The Place of Ivermectin in the Management of Covid-19: State of the Evidence
PhD Ajayi A A Md
doi:10.18103/mra.v
Background and aims. The covid 19 pandemic necessitated the use of old, repurposed, and new drugs, in addition to vaccines and public health measures. There are still many controversies about the efficacy and impact of some of the medications used, which need further elucidation. We review the pharmacological properties and the place of the repurposed drug, Ivermectin (IVM) in the prophylaxis and treatment of SARS -CoV-2 (severe acute respiratory syndrome coronavirus 2.) infection or Covid 19 disease.
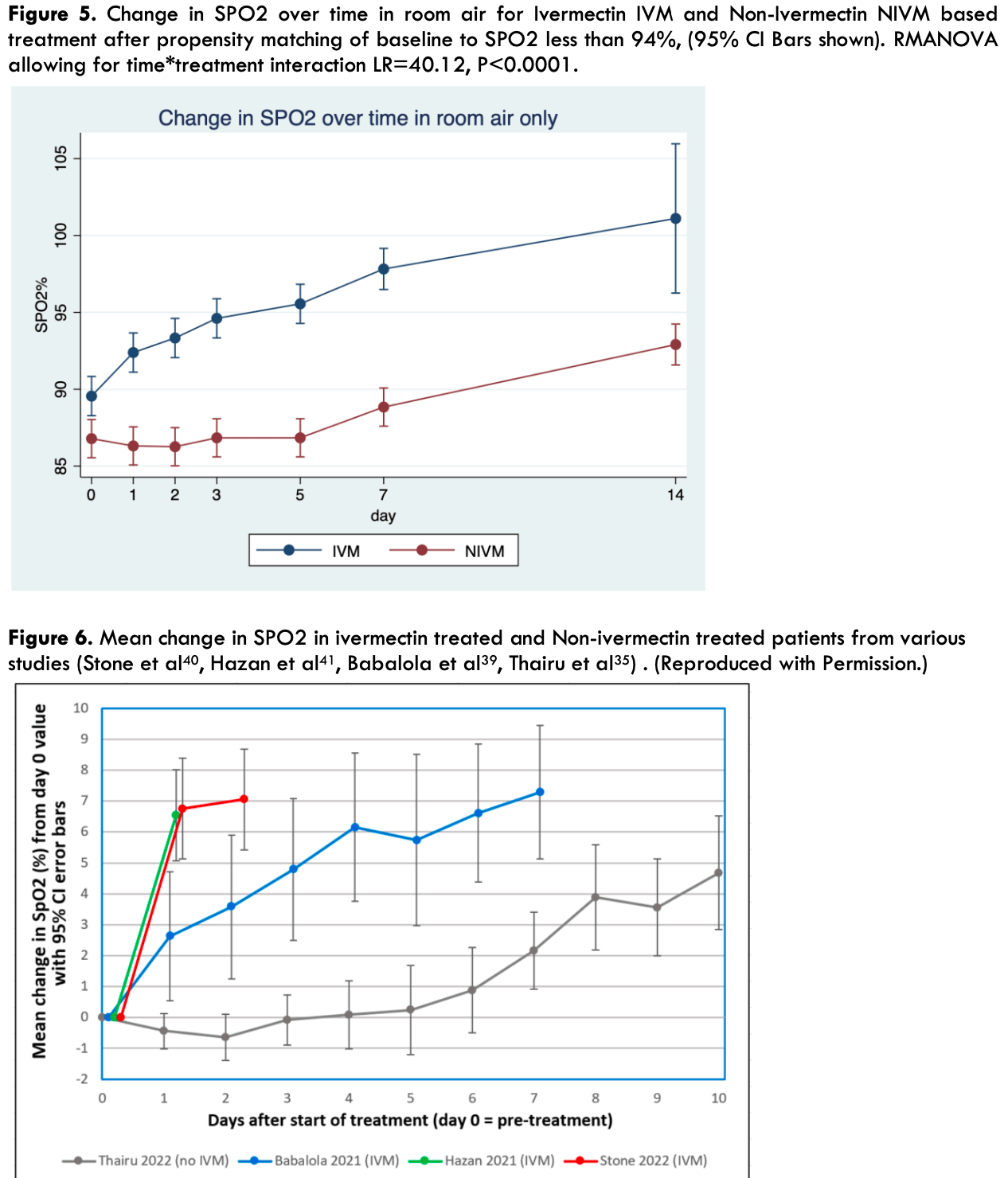
Major findings: in-vitro, in-vivo, and human studies In vitro studies in Vero/hSlam cells caused a 99.98 % inhibition of SARS -Cov-2 (5000-fold) within 48 hours. The IC50 (half maximal inhibitory concentration) for this virucidal action was 2.8μM, which was thought unattainable in humans in-vivo. Thus, there was initial skepticism on pharmacokinetic grounds as to possible efficacy of IVM in humans with Covid 19. There are, however, a multiplicity of anti-covid 19 mechanisms, beyond mere anti-viral effects, such as blockade of ACE2 receptor viral entry, and the anti-cytokine and anti-inflammatory effects of IVM. IVM has a long half-life of 18 -24 hours, Mean Residence Time (MRT) of 3.4 days and a preferential site of lung accumulation. In-vivo studies in Syrian Golden hamsters confirmed the symptomatic, antiinflammatory, anti-cytokine, histopathological and survival benefit of IVM, which was more manifest in female animals. In a January 2023 meta-analysis of studies (s) in total number of patients (n) for various parameters (p), the reduction in risk relative to placebo or controls were as follows: 1. Overall improvement (s= 95) (n= 134,554) was 62% [95%CI 54-69]. 2. Mortality (s=48) (n=120,000) there was 51% reduction [95%CI 37-62]. 3. Hospitalization (s=29) (n = 44, 784), there was 34% reduction [ 95% CI 20-45]. 4. Viral clearance (s=20) (n= 3945) there was 45% reduction [95%CI 31-55]. 5. Prophylaxis (s=17) (n=19,764) showed 82% reduction [95%CI 73-88] 6 Randomised Control Trial (RCT) studies (s= 45) (n=2173) showed a 54 % mortality reduction [95% CI 39-65] In addition, IVM has been shown in studies to cause a rapid reversal of hypoxemia (SPO2 < 94%) and a rapid increase in SPO2, an effect exhibiting a gender dichotomy. (SPO2 is the percentage of the maximum carrying capacity of the blood). This effect on SPO2 has been attributed to IVM's reversal and prevention of SARS-CoV-2 virus induced hemagglutination. The dosage used for treatment of covid 19 varied widely within studies, but doses of 200-400 μg/kg twice weekly or daily for 5 consecutive days, caused significant viral clearance and clinical improvement, with minimal safety concerns. For prophylaxis, a dose of 200μg/kg for two consecutive days every 15 days was found effective in studies.
Conclusion: This review provides powerful evidence that IVM is efficacious singly or as a part of a regimen for covid 19. IVM could potentially be combined with newer oral anti-covid 19 agents, such as..
References
Ajayi, Drugs Shown to Inhibit SARS-CoV-2 in COVID-19 Disease: Comparative Basic and Clinical Pharmacology of Molnupiravir and Ivermectin, Austin J Pharmacol Ther
Alam, Murshed, Gomes, Masud, Ivermectin as Pre-exposure Prophylaxis for COVID-19 among Healthcare Providers in a Selected Tertiary Hospital in Dhaka -An Observational Study, European Journal of Medical and Health Sciences,
doi:10.24018/ejmed.2020.2.6.599Annunziata, Coppola, Carannante, Simioli, Lanza, Home management of patients with moderate or severe respiratory failure secondary to COVID-19, using remote monitoring and oxygen with or without HFNC, Pathogens
Arshad, Pertinez, Box, Prioritization of Anti-SARS-Cov-2 drug repurposing opportunities based on plasma and target site concentrations derived from their established human pharmacokinetics, Clin Pharmacol Ther,
doi:10.1002/cpt.1909Babalola, Ajayi, Yunusa, Ndanusa, Ogedengbe et al., Ivermectin is associated with Increase in SPO 2 in Hypoxemic SARS-CoV-2 Patients: Pharmacodynamic Profile and Correlates, J Clin Chem Lab Med
Babalola, Bode, Ajayi, Alakaloko, Akase et al., Ivermectin shows clinical benefits in mild to moderate COVID19: a randomized controlled double-blind, dose-response study in Lagos, QJM: An International Journal of Medicine,
doi:10.1093/qjmed/hcab035Behera, Patro, Padhy, Prophylactic Role of Ivermectin in Severe Acute Respiratory Syndrome Coronavirus 2 Infection Among Healthcare Workers, Cureus,
doi:10.7759/cureus.16897Behera, Patro, Singh, Chandanshive, Pradhan, Role of ivermectin in the prevention of SARS-CoV-2 infection among healthcare workers in India: A matched case-control study, PLoS ONE,
doi:10.1371/journal.pone.0247163Boschi, Scheim, Bancod, Militello, Bideau et al., SARS-CoV-2 Spike Protein Induces Hemagglutination: Implications for COVID-19 Morbidities and Therapeutics and for Vaccine Adverse Effects, International Journal of Molecular Sciences,
doi:10.3390/ijms232415480Butler, Molnupiravir plus usual care versus usual care alone as early treatment for adults with COVID-19 at increased risk of adverse outcomes (PANORAMIC): an openlabel, platform-adaptive randomised controlled trial, The Lancet
Caly, Druce, Catton, Jans, Wagstaff et al., Ivermectin as a potential COVID-19 treatment from the pharmacokinetic point of view: antiviral levels are not likely attainable with known dosing regimens, Biotechnology & Biotechnological Equipment,
doi:10.1016/j.antiviral.2020.104787Canga, Prieto, Liébana, Martínez, Vega et al., The pharmacokinetics and interactions of ivermectin in humans--a mini-review, AAPS J,
doi:10.1208/s12248-007-9000-9Canga, Prieto, Liébana, Martínez, Vega et al., The pharmacokinetics and interactions of ivermectin in humans--a mini-review, AAPS J,
doi:10.1208/s12248-007-9000-9Carvallo, Study of the Efficacy and Safety of Topical Ivermectin + Iota-Carrageenan in the Prophylaxis against COVID-19 in Health Personnel,
doi:10.31546/2633-8653Coffeng, Stolk, Zouré, Veerman, Agblewonu et al., African Programme For Onchocerciasis Control 1995-2015: modelestimated health impact and cost, PLoS Negl Trop Dis,
doi:10.1371/journal.pntd.0002032Crump, Ōmura, Ivermectin, drug' from Japan: the human use perspective, Proc Jpn Acad Ser B Phys Biol Sci,
doi:10.2183/pjab.87.13Dias De Melo, Lazarini, Marchio, Pineau, Lecuit et al., Attenuation of clinical and immunological outcomes during SARS-CoV-2 infection by ivermectin, Embo Molecular Medicine,
doi:10.15252/Emmm202114122Espitia-Hernandez, Effects of Ivermectinazithromycin-cholecalciferol combined therapy on COVID-19 infected patients: A proof of concept study
Gottlieb, Vaca, Paredes, Mera, Webb et al., Early Remdesivir to Prevent Progression to Severe Covid-19 in Outpatients, N Engl J Med,
doi:10.1056/NEJMoa2116846Hay, Arnott, Ivermectin and coagulation: an in vitro study, Ann Trop Med Parasitol
Hazan, Dave, Gunaratne, Dolai, Clancy et al., Effectiveness of ivermectin-based multidrug therapy in severely hypoxic, ambulatory COVID-19 patients, Future Microbiol
Hazan, Mmicrobiome -based hypothesis on Ivermectin Mechanisms in COVID -19 : Ivermectin feeds bifidobacteria to boost immunity
Justus, Ivermectin obliterates 97% of Delhi cases
Kerr, Cadegiani, Baldi, Ivermectin Prophylaxis Used for COVID-19: A Citywide, Prospective, Observational Study of 223,128 Subjects Using Propensity Score Matching, Cureus,
doi:10.7759/cureus.21272Kory, Meduri, Varon, Jose, Marik, Review of the Emerging Evidence Demonstrating the Efficacy of Ivermectin in the Prophylaxis and Treatment of COVID-19, American Journal of Therapeutics,
doi:10.1097/MJT.0000000000001377Krolewiecki, Lifschitz, Moragas, Travacio, Valentini et al., Antiviral effect of high-dose ivermectin in adults with COVID-19: A proof-of-concept randomized trial, EClinicalMedicine,
doi:10.1016/j.eclinm.2021.100959Lehrer, Rheinstein, Ivermectin Docks to the SARS-CoV-2 Spike Receptor-binding Domain Attached to ACE2, Vivo,
doi:10.21873/invivo.12134Marathea, Mendez-Lopeza, Potential impact of 5 years of ivermectin mass drug administration on malaria outcomes in high burden countries, BMJ Global Health2021
Mohan, Tiwari, Suri, Single-dose oral ivermectin in mild and moderate COVID-19 (RIVET-COV): A single-centre randomized, placebo-controlled trial, Journal of Infection and Chemotherapy,
doi:10.1016/j.jiac.2021.08.021Najjar-Debbiny, Gronich, Weber, Effectiveness of Paxlovid in reducing severe coronavirus disease 2019 and mortality in highrisk patients, Clin Infect Dis,
doi:10.1093/cid/ciac443€PMID:35653428Osman, Farouk, Osman, Abdrabou, Longitudinal assessment of chest computerized tomography and oxygen saturation for patients with COVID-19, The Egyptian Journal of Radiology and Nuclear Medicine
Richards, Mcneeley, Bryan, Ivermectin and prothrombin time, The Lancet
Santin, Scheim, Mccullough, Yagisawa, Borody, Ivermectin: a multifaceted drug of Nobel prize-honored distinction with indicated efficacy against a new global scourge, COVID-19, New Microbes and New Infections
Scheim, A Deadly Embrace: Hemagglutination Mediated by SARS-CoV-2
Seth, Mas, Conod, Mueller, Siems et al., LongLasting WNT-TCF response blocking and epigenetic modifying activities of withanolide f in human cancer cells, PLoS One
Shah, Joyce, Plumb, Paxlovid is associated with decreased hospitalization rate among adults with Covid-19-United States, April-September 2022, MMWR MORB MORTAL WKLY REP,
doi:10.15585/mmwr.mm7148e2Shuhan, Xinxin, Chen, Chi, Xiangchao et al., Anti-inflammatory effects of ivermectin in mouse model of allergic asthma, Inflamm Res,
doi:10.1007/s00011-011-0307-8Spagnuolo, Voarino, Tonelli, Galli, Poli et al., Impact of Remdesivir on SARS-CoV-2 Clearance in a Real-Life Setting: A Matched-Cohort Study, Drug Des Devel Ther,
doi:10.2147/DDDT.S369473Stone, Ndarukwa, Scheim, Dancis, Dancis et al., Changes in SpO2 on Room Air for 34 Severe COVID-19 Patients after Ivermectin-Based Combination Treatment: 62% Normalization within 24 Hours, Biologics,
doi:10.3390/biologics2030015Suputtamongkol, Avirutnan, Mairiang, Angkasekwinai, Niwattayakul et al., Ivermectin Accelerates Circulating Nonstructural Protein 1 (NS1) Clearance in Adult Dengue Patients: A Combined Phase 2/3
Thacker, The covid-19 lab leak hypothesis: did the media fall victim to a misinformation campaign?, BMJ2021,
doi:10.1136/bmj.n1656Thairu, Babalola, Ajayi, A Comparison of Ivermectin and Non Ivermectin Based Regimen for COVID-19 in Abuja: Effects on Virus Clearance, Days-todischarge and Mortality, Journal of Pharmaceutical Research International,
doi:10.9734/jpri/2022/v34i44A36328Venco, Mccall, Guerrero, Genchi, Efficacy of long-term monthly administration of ivermectin on the progress of naturally acquired heartworm infections in dogs, Vet Parasitol,
doi:10.1016/j.vetpar.2004.06.024Wagstaff, Ivermectin Global Summit
Whitworth, Hay, Mcnicholas, Morgan, Maude et al., Coagulation abnormalities and ivermectin, Ann Trop Med Parasitol
Yan, Ci, Chen, Anti-Inflammatory effects of ivermectin in mouse model of allergic asthma, Inflamm Res
Yang, Atkinson, Wang, Lee, Bogoyevitch et al., The broad spectrum antiviral ivermectin targets the host nuclear transport importin α/β1 heterodimer, Antivir Res
Zhang, Song, Ci, Ivermectin inhibits LPS-induced production of inflammatory cytokines and improves LPS-induced survival in mice, Inflamm Res,
doi:10.1007/s00011-008-8007-Zhang, Song, Ci, Ju, Li, Ivermectin Inhibits LPS-induced Production of Inflammatory Cytokines and Improves LPSinduced Survival in Mice, Inflamm Res,
doi:10.1007/s00011-008-8007-8Zou, Peng, Shu, Zhao, Lan et al., Antiviral Efficacy and Safety of Molnupiravir Against Omicron Variant Infection: A Randomized Controlled Clinical Trial, Front Pharmacol,
doi:10.3389/fphar.2022.939573DOI record:
{
"DOI": "10.18103/mra.v11i4.3778",
"ISSN": [
"2375-1916",
"2375-1924"
],
"URL": "http://dx.doi.org/10.18103/mra.v11i4.3778",
"abstract": "<jats:p>Background and aims. The covid 19 pandemic necessitated the use of old, repurposed, and new drugs, in addition to vaccines and public health measures. There are still many controversies about the efficacy and impact of some of the medications used, which need further elucidation. We review the pharmacological properties and the place of the repurposed drug, Ivermectin (IVM) in the prophylaxis and treatment of SARS - CoV- 2 (severe acute respiratory syndrome coronavirus 2.) infection or Covid 19 disease. Major findings: in-vitro, in-vivo, and human studies In vitro studies in Vero/hSlam cells caused a 99.98 % inhibition of SARS - Cov-2 (5000-fold) within 48 hours. The IC50 (half maximal inhibitory concentration) for this virucidal action was 2.8μM, which was thought unattainable in humans in-vivo. Thus, there was initial skepticism on pharmacokinetic grounds as to possible efficacy of IVM in humans with Covid 19. There are, however, a multiplicity of anti-covid 19 mechanisms, beyond mere anti-viral effects, such as blockade of ACE2 receptor viral entry, and the anti-cytokine and anti-inflammatory effects of IVM. IVM has a long half-life of 18 - 24 hours, Mean Residence Time (MRT) of 3.4 days and a preferential site of lung accumulation. In-vivo studies in Syrian Golden hamsters confirmed the symptomatic, anti-inflammatory, anti-cytokine, histopathological and survival benefit of IVM, which was more manifest in female animals. In a January 2023 meta-analysis of studies (s) in total number of patients (n) for various parameters (p), the reduction in risk relative to placebo or controls were as follows: Overall improvement (s= 95) (n= 134,554) was 62% [95%CI 54-69]. Mortality (s=48) (n=120,000) there was 51% reduction [95%CI 37-62]. Hospitalization (s=29) (n = 44, 784), there was 34% reduction [ 95% CI 20-45]. Viral clearance (s=20) (n= 3945) there was 45% reduction [95%CI 31-55]. Prophylaxis (s=17) (n=19,764) showed 82% reduction [95%CI 73-88] 6 Randomised Control Trial (RCT) studies (s= 45) (n=2173) showed a 54 % mortality reduction [95% CI 39-65] In addition, IVM has been shown in studies to cause a rapid reversal of hypoxemia (SPO2 < 94%) and a rapid increase in SPO2, an effect exhibiting a gender dichotomy. (SPO2 is the percentage of the maximum carrying capacity of the blood). This effect on SPO2 has been attributed to IVM’s reversal and prevention of SARS-CoV-2 virus induced hemagglutination. The dosage used for treatment of covid 19 varied widely within studies, but doses of 200-400 μg/kg twice weekly or daily for 5 consecutive days, caused significant viral clearance and clinical improvement, with minimal safety concerns. For prophylaxis, a dose of 200μg/kg for two consecutive days every 15 days was found effective in studies. Conclusion: This review provides powerful evidence that IVM is efficacious singly or as a part of a regimen for covid 19. IVM could potentially be combined with newer oral anti-covid 19 agents, such as Paxlovid, for effective and life-saving regimen in patients infected with covid-19. The anti-viral properties of these drugs can synergize with the anti-inflammatory and anti-cytokine properties of Ivermectin. Ivermectin is also useful prophylactically, especially where vaccines are unavailable or undesirable.</jats:p>",
"author": [
{
"affiliation": [],
"family": "O.E",
"given": "Babalola",
"sequence": "first"
},
{
"affiliation": [],
"family": "A.A",
"given": "Ajayi",
"sequence": "additional"
}
],
"container-title": "Medical Research Archives",
"container-title-short": "MRAJ",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2023,
5,
1
]
],
"date-time": "2023-05-01T17:14:06Z",
"timestamp": 1682961246000
},
"deposited": {
"date-parts": [
[
2023,
5,
1
]
],
"date-time": "2023-05-01T17:15:12Z",
"timestamp": 1682961312000
},
"indexed": {
"date-parts": [
[
2023,
5,
2
]
],
"date-time": "2023-05-02T04:23:36Z",
"timestamp": 1683001416473
},
"is-referenced-by-count": 0,
"issue": "4",
"issued": {
"date-parts": [
[
2023
]
]
},
"journal-issue": {
"issue": "4",
"published-online": {
"date-parts": [
[
2023
]
]
},
"published-print": {
"date-parts": [
[
2023
]
]
}
},
"member": "7483",
"original-title": [],
"prefix": "10.18103",
"published": {
"date-parts": [
[
2023
]
]
},
"published-online": {
"date-parts": [
[
2023
]
]
},
"published-print": {
"date-parts": [
[
2023
]
]
},
"publisher": "Knowledge Enterprise Journals",
"reference-count": 0,
"references-count": 0,
"relation": {},
"resource": {
"primary": {
"URL": "https://esmed.org/MRA/mra/article/view/3778"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"General Arts and Humanities"
],
"subtitle": [],
"title": "The Place of Ivermectin in the Management of Covid-19: State of the Evidence",
"type": "journal-article",
"volume": "11"
}