Ivermectin as a possible treatment for COVID-19: a review of the 2022 protocols
L L M Marques, S C Beneti, C Pinzon, F A R Cardoso
Brazilian Journal of Biology, doi:10.1590/1519-6984.258325
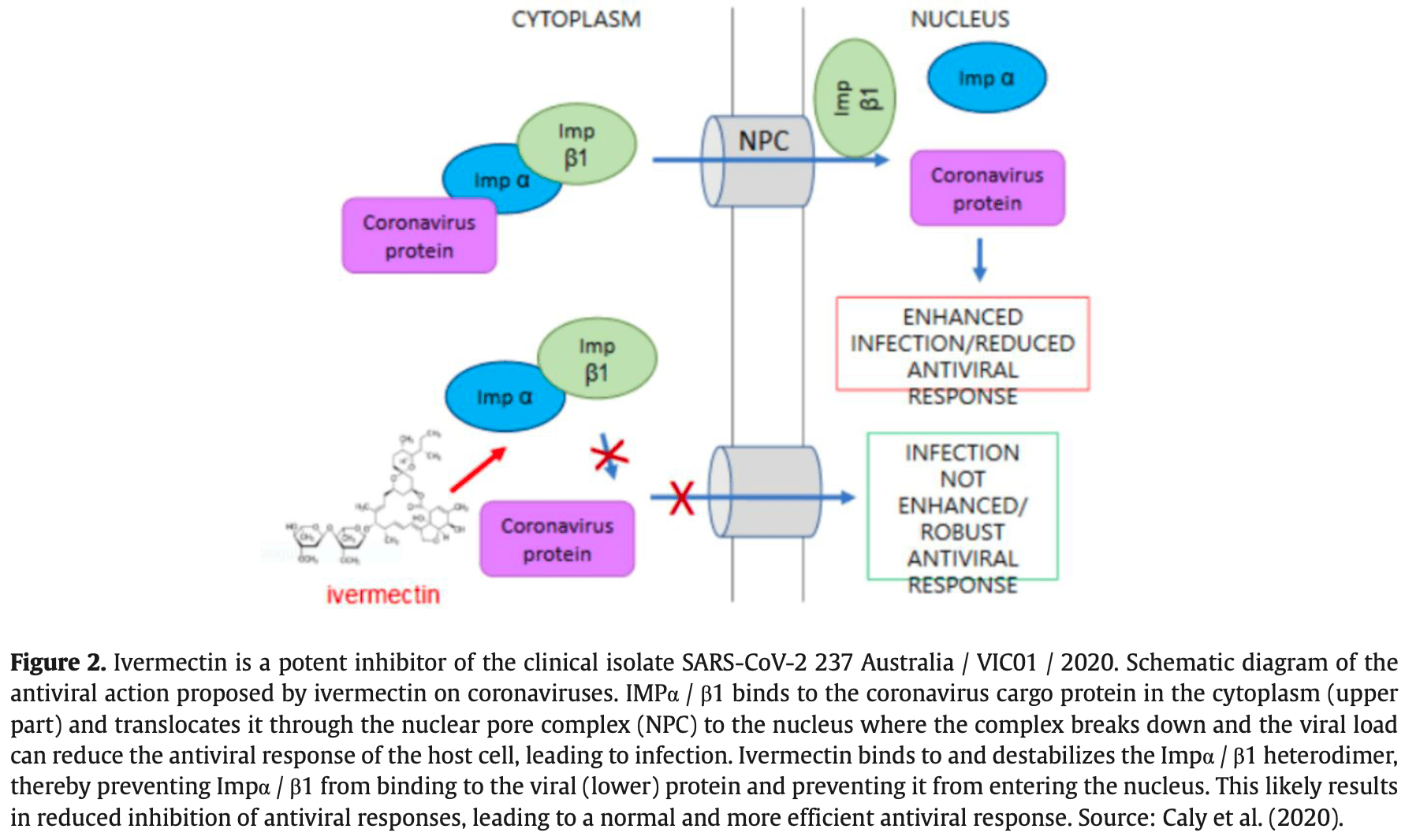
Ivermectin is a safe and effective drug in humans and has been approved for use in numerous parasitic infections for over 50 years. In addition, many studies have already shown its antiviral activity. Ivermectin is generally well tolerated, with no indication of central nervous system-associated toxicity at doses up to 10 times the highest FDAapproved dose of 200 µg/kg. The in vitro results of ivermectin for reducing SARS-CoV-2 viral load are promising and show that Ivermectin kills SARS-CoV-2 within 48 hours. A hypothesized mechanism of action for this drug is a likely inhibition of IMPα/β1-mediated nuclear import of viral proteins as demonstrated for other RNA viruses. However, controlled and randomized studies are needed to prove its effectiveness in COVID-19 in humans. In a single in vivo study with published results, patients confirmed to be infected with SARS-CoV-2 received at least one dose of ivermectin at any time during hospitalization. The use of ivermectin was associated with lower mortality during treatment with COVID-19, especially in patients who required increased inspired oxygen or ventilatory support. Additionally, 81 studies with the clinical use of ivermectin in humans are being carried out worldwide according to ClinicalTrials.gov. However, none of these data has been published so far. However, private and public entities in Brazil have been adopting this drug in their protocols as prophylaxis and in the initial phase of the disease. In addition, ivermectin has been used in mass treatment to prevent onchocerciasis and lymphatic filariasis in sub-Saharan Africa for many years. Surprisingly, this region has the lowest proportional mortality rate among the continents, despite the increasing numbers of infected people released by the World Health Organization.
References
Awadzi, Opoku, Addy, Quartey, The chemotherapy of onchocerciasis XIX: the clinical and laboratory tolerance of-high dose ivermectin
Baraka, Mahmoud, Marschke, Geary, Homeida et al., Ivermectin distribution in the plasma and tissues of patients infected with Onchocerca volvulus, European Journal of Clinical Pharmacology,
doi:10.1007/s002280050131Bezerra, Medeiros, Mota, Costa, Protocolo de manejo para síndromes gripais frente à pandemia de coronavírus (COVID-19)
Brasil, Dispõe sobre o protocolo para o uso dos medicamentos Ivermectina e Cloroquina/Hidroxicloroquina nos pacientes com suspeita ou confirmação de COVID-19
Brasil, None
Bryant, Lawrie, Dowswell, Fordham, Mitchell et al., Ivermectin for prevention and treatment of COVID-19 Infection: a systematic review, meta-analysis, and trial sequential analysis to inform clinical guidelines, American Journal of Therapeutics,
doi:10.1097/MJT.0000000000001402Cascella, Rajnik, Cuomo, Dulebohn, Napoli, Features, evaluation and treatment coronavirus (COVID-19)
Chaccour, Casellas, Blanco-Di, Matteo, Pineda et al., The effect of early treatment with ivermectin on viral load, symptoms and humoral response in patients with non-severe COVID-19: a pilot, double-blind, placebo-controlled, randomized clinical trial, EClinicalMedicine,
doi:10.1016/j.eclinm.2020.100720Chaccour, Hammann, Rabinovich, Ivermectin to reduce malaria transmission I. Pharmacokinetic and pharmacodynamic considerations regarding efficacy and safety, Malaria Journal,
doi:10.1186/s12936-017-1801-4Chaccour, Ruiz-Castillo, Richardson, Moncunill, Casellas et al., The SARS-CoV-2 ivermectin Navarra-ISGlobal Trial (SAINT) to evaluate the potential of ivermectin to reduce COVID-19 transmission in low risk, non-severe COVID-19 patients in the first 48 hours after symptoms onset: a structured summary of a study protocol, Trials,
doi:10.1186/s13063-020-04421-zCrump, Ivermectin: enigmatic multifaceted 'wonder' drug continues to surprise and exceed expectations, The Journal of Antibiotics,
doi:10.1038/ja.2017.11Daley, %E2%80%93%20shared,centuries%20 %E2%80%93%20elephantiasis%20and%20river%20blindness
Edwards, Dingsdale, Helsby, Orme, Breckenridge, The relative systemic availability of ivermectin after administration as capsule, tablet, and oral solution, European Journal of Clinical Pharmacology,
doi:10.1007/BF00637608González Canga, Prieto, Diez Liébana, Fernández Martínez, Sierra Vega et al., The pharmacokinetics and interactions of ivermectin in humans: a mini-review, The AAPS Journal,
doi:10.1208/s12248-007-9000-9Guzzo, Furtek, Porras, Chen, Tipping et al., Safety, tolerability, and pharmacokinetics of escalating high doses of ivermectin in healthy adult subjects, Journal of Clinical Pharmacology,
doi:10.1177/009127002401382731Heidary, Gharebaghi, Ivermectin: a systematic review from antiviral effects to COVID-19 complementary regimen, The Journal of Antibiotics,
doi:10.1038/s41429-020-0336-zKrolewiecki, Lifschitz, Moragas, Travacio, Alonso et al., Antiviral effect of highdose ivermectin in adults with COVID-19: A proof-of-concept randomized trial, EClinicalMedicine,
doi:10.1016/j.eclinm.2021.100959Merck, Tablets Stromectol (Ivermectina)
Ménez, Sutra, Prichard, Lespine, Relative neurotoxicity of ivermectin and moxidectin in Mdr1ab (-/-) mice and effects on mammalian GABA(A) channel activity, PLoS Neglected Tropical Diseases,
doi:10.1371/journal.pntd.0001883National, Coronavírus: Unimed Belém tem cerca de 450 pacientes recuperados
Navarro, Camprubí, Requena-Méndez, Buonfrate, Giorli et al., Safety of high-dose ivermectin: a systematic review and meta-analysis, The Journal of Antimicrobial Chemotherapy,
doi:10.1093/jac/dkz524Nicolas, Maia, Bassat, Kobylinski, Monteiro et al., Safety of oral ivermectin during pregnancy: a systematic review and meta-analysis, The Lancet. Global Health,
doi:10.1016/S2214-109X(19)30453-XNunes, Lima, harmacotherapy for COVID-19 treatment in patients with renal impairment: a updated review, Scielo Preprints
Oosting, Njoo, Kijlstra, Van Wilgenburg, Keukens et al., Ivermectin detection in serum of onchocerciasis patients: relationship to adverse reactions, The American Journal of Tropical Medicine and Hygiene,
doi:10.4269/ajtmh.1995.52.94Patrì, Fabbrocini, Hydroxychloroquine and ivermectin: a synergistic combination for COVID-19 chemoprophylaxis and treatment, Journal of the American Academy of Dermatology,
doi:10.1016/j.jaad.2020.04.017Rajter, Sherman, Fatteh, Vogel, Sacks et al., ICON (Ivermectin in COvid Nineteen) study: use of ivermectin is associated with lower mortality in hospitalized patients with COVID19, Chest Journal,
doi:10.1016/j.chest.2020.10.009Richards, Eigege, Umaru, Kahansim, Adelamo et al., The interruption of transmission of human onchocerciasis by an annual mass drug administration program in Plateau and Nasarawa States, Nigeria, The American Journal of Tropical Medicine and Hygiene,
doi:10.4269/ajtmh.19-0577Romani, Marks, Sokana, Nasi, Kamoriki et al., Efficacy of mass drug administration with ivermectin for control of scabies and impetigo, with coadministration of azithromycin: a single-arm community intervention trial, The Lancet. Infectious Diseases,
doi:10.1016/S1473-3099(18)30790-4Rupp, Balneário Camboriú vai oferecer tratamento em fase inicial a pacientes com Covid-19
Sharun, Dhama, Patel, Pathak, Tiwari et al., Ivermectin, a new candidate therapeutic against SARS-CoV-2/COVID-19, Ann Clin Microbiol Antimicrob,
doi:10.1186/s12941-020-00368-wVilanova, Baía, Abelardo Santos' registra mais de 900 recuperados da Covid-19
Walker, Whittaker, Watson, Baguelin, Winskill et al., The global impact of COVID-19 and strategies for mitigation and suppression, Science, Brazilian Journal of Biology,
doi:10.1126/science.abc0035Zeng, Andrew, Arison, Luffer-Atlas, Wang, Identification of cytochrome P4503A4 as the major enzyme responsible for the metabolism of ivermectin by human liver microsomes, Xenobiotica,
doi:10.1080/004982598239597DOI record:
{
"DOI": "10.1590/1519-6984.258325",
"ISSN": [
"1678-4375",
"1519-6984"
],
"URL": "http://dx.doi.org/10.1590/1519-6984.258325",
"abstract": "<jats:p>Abstract Ivermectin is a safe and effective drug in humans and has been approved for use in numerous parasitic infections for over 50 years. In addition, many studies have already shown its antiviral activity. Ivermectin is generally well tolerated, with no indication of central nervous system-associated toxicity at doses up to 10 times the highest FDA-approved dose of 200 µg/kg. The in vitro results of ivermectin for reducing SARS-CoV-2 viral load are promising and show that Ivermectin kills SARS-CoV-2 within 48 hours. A hypothesized mechanism of action for this drug is a likely inhibition of IMPα/β1-mediated nuclear import of viral proteins as demonstrated for other RNA viruses. However, controlled and randomized studies are needed to prove its effectiveness in COVID-19 in humans. In a single in vivo study with published results, patients confirmed to be infected with SARS-CoV-2 received at least one dose of ivermectin at any time during hospitalization. The use of ivermectin was associated with lower mortality during treatment with COVID-19, especially in patients who required increased inspired oxygen or ventilatory support. Additionally, 81 studies with the clinical use of ivermectin in humans are being carried out worldwide according to ClinicalTrials.gov. However, none of these data has been published so far. However, private and public entities in Brazil have been adopting this drug in their protocols as prophylaxis and in the initial phase of the disease. In addition, ivermectin has been used in mass treatment to prevent onchocerciasis and lymphatic filariasis in sub-Saharan Africa for many years. Surprisingly, this region has the lowest proportional mortality rate among the continents, despite the increasing numbers of infected people released by the World Health Organization.</jats:p>",
"alternative-id": [
"S1519-69842024000100303"
],
"author": [
{
"ORCID": "http://orcid.org/0000-0002-5024-0542",
"affiliation": [
{
"name": "Universidade Tecnológica Federal do Paraná, Brasil"
}
],
"authenticated-orcid": false,
"family": "Marques",
"given": "L. L. M.",
"sequence": "first"
},
{
"ORCID": "http://orcid.org/0000-0001-7888-5820",
"affiliation": [
{
"name": "Universidade Tecnológica Federal do Paraná, Brasil"
}
],
"authenticated-orcid": false,
"family": "Beneti",
"given": "S. C.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-5719-5633",
"affiliation": [
{
"name": "Universidade Tecnológica Federal do Paraná, Brasil"
}
],
"authenticated-orcid": false,
"family": "Pinzon",
"given": "C.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-0432-9191",
"affiliation": [
{
"name": "Universidade Tecnológica Federal do Paraná, Brasil"
}
],
"authenticated-orcid": false,
"family": "Cardoso",
"given": "F. A. R.",
"sequence": "additional"
}
],
"container-title": "Brazilian Journal of Biology",
"container-title-short": "Braz. J. Biol.",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2022,
5,
13
]
],
"date-time": "2022-05-13T12:33:35Z",
"timestamp": 1652445215000
},
"deposited": {
"date-parts": [
[
2022,
5,
13
]
],
"date-time": "2022-05-13T12:34:12Z",
"timestamp": 1652445252000
},
"indexed": {
"date-parts": [
[
2024,
7,
31
]
],
"date-time": "2024-07-31T18:35:45Z",
"timestamp": 1722450945579
},
"is-referenced-by-count": 3,
"issued": {
"date-parts": [
[
2024
]
]
},
"license": [
{
"URL": "http://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
1,
1
]
],
"date-time": "2024-01-01T00:00:00Z",
"timestamp": 1704067200000
}
},
{
"URL": "http://creativecommons.org/licenses/by/4.0/",
"content-version": "am",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
1,
1
]
],
"date-time": "2024-01-01T00:00:00Z",
"timestamp": 1704067200000
}
},
{
"URL": "http://creativecommons.org/licenses/by/4.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
1,
1
]
],
"date-time": "2024-01-01T00:00:00Z",
"timestamp": 1704067200000
}
}
],
"link": [
{
"URL": "http://www.scielo.br/scielo.php?script=sci_pdf&pid=S1519-69842024000100303&tlng=en",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "530",
"original-title": [
"Ivermectina como um possível tratamento para COVID-19: uma revisão dos protocolos de 2022"
],
"prefix": "10.1590",
"published": {
"date-parts": [
[
2024
]
]
},
"published-online": {
"date-parts": [
[
2024
]
]
},
"publisher": "FapUNIFESP (SciELO)",
"reference": [
{
"key": "ref1",
"series-title": "Bulário eletrônico",
"year": "2021"
},
{
"key": "ref2",
"series-title": "Secretaria de Saúde de Natal recomenda usar ivermectina para prevenir e tratar coronavírus",
"year": "2020"
},
{
"article-title": "The chemotherapy of onchocerciasis XIX: the clinical and laboratory tolerance of-high dose ivermectin",
"author": "AWADZI K.",
"first-page": "131",
"issue": "2",
"journal-title": "Tropical Medicine and Parasitology",
"key": "ref3",
"volume": "46",
"year": "1995"
},
{
"DOI": "10.1007/s002280050131",
"article-title": "Ivermectin distribution in the plasma and tissues of patients infected with Onchocerca volvulus",
"author": "BARAKA O.Z.",
"doi-asserted-by": "crossref",
"first-page": "407",
"issue": "5",
"journal-title": "European Journal of Clinical Pharmacology",
"key": "ref4",
"volume": "50",
"year": "1996"
},
{
"author": "BEZERRA H.M.",
"key": "ref5",
"series-title": "Protocolo de manejo para síndromes gripais frente à pandemia de coronavírus (COVID-19).",
"year": "2020"
},
{
"key": "ref6",
"series-title": "Decreto no 11.940, de 07 de Julho de 2020, que regulamenta a distribuição do medicamento ivermectina no Município de Itajaí",
"year": "2020"
},
{
"key": "ref7",
"series-title": "Portaria no 022/2020. Dispõe sobre o protocolo para o uso dos medicamentos Ivermectina e Cloroquina/Hidroxicloroquina nos pacientes com suspeita ou confirmação de COVID-19.",
"year": "2020"
},
{
"DOI": "10.1097/MJT.0000000000001402",
"article-title": "Ivermectin for prevention and treatment of COVID-19 Infection: a systematic review, meta-analysis, and trial sequential analysis to inform clinical guidelines",
"author": "BRYANT A.",
"doi-asserted-by": "crossref",
"first-page": "e434",
"issue": "4",
"journal-title": "American Journal of Therapeutics",
"key": "ref8",
"volume": "28",
"year": "2021"
},
{
"DOI": "10.1016/j.antiviral.2020.104787",
"article-title": "The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro",
"author": "CALY L.",
"doi-asserted-by": "crossref",
"journal-title": "Antiviral Research",
"key": "ref9",
"volume": "178",
"year": "2020"
},
{
"author": "CASCELLA M.",
"key": "ref10",
"series-title": "Features, evaluation and treatment coronavirus (COVID-19).",
"year": "2020"
},
{
"DOI": "10.1016/j.eclinm.2020.100720",
"article-title": "The effect of early treatment with ivermectin on viral load, symptoms and humoral response in patients with non-severe COVID-19: a pilot, double-blind, placebo-controlled, randomized clinical trial",
"author": "CHACCOUR C.",
"doi-asserted-by": "crossref",
"journal-title": "EClinicalMedicine",
"key": "ref11",
"volume": "32",
"year": "2021"
},
{
"DOI": "10.1186/s12936-017-1801-4",
"article-title": "Ivermectin to reduce malaria transmission I. Pharmacokinetic and pharmacodynamic considerations regarding efficacy and safety",
"author": "CHACCOUR C.",
"doi-asserted-by": "crossref",
"first-page": "161",
"issue": "1",
"journal-title": "Malaria Journal",
"key": "ref12",
"volume": "16",
"year": "2017"
},
{
"DOI": "10.1186/s13063-020-04421-z",
"article-title": "The SARS-CoV-2 ivermectin Navarra-ISGlobal Trial (SAINT) to evaluate the potential of ivermectin to reduce COVID-19 transmission in low risk, non-severe COVID-19 patients in the first 48 hours after symptoms onset: a structured summary of a study protocol",
"author": "CHACCOUR C.",
"doi-asserted-by": "crossref",
"first-page": "498",
"issue": "1",
"journal-title": "Trials",
"key": "ref13",
"volume": "21",
"year": "2020"
},
{
"key": "ref14",
"series-title": "Boletim ética na pesquisa",
"year": "2020"
},
{
"DOI": "10.1038/ja.2017.11",
"article-title": "Ivermectin: enigmatic multifaceted ‘wonder’ drug continues to surprise and exceed expectations",
"author": "CRUMP A.",
"doi-asserted-by": "crossref",
"first-page": "495",
"issue": "5",
"journal-title": "The Journal of Antibiotics",
"key": "ref15",
"volume": "70",
"year": "2017"
},
{
"author": "DALEY B.",
"key": "ref16",
"series-title": "How 2015 Nobel Prize drug might rid Africa of ancient scourges",
"year": "2015"
},
{
"DOI": "10.1007/BF00637608",
"article-title": "The relative systemic availability of ivermectin after administration as capsule, tablet, and oral solution",
"author": "EDWARDS G.",
"doi-asserted-by": "crossref",
"first-page": "681",
"issue": "6",
"journal-title": "European Journal of Clinical Pharmacology",
"key": "ref17",
"volume": "35",
"year": "1988"
},
{
"author": "FINK D.W.",
"first-page": "113",
"key": "ref18",
"series-title": "Ivermectin and abamectin",
"volume-title": "Pharmacokinetics of ivermectin in animals and humans.",
"year": "1989"
},
{
"DOI": "10.1208/s12248-007-9000-9",
"article-title": "The pharmacokinetics and interactions of ivermectin in humans: a mini-review",
"author": "GONZÁLEZ CANGA A.",
"doi-asserted-by": "crossref",
"first-page": "42",
"issue": "1",
"journal-title": "The AAPS Journal",
"key": "ref19",
"volume": "10",
"year": "2008"
},
{
"DOI": "10.1177/009127002401382731",
"article-title": "Safety, tolerability, and pharmacokinetics of escalating high doses of ivermectin in healthy adult subjects",
"author": "GUZZO C.A.",
"doi-asserted-by": "crossref",
"first-page": "1122",
"issue": "10",
"journal-title": "Journal of Clinical Pharmacology",
"key": "ref20",
"volume": "42",
"year": "2002"
},
{
"DOI": "10.1038/s41429-020-0336-z",
"article-title": "Ivermectin: a systematic review from antiviral effects to COVID-19 complementary regimen",
"author": "HEIDARY F.",
"doi-asserted-by": "crossref",
"first-page": "593",
"issue": "9",
"journal-title": "The Journal of Antibiotics",
"key": "ref21",
"volume": "73",
"year": "2020"
},
{
"DOI": "10.1016/S2214-109X(19)30490-5",
"article-title": "Is ivermectin safe in pregnancy?",
"author": "KING C.L.",
"doi-asserted-by": "crossref",
"first-page": "e12",
"issue": "1",
"journal-title": "The Lancet. Global Health",
"key": "ref22",
"volume": "8",
"year": "2020"
},
{
"DOI": "10.1016/j.eclinm.2021.100959",
"article-title": "Antiviral effect of high-dose ivermectin in adults with COVID-19: A proof-of-concept randomized trial",
"author": "KROLEWIECKI A.",
"doi-asserted-by": "crossref",
"journal-title": "EClinicalMedicine",
"key": "ref23",
"volume": "37",
"year": "2021"
},
{
"DOI": "10.1371/journal.pntd.0001883",
"article-title": "Relative neurotoxicity of ivermectin and moxidectin in Mdr1ab (−/−) mice and effects on mammalian GABA(A) channel activity",
"author": "MÉNEZ C.",
"doi-asserted-by": "crossref",
"issue": "11",
"journal-title": "PLoS Neglected Tropical Diseases",
"key": "ref24",
"volume": "6",
"year": "2012"
},
{
"key": "ref25",
"series-title": "Tablets Stromectol (Ivermectina) FDA approved Package insert 2009",
"year": "2009"
},
{
"DOI": "10.1093/jac/dkz524",
"article-title": "Safety of high-dose ivermectin: a systematic review and meta-analysis",
"author": "NAVARRO M.",
"doi-asserted-by": "crossref",
"first-page": "827",
"issue": "4",
"journal-title": "The Journal of Antimicrobial Chemotherapy",
"key": "ref26",
"volume": "75",
"year": "2020"
},
{
"DOI": "10.1016/S2214-109X(19)30453-X",
"article-title": "Safety of oral ivermectin during pregnancy: a systematic review and meta-analysis",
"author": "NICOLAS P.",
"doi-asserted-by": "crossref",
"first-page": "e92",
"issue": "1",
"journal-title": "The Lancet. Global Health",
"key": "ref27",
"volume": "8",
"year": "2020"
},
{
"article-title": "harmacotherapy for COVID-19 treatment in patients with renal impairment: a updated review",
"author": "NUNES L.",
"journal-title": "Scielo Preprints.",
"key": "ref28",
"year": "2020"
},
{
"DOI": "10.4269/ajtmh.1995.52.94",
"article-title": "Ivermectin detection in serum of onchocerciasis patients: relationship to adverse reactions",
"author": "OOSTING J.",
"doi-asserted-by": "crossref",
"first-page": "94",
"issue": "1",
"journal-title": "The American Journal of Tropical Medicine and Hygiene",
"key": "ref29",
"volume": "52",
"year": "1995"
},
{
"DOI": "10.1016/j.jaad.2020.04.017",
"article-title": "Hydroxychloroquine and ivermectin: a synergistic combination for COVID-19 chemoprophylaxis and treatment?",
"author": "PATRÌ A.",
"doi-asserted-by": "crossref",
"issue": "6",
"journal-title": "Journal of the American Academy of Dermatology",
"key": "ref30",
"volume": "82",
"year": "2020"
},
{
"DOI": "10.1016/j.chest.2020.10.009",
"article-title": "ICON (Ivermectin in COvid Nineteen) study: use of ivermectin is associated with lower mortality in hospitalized patients with COVID19",
"author": "RAJTER J.C.",
"doi-asserted-by": "crossref",
"first-page": "85",
"issue": "1",
"journal-title": "Chest Journal",
"key": "ref31",
"volume": "159",
"year": "2020"
},
{
"DOI": "10.4269/ajtmh.19-0577",
"article-title": "The interruption of transmission of human onchocerciasis by an annual mass drug administration program in Plateau and Nasarawa States, Nigeria",
"author": "RICHARDS F.O.",
"doi-asserted-by": "crossref",
"first-page": "582",
"issue": "3",
"journal-title": "The American Journal of Tropical Medicine and Hygiene",
"key": "ref32",
"volume": "102",
"year": "2020"
},
{
"DOI": "10.1016/S1473-3099(18)30790-4",
"article-title": "Efficacy of mass drug administration with ivermectin for control of scabies and impetigo, with coadministration of azithromycin: a single-arm community intervention trial",
"author": "ROMANI L.",
"doi-asserted-by": "crossref",
"first-page": "510",
"issue": "5",
"journal-title": "The Lancet. Infectious Diseases",
"key": "ref33",
"volume": "19",
"year": "2019"
},
{
"author": "RUPP I.",
"key": "ref34",
"series-title": "Balneário Camboriú vai oferecer tratamento em fase inicial a pacientes com Covid-19",
"year": "2020"
},
{
"article-title": "Ivermectin, a new candidate therapeutic against SARS-CoV-2/COVID-19",
"author": "SHARUN K.",
"issue": "23",
"journal-title": "Ann Clin Microbiol Antimicrob",
"key": "ref35",
"volume": "19",
"year": "2020"
},
{
"key": "ref36",
"series-title": "ClinicalTrials.gov",
"year": "2022"
},
{
"key": "ref37",
"series-title": "Coronavírus: Unimed Belém tem cerca de 450 pacientes recuperados",
"year": "2020"
},
{
"key": "ref38",
"series-title": "Distribuição de medicamentos para tratamento da Covid-19",
"year": "2020"
},
{
"edition": "5.",
"key": "ref39",
"series-title": "Proposta de tratamento da COVID-19 dependendo da fase, no momento do diagnóstico",
"year": "2020"
},
{
"author": "VILANOVA R.",
"key": "ref40",
"series-title": "Em dois meses, ‘Abelardo Santos’ registra mais de 900 recuperados da Covid-19.",
"year": "2020"
},
{
"DOI": "10.1126/science.abc0035",
"article-title": "The global impact of COVID-19 and strategies for mitigation and suppression",
"author": "WALKER P.G.T.",
"doi-asserted-by": "crossref",
"first-page": "413",
"issue": "6502",
"journal-title": "Science",
"key": "ref41",
"volume": "369",
"year": "2020"
},
{
"key": "ref42",
"series-title": "Novel Coronavirus (COVID-19) health sector preparedness & response.",
"year": "2020"
},
{
"key": "ref43",
"series-title": "WHO Coronavirus disease (COVID-19) dashboard-situation by WHO Region.",
"year": "2020"
},
{
"DOI": "10.1080/004982598239597",
"article-title": "Identification of cytochrome P4503A4 as the major enzyme responsible for the metabolism of ivermectin by human liver microsomes",
"author": "ZENG Z.",
"doi-asserted-by": "crossref",
"first-page": "313",
"issue": "3",
"journal-title": "Xenobiotica",
"key": "ref44",
"volume": "28",
"year": "1998"
}
],
"reference-count": 44,
"references-count": 44,
"relation": {},
"resource": {
"primary": {
"URL": "http://www.scielo.br/scielo.php?script=sci_arttext&pid=S1519-69842024000100303&tlng=en"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Ivermectin as a possible treatment for COVID-19: a review of the 2022 protocols",
"type": "journal-article",
"volume": "84"
}