
{
"key": "ref14",
"unstructured": "Death seasonality, Google community mobility trends, seropositivity rates, comparisons of SINADEF data with who summary data, and other data items as useful in analysis of excess deaths during the COVID-19 pandemic in Peru, 2020-2021 (Chamie-Quintero JJ, Hibberd, JA, Scheim DE) [PREPRINT]. (2023). Accessed. July 13, 2023: https://osf.io/a9ex5/."
},
{
"key": "ref15",
"unstructured": "Covid-19. second report to update the death toll will be released this week. Andina Peruvian News Agency [Article in Spanish]. (July 26, 2020). Accessed: July 3, 2023: https://andina.pe/agencia/noticia-covid19-segundo-informe-para-actualizar-cifra-fallecidos-se-conocera-esta-semana-80...."
},
{
"DOI": "10.1002/jmv.27167",
"article-title": "Phylogenomics reveals multiple introductions and early spread of SARS-CoV-2 into Peru",
"author": "Juscamayta-López E",
"doi-asserted-by": "publisher",
"journal-title": "J Med Virol",
"key": "ref16",
"unstructured": "Juscamayta-López E, Carhuaricra D, Tarazona D, Valdivia F, Rojas N, Maturrano L, Gavilán R. Phylogenomics reveals multiple introductions and early spread of SARS-CoV-2 into Peru. J Med Virol. 2021, 93:5961-8. 10.1002/jmv.27167",
"volume": "93",
"year": "2021"
},
{
"DOI": "10.1101/2020.09.05.284604",
"article-title": "Genomic analysis reveals local transmission of SARS-CoV-2 in early pandemic phase in Peru",
"author": "Padilla-Rojas C",
"doi-asserted-by": "publisher",
"journal-title": "bioRxiv",
"key": "ref17",
"unstructured": "Padilla-Rojas C, Vega-Chozo K, Galarza-Perez M, et al.. Genomic analysis reveals local transmission of SARS-CoV-2 in early pandemic phase in Peru. bioRxiv. 2020, 10.1101/2020.09.05.284604",
"year": "2020"
},
{
"DOI": "10.1016/j.lana.2021.100039",
"article-title": "Estimation of all-cause excess mortality by age-specific mortality patterns for countries with incomplete vital statistics: a population-based study of the case of Peru during the first wave of the COVID-19 pandemic",
"author": "Sempé L",
"doi-asserted-by": "publisher",
"journal-title": "Lancet Reg Health Am",
"key": "ref18",
"unstructured": "Sempé L, Lloyd-Sherlock P, Martínez R, Ebrahim S, McKee M, Acosta E. Estimation of all-cause excess mortality by age-specific mortality patterns for countries with incomplete vital statistics: a population-based study of the case of Peru during the first wave of the COVID-19 pandemic. Lancet Reg Health Am. 2021, 2:10.1016/j.lana.2021.100039",
"volume": "2",
"year": "2021"
},
{
"article-title": "",
"author": "Republic of Peru",
"key": "ref19",
"unstructured": "Republic of Peru. Political Constitution of Peru Edition of the Congress of the Republic [Article in Spanish]. 2016. http://www.congreso.gob.pe/Docs/files/documentos/constitucion1993-01.pdf.",
"year": "2016"
},
{
"key": "ref20",
"unstructured": "Sharp reductions in COVID-19 case fatalities and excess deaths in Peru in close time conjunction, state-by-state, with ivermectin treatments (Chamie-Quintero JJ, Hibberd, JA, Scheim DE). (2022). Accessed. September 27, 2022: https://osf.io/h7zbg/."
},
{
"key": "ref21",
"unstructured": "Ivermectina. crece la demanda de fármaco antiparasitario para casos de covid-19. Realidadpe | Noticias relevantes del Perú | Francisco Sagasti, Elecciones 2021, COVID-19, Reactiva Perú, Arranca Perú, Cuarentena focalizada. April 29, 2020. Accessed: July 3, 2023: https://realidad.pe/salud/ivermectina-crece-la-demanda-de-farmaco-antiparasitario-para-casos-de-covid-19/."
},
{
"key": "ref22",
"unstructured": "Piura. a couple is intervened with more than 200 doses of contraband ivermectin [Article in Spanish]. (June 19, 2020). Accessed: July 3, 2023: https://elcomercio.pe/peru/piura-detienen-a-dos-personas-con-mas-de-200-dosis-de-ivermectina-de-contrabando-coronavir...."
},
{
"key": "ref23",
"unstructured": "San Martín de Porres. 20,000 vials of ivermectin seized and 12 people arrested [Article in Spanish]. (May 27, 2020). Accessed: July 3, 2023: https://andina.pe/agencia/noticia-san-martin-porres-incautan-20000-frascos-ivermectina-y-detienen-a-12-personas-79910...."
},
{
"key": "ref24",
"unstructured": "El Comercio. A trip to the black market of COVID-19 [Article in Spanish]. (May 17, 2020). Accessed: July 3, 2023: https://www.facebook.com/watch/?v=292349115157434."
},
{
"key": "ref25",
"unstructured": "COVID-19 in Peru. Pharmacy in SJM that sold adulterated ivermectin and drugs stolen from the State intervened [Article in Spanish]. (June 14, 2020). Accessed: July 3, 2023: https://elcomercio.pe/lima/sucesos/intervienen-farmacia-en-sjm-que-vendia-invermectina-adulterada-y-medicamentos-sus...."
},
{
"key": "ref26",
"unstructured": "COVID-19 Open Data; National Open Data Platform. Digital Government Secretariat Presidency of the Council of Ministers [Article in Spanish]. (2020). Accessed. July 3, 2023: https://www.datosabiertos.gob.pe/group/datos-abiertos-de-covid-19."
},
{
"key": "ref27",
"unstructured": "More than 500 older adults are cared for by the Mimp during Operation Tayta in Pasco [Article in Spanish]. (July 30, 2020). Accessed. July 3, 2023: https://andina.pe/agencia/noticia-mas-500-adultos-mayores-son-atendidos-por-mimp-durante-operacion-tayta-pasco-807916...."
},
{
"key": "ref28",
"unstructured": "Management Resolution, No. 133-2020-HMPP-A/GM. Municipal, Provincial Management of Pasco [Article in Spanish]. (August 5, 2020). Accessed. July 3, 2023: https://www.munipasco.gob.pe/admin/files/1669x9xf7f741e17z7417z7ze17a79xf79xf78179x9xa73670E4g3670E4g3670E4g0E4g.pdf."
},
{
"key": "ref29",
"unstructured": "Management Resolution, No. 154-2020-HMPP-A/GM. Municipal, Provincial Management of Pasco [Article in Spanish]. (August 25, 2020). Accessed. July 3, 2023: https://cdn.www.gob.pe/uploads/document/file/2065537/Resoluci%C3%B3n%20Gerencial%20%20N%C2%B0%20133%20-%20Aprobar%20e...."
},
{
"key": "ref30",
"unstructured": "Junín. regional government produces ivermectin for early treatment of COVID-19 [Article in Spanish]. (July 22, 2020). Accessed: July 3, 2023: https://andina.pe/agencia/noticia-junin-gobierno-regional-elabora-ivermectina-para-tratamiento-temprano-covid19-80686...."
},
{
"key": "ref31",
"unstructured": "Hospital Unanue maintains regular production of ivermectin. Regional Government of Tacna [Article in Spanish]. (August 6, 2020). Accessed. July 3, 2023: https://www.gob.pe/institucion/regiontacna/noticias/548716-hospital-unanue-mantiene-produccion-regular-de-ivermectina."
},
{
"key": "ref32",
"unstructured": "Government starts Operation TAYTA to protect the vulnerable population against COVID-19 in risk areas. Lima, Peru. Ministry of Defense of Peru [Article in Spanish]. (June 1, 2020). Accessed: July 3, 2023: https://www.gob.pe/institucion/mindef/noticias/168091-gobierno-inicia-operacion-tayta-para-proteger-a-la-poblacion-vu...."
},
{
"key": "ref33",
"unstructured": "Plan for the actions of the multisectoral working group called \"I take care of you Peru,\" Ministry of Defense, General Directorate of Policy and Strategy [Article in Spanish]. (2020). Accessed. July 3, 2023: https://drive.google.com/file/d/1s-EQwuT59Na8umvxqWthXCo2Borl0JPG/view?usp=sharing."
},
{
"key": "ref34",
"unstructured": "Mayor of Lima must convene business and government to apply massive family fences [Article in Spanish]. (August 31, 2020). Accessed. July 3, 2023: https://www.lampadia.com/analisis/gobernanza/alcalde-de-lima-debe-convocar-a-empresariado-y-gobierno-para-aplicar-cer...."
},
{
"key": "ref35",
"unstructured": "Coronavirus in Peru. find out how Operation Tayta works. (August 12, 2020). Accessed: July 3, 2023: https://rpp.pe/peru/actualidad/coronavirus-en-peru-conoce-como-funciona-la-operacion-tayta-noticia-1286058."
},
{
"key": "ref36",
"unstructured": "Operation Tayta arrived in Huánuco to strengthen the fight against COVID-19 [Article in Spanish]. (August 12, 2020). Accessed. July 3, 2023: https://www.gob.pe/institucion/regionhuanuco/noticias/288607-operacion-tayta-llego-a-huanuco-para-fortalecer-lucha-co...."
},
{
"key": "ref37",
"unstructured": "Covid-19. more than 22,000 people were attended by Operation Tayta-Yo Me Apunto in Cajamarca. Andina, Peruvian News Agency [Article in Spanish]. (August 2, 2020). Accessed: July 3, 2023: https://andina.pe/agencia/noticia-covid19-a-mas-22000-personas-atendio-operacion-taytayo-me-apunto-cajamarca-808221.aspx."
},
{
"key": "ref38",
"unstructured": "Moquegua. another 45 doctors arrive from Lima to reinforce the fight against the coronavirus. Editorial The Republic [Article in Spanish]. (August 5, 2020). Accessed: July 3, 2023: https://larepublica.pe/sociedad/2020/08/05/moquegua-llegan-otros-45-medicos-desde-lima-para-reforzar-lucha-contra-el-...."
},
{
"key": "ref39",
"unstructured": "A team from the Ministry of Health carries out Operation Tayta and provides technical assistance in Moquegua. Ministry of Health [Article in Spanish]. (August 6, 2020). Accessed. July 3, 2023: https://www.gob.pe/institucion/minsa/noticias/286591-equipo-del-ministerio-de-salud-realiza-operacion-tayta-y-brinda-...."
},
{
"key": "ref40",
"unstructured": "Through enrollment executed by 60 brigadistas of the mph and “protection”. Peru Provincial Municipality of Huancayo [Article in Spanish]. (August 6, 2020). Accessed. July 3, 2023: https://www.munihuancayo.gob.pe/portal/s2/noticias-1/593-mediante-empadronamiento-ejecutado-por-60-brigadistas-de-la-...."
},
{
"key": "ref41",
"unstructured": "Minister of Defense. Operation Tayta allows the strengthening of primary care for COVID-19 cases [Article in Spanish]. (August 15, 2020). Accessed: July 3, 2023: https://www.gob.pe/institucion/mindef/noticias/294084-ministro-de-defensa-operacion-tayta-permite-fortalecer-la-atenc...."
},
{
"key": "ref42",
"unstructured": "Puno. Starting tomorrow they will implement the Tayta operation led by the Peruvian Army [Article in Spanish]. (August 13, 2020). Accessed: July 3, 2023: https://radioondaazul.com/puno-desde-manana-implementaran-la-operacion-tayta-liderado-por-el-ejercito-peruano/."
},
{
"key": "ref43",
"unstructured": "Operation Tayta will serve 10,000 vulnerable people in Huánuco. Andina, Peruvian News Agency [Article in Spanish]. (August 14, 2020). Accessed. July 3, 2023: https://andina.pe/agencia/noticia-operacion-tayta-atendera-a-10000-personas-vulnerables-huanuco-810025.aspx."
},
{
"key": "ref44",
"unstructured": "Government delivers to Huancavelica more than 17,000 units of PPE and medicines to fight Covid-19 [Article in Spanish]. (August 27, 2020). Accessed. July 3, 2023: https://www.gob.pe/institucion/produce/noticias/296401-gobierno-entrega-a-huancavelica-mas-de-17-000-unidades-de-epp-...."
},
{
"DOI": "10.1016/j.fopow.2020.01.008",
"doi-asserted-by": "crossref",
"key": "ref45",
"unstructured": "The Mega Tayta 2020 plan begins in ayacucho to diagnose, treat, isolate and assist positive cases with food [Article in Spanish]. (August 20, 2020). Accessed. July 3, 2023: https://www.gob.pe/institucion/regionayacucho/noticias/294735-inician-plan-mega-tayta-2020-en-ayacucho-para-diagnosti...."
},
{
"key": "ref46",
"unstructured": "Covid-19. Tayta operation began with the application of 3,000 discard tests [Article in Spanish]. (August 20, 2020). Accessed: July 3, 2023: https://www.apnoticias.pe/peru/exitosa-noticias/covid-19-inicio-operacion-tayta-con-aplicacion-de-3000-pruebas-de-des...."
},
{
"DOI": "10.1097/MJT.0000000000001377",
"article-title": "Review of the emerging evidence demonstrating the efficacy of ivermectin in the prophylaxis and treatment of COVID-19",
"author": "Kory P",
"doi-asserted-by": "publisher",
"journal-title": "Am J Ther",
"key": "ref47",
"unstructured": "Kory P, Meduri GU, Varon J, Iglesias J, Marik PE. Review of the emerging evidence demonstrating the efficacy of ivermectin in the prophylaxis and treatment of COVID-19. Am J Ther. 2021, 28:e299-318. 10.1097/MJT.0000000000001377",
"volume": "28",
"year": "2021"
},
{
"DOI": "10.1016/j.nmni.2021.100924",
"article-title": "Ivermectin: a multifaceted drug of Nobel prize-honoured distinction with indicated efficacy against a new global scourge, COVID-19",
"author": "Santin AD",
"doi-asserted-by": "publisher",
"journal-title": "New Microbes New Infect",
"key": "ref48",
"unstructured": "Santin AD, Scheim DE, McCullough PA, Yagisawa M, Borody TJ. Ivermectin: a multifaceted drug of Nobel prize-honoured distinction with indicated efficacy against a new global scourge, COVID-19. New Microbes New Infect. 2021, 43:100924. 10.1016/j.nmni.2021.100924",
"volume": "43",
"year": "2021"
},
{
"DOI": "10.1001/jama.2021.3071",
"article-title": "Effect of ivermectin on time to resolution of symptoms among adults with mild COVID-19: a randomized clinical trial",
"author": "López-Medina E",
"doi-asserted-by": "publisher",
"journal-title": "JAMA",
"key": "ref49",
"unstructured": "López-Medina E, López P, Hurtado IC, et al.. Effect of ivermectin on time to resolution of symptoms among adults with mild COVID-19: a randomized clinical trial. JAMA. 2021, 325:1426-35. 10.1001/jama.2021.3071",
"volume": "325",
"year": "2021"
},
{
"DOI": "10.1186/s12879-021-06348-5",
"article-title": "Ivermectin to prevent hospitalizations in patients with COVID-19 (IVERCOR-COVID19) a randomized, double-blind, placebo-controlled trial",
"author": "Vallejos J",
"doi-asserted-by": "publisher",
"journal-title": "BMC Infect Dis",
"key": "ref50",
"unstructured": "Vallejos J, Zoni R, Bangher M, et al.. Ivermectin to prevent hospitalizations in patients with COVID-19 (IVERCOR-COVID19) a randomized, double-blind, placebo-controlled trial. BMC Infect Dis. 2021, 21:635. 10.1186/s12879-021-06348-5",
"volume": "21",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2115869",
"article-title": "Effect of early treatment with ivermectin among patients with COVID-19",
"author": "Reis G",
"doi-asserted-by": "publisher",
"journal-title": "N Engl J Med",
"key": "ref51",
"unstructured": "Reis G, Silva EA, Silva DC, et al.. Effect of early treatment with ivermectin among patients with COVID-19. N Engl J Med. 2022, 386:1721-31. 10.1056/NEJMoa2115869",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.1001/jamainternmed.2022.0189",
"article-title": "Efficacy of ivermectin treatment on disease progression among adults with mild to moderate covid-19 and comorbidities: the I-TECH randomized clinical trial",
"author": "Lim SC",
"doi-asserted-by": "publisher",
"journal-title": "JAMA Intern Med",
"key": "ref52",
"unstructured": "Lim SC, Hor CP, Tay KH, et al.. Efficacy of ivermectin treatment on disease progression among adults with mild to moderate covid-19 and comorbidities: the I-TECH randomized clinical trial. JAMA Intern Med. 2022, 182:426-35. 10.1001/jamainternmed.2022.0189",
"volume": "182",
"year": "2022"
},
{
"key": "ref53",
"unstructured": "Protocol violations in López-Medina et al.. 38 switched ivermectin (IVM) and placebo doses, failure of blinding, ubiquitous IVM use OTC in Cali, and nearly identical AEs for the IVM and control groups (Scheim DE, Hibberd JA, Chamie-Quintero JJ) [PREPRINT]. (2021). Accessed: March 20, 2023: https://osf.io/u7ewz/."
},
{
"key": "ref54",
"unstructured": "The drug used in Lim et al. 2022, source not specified, had <1% incidence of AEs distinctive and common for ivermectin at this study’s very high dose, 2 mg/kg (Scheim DE) [PREPRINT]. (2022). Accessed. September 20, 2022: https://osf.io/5cwmr/."
},
{
"DOI": "10.3390/jcm12113625",
"article-title": "When characteristics of clinical trials require per-protocol as well as intention-to-treat outcomes to draw reliable conclusions: three examples",
"author": "Scheim DE",
"doi-asserted-by": "publisher",
"journal-title": "J Clin Med",
"key": "ref55",
"unstructured": "Scheim DE, Aldous C, Osimani B, Fordham EJ, Hoy WE. When characteristics of clinical trials require per-protocol as well as intention-to-treat outcomes to draw reliable conclusions: three examples. J Clin Med. 2023, 12:10.3390/jcm12113625",
"volume": "12",
"year": "2023"
},
{
"key": "ref56",
"unstructured": "NIH COVID-19 treatment guidelines. Fluvoxamine. selected clinical data, limitations and interpretation. Table 4c. (2022). Accessed: July 3, 2023: https://www.covid19treatmentguidelines.nih.gov/tables/fluvoxamine-data/."
},
{
"key": "ref57",
"unstructured": "Memorandum explaining basis for declining request for emergency use authorization of fluvoxamine maleate. U.S. Food & Drug Administration. (2022). Accessed. March 20, 2023: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2020/EUA%20110%20Fluvoxamine%20Decisional%20Memo_Redacted.pdf."
},
{
"DOI": "10.1056/NEJMoa2201662",
"article-title": "Randomized trial of metformin, ivermectin, and fluvoxamine for Covid-19",
"author": "Bramante CT",
"doi-asserted-by": "publisher",
"journal-title": "N Engl J Med",
"key": "ref58",
"unstructured": "Bramante CT, Huling JD, Tignanelli CJ, et al.. Randomized trial of metformin, ivermectin, and fluvoxamine for Covid-19. N Engl J Med. 2022, 387:599-610. 10.1056/NEJMoa2201662",
"volume": "387",
"year": "2022"
},
{
"DOI": "10.1001/jama.2022.18590",
"article-title": "Effect of ivermectin vs placebo on time to sustained recovery in outpatients with mild to moderate COVID-19: a randomized clinical trial",
"author": "Naggie S",
"doi-asserted-by": "publisher",
"journal-title": "JAMA",
"key": "ref59",
"unstructured": "Naggie S, Boulware DR, Lindsell CJ, et al.. Effect of ivermectin vs placebo on time to sustained recovery in outpatients with mild to moderate COVID-19: a randomized clinical trial. JAMA. 2022, 328:1595-603. 10.1001/jama.2022.18590",
"volume": "328",
"year": "2022"
},
{
"DOI": "10.1001/jama.2023.1650",
"article-title": "Effect of higher-dose ivermectin for 6 days vs placebo on time to sustained recovery in outpatients with COVID-19: a randomized clinical trial",
"author": "Naggie S",
"doi-asserted-by": "publisher",
"journal-title": "JAMA",
"key": "ref60",
"unstructured": "Naggie S, Boulware DR, Lindsell CJ, et al.. Effect of higher-dose ivermectin for 6 days vs placebo on time to sustained recovery in outpatients with COVID-19: a randomized clinical trial. JAMA. 2023, 329:888-97. 10.1001/jama.2023.1650",
"volume": "329",
"year": "2023"
},
{
"DOI": "10.1056/NEJMe2209017",
"article-title": "Time to stop using ineffective COVID-19 drugs",
"author": "Abdool Karim SS",
"doi-asserted-by": "publisher",
"journal-title": "N Engl J Med",
"key": "ref61",
"unstructured": "Abdool Karim SS, Devnarain N. Time to stop using ineffective COVID-19 drugs. N Engl J Med. 2022, 387:654-5. 10.1056/NEJMe2209017",
"volume": "387",
"year": "2022"
},
{
"DOI": "10.1186/s12985-022-01829-8",
"article-title": "Ivermectin under scrutiny: a systematic review and meta-analysis of efficacy and possible sources of controversies in COVID-19 patients",
"author": "Shafiee A",
"doi-asserted-by": "publisher",
"journal-title": "Virol J",
"key": "ref62",
"unstructured": "Shafiee A, Teymouri Athar MM, Kohandel Gargari O, Jafarabady K, Siahvoshi S, Mozhgani SH. Ivermectin under scrutiny: a systematic review and meta-analysis of efficacy and possible sources of controversies in COVID-19 patients. Virol J. 2022, 19:102. 10.1186/s12985-022-01829-8",
"volume": "19",
"year": "2022"
},
{
"DOI": "10.3390/computation10040051",
"article-title": "In silico analysis of the multi-targeted mode of action of ivermectin and related compounds",
"author": "Aminpour M",
"doi-asserted-by": "publisher",
"journal-title": "Computation",
"key": "ref63",
"unstructured": "Aminpour M, Cannariato M, Safaeeardebili ME, et al.. In silico analysis of the multi-targeted mode of action of ivermectin and related compounds. Computation. 2022, 10:51. 10.3390/computation10040051",
"volume": "10",
"year": "2022"
},
{
"DOI": "10.3390/ijms23052558",
"article-title": "A deadly embrace: hemagglutination mediated by SARS-COV-2 spike protein at its 22 n-glycosylation sites, red blood cell surface sialoglycoproteins, and antibody",
"author": "Scheim DE",
"doi-asserted-by": "publisher",
"journal-title": "Int J Mol Sci",
"key": "ref64",
"unstructured": "Scheim DE. A deadly embrace: hemagglutination mediated by SARS-COV-2 spike protein at its 22 n-glycosylation sites, red blood cell surface sialoglycoproteins, and antibody. Int J Mol Sci. 2022, 23:10.3390/ijms23052558",
"volume": "23",
"year": "2022"
},
{
"DOI": "10.21873/invivo.12134",
"article-title": "Ivermectin docks to the SARS-CoV-2 spike receptor-binding domain attached to ACE2",
"author": "Lehrer S",
"doi-asserted-by": "publisher",
"journal-title": "In Vivo",
"key": "ref65",
"unstructured": "Lehrer S, Rheinstein PH. Ivermectin docks to the SARS-CoV-2 spike receptor-binding domain attached to ACE2. In Vivo. 2020, 34:3023-6. 10.21873/invivo.12134",
"volume": "34",
"year": "2020"
},
{
"DOI": "10.3390/biologics2030015",
"article-title": "Changes in SpO2 on room air for 34 severe COVID-19 patients after ivermectin-based combination treatment: 62% normalization within 24 hours",
"author": "Stone JC",
"doi-asserted-by": "publisher",
"journal-title": "Biologics",
"key": "ref66",
"unstructured": "Stone JC, Ndarukwa P, Scheim DE, et al.. Changes in SpO2 on room air for 34 severe COVID-19 patients after ivermectin-based combination treatment: 62% normalization within 24 hours. Biologics. 2022, 2:196-210. 10.3390/biologics2030015",
"volume": "2",
"year": "2022"
},
{
"DOI": "10.2217/fmb-2022-0014",
"article-title": "Effectiveness of ivermectin-based multidrug therapy in severely hypoxic, ambulatory COVID-19 patients",
"author": "Hazan S",
"doi-asserted-by": "publisher",
"journal-title": "Future Microbiol",
"key": "ref67",
"unstructured": "Hazan S, Dave S, Gunaratne AW, Dolai S, Clancy RL, McCullough PA, Borody TJ. Effectiveness of ivermectin-based multidrug therapy in severely hypoxic, ambulatory COVID-19 patients. Future Microbiol. 2022, 17:339-50. 10.2217/fmb-2022-0014",
"volume": "17",
"year": "2022"
},
{
"DOI": "10.23937/2474-3658/1510233",
"article-title": "A randomized controlled trial of ivermectin monotherapy versus HCQ, IVM, and AZ combination therapy in Covid-19 patients in Nigeria",
"author": "Babalola OE",
"doi-asserted-by": "publisher",
"journal-title": "J Infect Dis Epidemiol",
"key": "ref68",
"unstructured": "Babalola OE, Ndanusa Y, Adesuyi A, Ogedengbe OJ, Thairu Y, Ogu O. A randomized controlled trial of ivermectin monotherapy versus HCQ, IVM, and AZ combination therapy in Covid-19 patients in Nigeria. J Infect Dis Epidemiol. 2021, 7:233. 10.23937/2474-3658/1510233",
"volume": "7",
"year": "2021"
},
{
"DOI": "10.2183/pjab.87.13",
"article-title": "Ivermectin, 'wonder drug' from Japan: the human use perspective",
"author": "Crump A",
"doi-asserted-by": "publisher",
"journal-title": "Proc Jpn Acad Ser B Phys Biol Sci",
"key": "ref69",
"unstructured": "Crump A, Ōmura S. Ivermectin, 'wonder drug' from Japan: the human use perspective. Proc Jpn Acad Ser B Phys Biol Sci. 2011, 87:13-28. 10.2183/pjab.87.13",
"volume": "87",
"year": "2011"
},
{
"DOI": "10.1086/653208",
"article-title": "Effect of ivermectin on Anopheles gambiae mosquitoes fed on humans: the potential of oral insecticides in malaria control",
"author": "Chaccour C",
"doi-asserted-by": "publisher",
"journal-title": "J Infect Dis",
"key": "ref70",
"unstructured": "Chaccour C, Lines J, Whitty CJ. Effect of ivermectin on Anopheles gambiae mosquitoes fed on humans: the potential of oral insecticides in malaria control. J Infect Dis. 2010, 202:113-6. 10.1086/653208",
"volume": "202",
"year": "2010"
},
{
"DOI": "10.1177/009127002401382731",
"article-title": "Safety, tolerability, and pharmacokinetics of escalating high doses of ivermectin in healthy adult subjects",
"author": "Guzzo CA",
"doi-asserted-by": "publisher",
"journal-title": "J Clin Pharmacol",
"key": "ref71",
"unstructured": "Guzzo CA, Furtek CI, Porras AG, et al.. Safety, tolerability, and pharmacokinetics of escalating high doses of ivermectin in healthy adult subjects. J Clin Pharmacol. 2002, 42:1122-33. 10.1177/009127002401382731",
"volume": "42",
"year": "2002"
},
{
"DOI": "10.1093/jac/dkz524",
"article-title": "Safety of high-dose ivermectin: a systematic review and meta-analysis",
"author": "Navarro M",
"doi-asserted-by": "publisher",
"journal-title": "J Antimicrob Chemother",
"key": "ref72",
"unstructured": "Navarro M, Camprubí D, Requena-Méndez A, et al.. Safety of high-dose ivermectin: a systematic review and meta-analysis. J Antimicrob Chemother. 2020, 75:827-34. 10.1093/jac/dkz524",
"volume": "75",
"year": "2020"
},
{
"DOI": "10.1080/10428194.2020.1786559",
"article-title": "Continuous high-dose ivermectin appears to be safe in patients with acute myelogenous leukemia and could inform clinical repurposing for COVID-19 infection",
"author": "de Castro CG Jr",
"doi-asserted-by": "publisher",
"journal-title": "Leuk Lymphoma",
"key": "ref73",
"unstructured": "de Castro CG Jr, Gregianin LJ, Burger JA. Continuous high-dose ivermectin appears to be safe in patients with acute myelogenous leukemia and could inform clinical repurposing for COVID-19 infection. Leuk Lymphoma. 2020, 61:2536-7. 10.1080/10428194.2020.1786559",
"volume": "61",
"year": "2020"
},
{
"DOI": "10.1016/j.eclinm.2021.100959",
"article-title": "Antiviral effect of high-dose ivermectin in adults with COVID-19: A proof-of-concept randomized trial",
"author": "Krolewiecki A",
"doi-asserted-by": "publisher",
"journal-title": "EClinicalMedicine",
"key": "ref74",
"unstructured": "Krolewiecki A, Lifschitz A, Moragas M, et al.. Antiviral effect of high-dose ivermectin in adults with COVID-19: A proof-of-concept randomized trial. EClinicalMedicine. 2021, 37:100959. 10.1016/j.eclinm.2021.100959",
"volume": "37",
"year": "2021"
},
{
"DOI": "10.1016/j.ijantimicag.2021.106516",
"article-title": "High-dose ivermectin for early treatment of COVID-19 (COVER study): a randomised, double-blind, multicentre, phase II, dose-finding, proof-of-concept clinical trial",
"author": "Buonfrate D",
"doi-asserted-by": "publisher",
"journal-title": "Int J Antimicrob Agents",
"key": "ref75",
"unstructured": "Buonfrate D, Chesini F, Martini D, et al.. High-dose ivermectin for early treatment of COVID-19 (COVER study): a randomised, double-blind, multicentre, phase II, dose-finding, proof-of-concept clinical trial. Int J Antimicrob Agents. 2022, 59:106516. 10.1016/j.ijantimicag.2021.106516",
"volume": "59",
"year": "2022"
},
{
"DOI": "10.5061/dryad.dv41ns1xr",
"article-title": "National Death Information System (SINADEF); this dataset, updated daily, was accessible by the public through December 2022. Frozen data snapshots as used here are available in the Dryad data repository at this URL",
"author": "Ministerio de Salud de Peru",
"doi-asserted-by": "publisher",
"key": "ref76",
"unstructured": "Ministerio de Salud de Peru. National Death Information System (SINADEF); this dataset, updated daily, was accessible by the public through December 2022. Frozen data snapshots as used here are available in the Dryad data repository at this URL. Dryad, USA; 2021. 10.5061/dryad.dv41ns1xr",
"year": "2021"
},
{
"key": "ref77",
"unstructured": "Estimated population by simple ages and age groups, according to department, province and district. Information Management Office, Ministry of Health, National Institute of Statistics and Informatics (INEI). National Censuses [Article in Spanish]. (2020). Accessed. September 27, 2022: https://www.gob.pe/institucion/inei/tema/censos."
},
{
"DOI": "10.1016/S1473-3099(20)30553-3",
"article-title": "Association between mobility patterns and COVID-19 transmission in the USA: a mathematical modelling study",
"author": "Badr HS",
"doi-asserted-by": "publisher",
"journal-title": "Lancet Infect Dis",
"key": "ref78",
"unstructured": "Badr HS, Du H, Marshall M, Dong E, Squire MM, Gardner LM. Association between mobility patterns and COVID-19 transmission in the USA: a mathematical modelling study. Lancet Infect Dis. 2020, 20:1247-54. 10.1016/S1473-3099(20)30553-3",
"volume": "20",
"year": "2020"
},
{
"DOI": "10.1186/s12889-022-13546-6",
"article-title": "The relative effects of non-pharmaceutical interventions on wave one Covid-19 mortality: natural experiment in 130 countries",
"author": "Stokes J",
"doi-asserted-by": "publisher",
"journal-title": "BMC Public Health",
"key": "ref79",
"unstructured": "Stokes J, Turner AJ, Anselmi L, Morciano M, Hone T. The relative effects of non-pharmaceutical interventions on wave one Covid-19 mortality: natural experiment in 130 countries. BMC Public Health. 2022, 22:1113. 10.1186/s12889-022-13546-6",
"volume": "22",
"year": "2022"
},
{
"DOI": "10.1038/s41598-021-92766-z",
"article-title": "Mobility restrictions were associated with reductions in COVID-19 incidence early in the pandemic: evidence from a real-time evaluation in 34 countries",
"author": "Oh J",
"doi-asserted-by": "publisher",
"journal-title": "Sci Rep",
"key": "ref80",
"unstructured": "Oh J, Lee HY, Khuong QL, et al.. Mobility restrictions were associated with reductions in COVID-19 incidence early in the pandemic: evidence from a real-time evaluation in 34 countries. Sci Rep. 2021, 11:13717. 10.1038/s41598-021-92766-z",
"volume": "11",
"year": "2021"
},
{
"DOI": "10.1038/s41467-020-19652-6",
"article-title": "State-level tracking of COVID-19 in the United States",
"author": "Unwin HJ",
"doi-asserted-by": "publisher",
"journal-title": "Nat Commun",
"key": "ref81",
"unstructured": "Unwin HJ, Mishra S, Bradley VC, et al.. State-level tracking of COVID-19 in the United States. Nat Commun. 2020, 11:6189. 10.1038/s41467-020-19652-6",
"volume": "11",
"year": "2020"
},
{
"key": "ref82",
"unstructured": "Wessa.net Free Statistics Software, Office for Research Development and Education, version 1.2.1. (2021). Accessed. July 3, 2023: https://www.wessa.net/."
},
{
"key": "ref83",
"unstructured": "Google COVID-19 Community Mobility Reports. (2020). Accessed. January 10, 2020: https://www.google.com/covid19/mobility/."
},
{
"key": "ref84",
"unstructured": "Peru confirms case of British variant of coronavirus. (January 9, 2020). https.//www.reuters.com/article/health-coronavirus-peru/peru-confirms-case-of-british-variant-of-coronavirus-idUSL1N2...."
},
{
"key": "ref85",
"unstructured": "Epidemiological update. occurrence of variants of SARS-CoV-2 in the Americas - 26 January 2021. (January 26, 2021). Accessed: July 3, 2023: https://www.paho.org/en/documents/epidemiological-update-occurrence-variants-sars-cov-2-americas-26-january-2021."
},
{
"key": "ref86",
"unstructured": "Covid-19. seroprevalence in the city of Iquitos is 75 percent. Andina Peruvian News Agency [Article in Spanish]. (September 25, 2020). Accessed: July 3, 2023: https://andina.pe/agencia/noticia-covid19-seroprevalencia-la-ciudad-iquitos-es-75-ciento-815197.aspx."
},
{
"key": "ref87",
"unstructured": "European Comission, Global Human Settlement, Urban centres database 2018 visualisation; Urban Centre Database UCDB R2019A. (November 30, 2020). Accessed. July 3, 2023: https://ghsl.jrc.ec.europa.eu/ucdb2018visual.php."
},
{
"key": "ref88",
"unstructured": "National Institute of Statistics and Informatics (INEI), National Censuses [Article in Spanish]. (2017). Accessed. July 3, 2023: https://www.inei.gob.pe/media/MenuRecursivo/publicaciones_digitales/Est/Lib1539/cap06.pdf."
},
{
"DOI": "10.1038/s41598-021-92263-3",
"article-title": "The impact of super-spreader cities, highways, and intensive care availability in the early stages of the COVID-19 epidemic in Brazil",
"author": "Nicolelis MA",
"doi-asserted-by": "publisher",
"journal-title": "Sci Rep",
"key": "ref89",
"unstructured": "Nicolelis MA, Raimundo RL, Peixoto PS, Andreazzi CS. The impact of super-spreader cities, highways, and intensive care availability in the early stages of the COVID-19 epidemic in Brazil. Sci Rep. 2021, 11:13001. 10.1038/s41598-021-92263-3",
"volume": "11",
"year": "2021"
},
{
"key": "ref90",
"unstructured": "Ministry of Health, National Center for Epidemiology, Disease Prevention and Control, Health Situation Room. Ministry of Health, National Epidemiology Center [Article in Spanish]. (2020). Accessed. July 3, 2023: https://www.dge.gob.pe/portal/docs/vigilancia/sala/2020/SE13/dengue.pdf."
},
{
"article-title": "Dengue in Peru: a quarter of a century after its reemergence [Article in Spanish]",
"author": "Cabezas C",
"journal-title": "Revista Peruana de Medicina Experimental y Salud Publica",
"key": "ref91",
"unstructured": "Cabezas C, Fiestas V, García-Mendoza M, Palomino M, Mamani E, Donaires F. Dengue in Peru: a quarter of a century after its reemergence [Article in Spanish]. Revista Peruana de Medicina Experimental y Salud Publica. 2015, 32:146-56.",
"volume": "32",
"year": "2015"
},
{
"article-title": "Use of ivermectin as a prophylactic option in asymptomatic family close contacts with patients of COVID-19 (NCT number: 04422561)",
"author": "Shouman W",
"journal-title": "J Clin Diagnostic Res",
"key": "ref92",
"unstructured": "Shouman W, Hegazy A, Nafae R, et al.. Use of ivermectin as a prophylactic option in asymptomatic family close contacts with patients of COVID-19 (NCT number: 04422561). J Clin Diagnostic Res. 2021, 15:27.",
"volume": "15",
"year": "2021"
},
{
"DOI": "10.1097/MJT.0000000000001433",
"article-title": "Intensive treatment with ivermectin and iota-carrageenan as pre-exposure prophylaxis for COVID-19 in health care workers from Tucuman, Argentina",
"author": "Chahla RE",
"doi-asserted-by": "publisher",
"journal-title": "Am J Ther",
"key": "ref93",
"unstructured": "Chahla RE, Medina Ruiz L, Ortega ES, et al.. Intensive treatment with ivermectin and iota-carrageenan as pre-exposure prophylaxis for COVID-19 in health care workers from Tucuman, Argentina. Am J Ther. 2021, 28:e601-4. 10.1097/MJT.0000000000001433",
"volume": "28",
"year": "2021"
},
{
"key": "ref94",
"unstructured": "The SAIVE Trial, post-exposure use of ivermectin in COVID-19 prevention. efficacy and safety results. Desort-Henin V, Kostova A, Babiker EA, Caramel A, Malamut R. Poster presentation, European Congress of Clinical Microbiology and Infectious Diseases. (April 18, 2023). Accessed: May 16, 2023: https://www.medincell.com/wp-content/uploads/2023/04/Poster-SAIVE-April2023-OK3.pdf."
},
{
"DOI": "10.1016/j.ijid.2021.04.035",
"article-title": "Positive impact of oral hydroxychloroquine and povidone-iodine throat spray for COVID-19 prophylaxis: An open-label randomized trial",
"author": "Seet RC",
"doi-asserted-by": "publisher",
"journal-title": "Int J Infect Dis",
"key": "ref95",
"unstructured": "Seet RC, Quek AM, Ooi DS, et al.. Positive impact of oral hydroxychloroquine and povidone-iodine throat spray for COVID-19 prophylaxis: An open-label randomized trial. Int J Infect Dis. 2021, 106:314-22. 10.1016/j.ijid.2021.04.035",
"volume": "106",
"year": "2021"
},
{
"DOI": "10.7759/cureus.28624",
"article-title": "Regular use of ivermectin as prophylaxis for COVID-19 led up to a 92% reduction in COVID-19 mortality rate in a dose-response manner: results of a prospective observational study of a strictly controlled population of 88,012 subjects",
"author": "Kerr L",
"doi-asserted-by": "publisher",
"journal-title": "Cureus",
"key": "ref96",
"unstructured": "Kerr L, Baldi F, Lobo R, et al.. Regular use of ivermectin as prophylaxis for COVID-19 led up to a 92% reduction in COVID-19 mortality rate in a dose-response manner: results of a prospective observational study of a strictly controlled population of 88,012 subjects. Cureus. 2022, 14:e28624. 10.7759/cureus.28624",
"volume": "14",
"year": "2022"
},
{
"key": "ref97",
"unstructured": "Uttar Pradesh going the last mile to stop COVID-19. World Health Organization. (May 7, 2021). Accessed. July 3, 2023: https://www.who.int/india/news/feature-stories/detail/uttar-pradesh-going-the-last-mile-to-stop-covid-19."
},
{
"DOI": "10.1016/j.jtemb.2022.126954",
"article-title": "Zinc augments the antiviral potential of HCQ/CQ and ivermectin to reduce the risks of more serious outcomes from COVID-19 infection",
"author": "Boretti A",
"doi-asserted-by": "publisher",
"journal-title": "J Trace Elem Med Biol",
"key": "ref98",
"unstructured": "Boretti A. Zinc augments the antiviral potential of HCQ/CQ and ivermectin to reduce the risks of more serious outcomes from COVID-19 infection. J Trace Elem Med Biol. 2022, 71:126954. 10.1016/j.jtemb.2022.126954",
"volume": "71",
"year": "2022"
},
{
"key": "ref99",
"unstructured": "The miracle not-heard around the world. the success of Uttar Pradesh - part 3. (August 14, 2022). Accessed: July 3, 2023: https://pierrekory.substack.com/p/the-miracle-not-heard-around-the-1ee."
},
{
"key": "ref100",
"unstructured": "Spreadsheet and screenshots of COVID-19 deaths, 7-day averages for selected dates, in Uttar Pradesh, all of India, and the United States, with underlying data (https.//covid19.healthdata.org/) from The Institute for Health Metrics and Evaluation (IHME) at the University of Washington (Seattle, USA). Accessed: July 3, 2023: https://drive.google.com/file/d/1ww64SlaXI5KPgmzNf2aNu6pVxJvjVCVJ/view?usp=drive_link."
},
{
"key": "ref101",
"unstructured": "Indian state will offer ivermectin to entire adult population — even as WHO warns against its use as COVID-19 treatment. (2021). Accessed. July 3, 2023: https://www.forbes.com/sites/siladityaray/2021/05/11/indian-state-will-offer-ivermectin-to-entire-adult-population---...."
},
{
"DOI": "10.1016/j.futures.2021.102860",
"article-title": "Science, the endless frontier of regulatory capture",
"author": "Saltelli A",
"doi-asserted-by": "publisher",
"journal-title": "Futures",
"key": "ref102",
"unstructured": "Saltelli A, Dankel DJ, Di Fiore M, Holland N, Pigeon M. Science, the endless frontier of regulatory capture. Futures. 2022, 135:102860. 10.1016/j.futures.2021.102860",
"volume": "135",
"year": "2022"
},
{
"DOI": "10.1016/j.jclinepi.2016.02.012",
"article-title": "Evidence-based medicine has been hijacked: a report to David Sackett",
"author": "Ioannidis JP",
"doi-asserted-by": "publisher",
"journal-title": "J Clin Epidemiol",
"key": "ref103",
"unstructured": "Ioannidis JP. Evidence-based medicine has been hijacked: a report to David Sackett. J Clin Epidemiol. 2016, 73:82-6. 10.1016/j.jclinepi.2016.02.012",
"volume": "73",
"year": "2016"
},
{
"key": "ref104",
"unstructured": "Merck’s deadly Vioxx playbook, redux. a debunked smear campaign against its competing drug—the FDA-approved, Nobel prize-honored ivermectin (Scheim DE). (September 7, 2021). Accessed: July 3, 2023: https://trialsitenews.com/mercks-deadly-vioxx-playbook-redux-a-debunked-smear-campaign-against-its-competing-drug-the...."
},
{
"key": "ref105",
"unstructured": "The 2015 Nobel Prize in Physiology or Medicine, Press release, Solna, Sweden. The Nobel Assembly at Karolinska Institutet. (October 5, 2015). Accessed: July 3, 2023: https://www.nobelprize.org/prizes/medicine/2015/press-release/."
},
{
"key": "ref106",
"unstructured": "Ivermectin for COVID-19 in Peru. 14-fold reduction in nationwide excess deaths, p<0.002 for effect by state, then 13-fold increase after ivermectin use restricted (Chamie-Quintero JJ, Hibberd JA, Scheim DE) [PREPRINT]. (2021). Accessed: July 3, 2023: https://osf.io/9egh4/."
}
],
"reference-count": 106,
"references-count": 106,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.cureus.com/articles/172991-covid-19-excess-deaths-in-perus-25-states-in-2020-nationwide-trends-confounding-factors-and-correlations-with-the-extent-of-ivermectin-treatment-by-state"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Aerospace Engineering"
],
"subtitle": [],
"title": "COVID-19 Excess Deaths in Peru’s 25 States in 2020: Nationwide Trends, Confounding Factors, and Correlations With the Extent of Ivermectin Treatment by State",
"type": "journal-article"
}