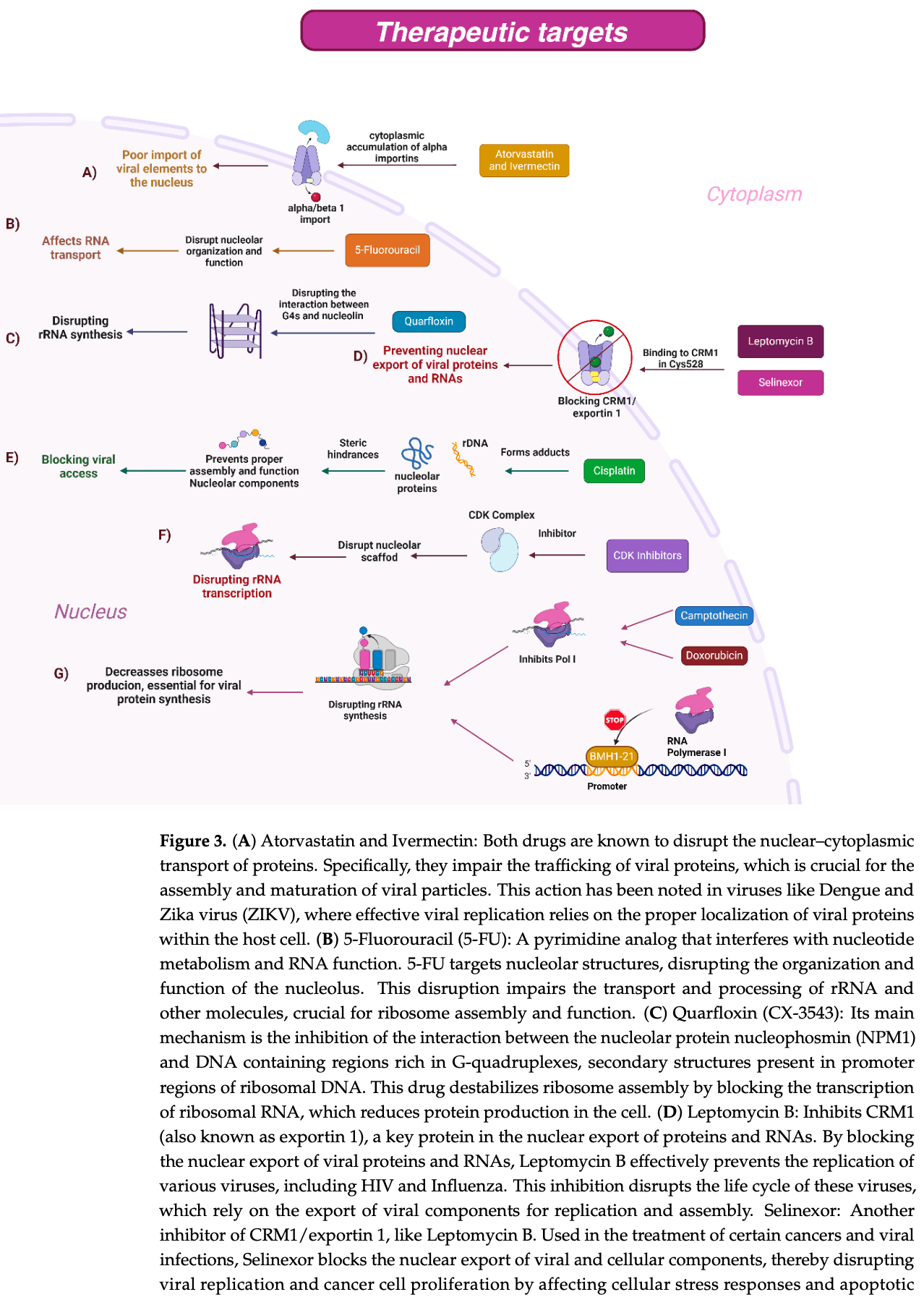
The Nucleolus and Its Interactions with Viral Proteins Required for Successful Infection
José Manuel Ulloa-Aguilar, Luis Herrera Moro Huitron, Rocío Yazmin Benítez-Zeferino, Jorge Francisco Cerna-Cortes, Julio García-Cordero, Guadalupe León-Reyes, Edgar Rodrigo Guzman-Bautista, Carlos Noe Farfan-Morales, José Manuel Reyes-Ruiz, Roxana U Miranda-Labra, Luis Adrián De Jesús-González, Moises León-Juárez
Cells, doi:10.3390/cells13181591
Nuclear bodies are structures in eukaryotic cells that lack a plasma membrane and are considered protein condensates, DNA, or RNA molecules. Known nuclear bodies include the nucleolus, Cajal bodies, and promyelocytic leukemia nuclear bodies. These bodies are involved in the concentration, exclusion, sequestration, assembly, modification, and recycling of specific components involved in the regulation of ribosome biogenesis, RNA transcription, and RNA processing. Additionally, nuclear bodies have been shown to participate in cellular processes such as the regulation of transcription of the cell cycle, mitosis, apoptosis, and the cellular stress response. The dynamics and functions of these bodies depend on the state of the cell. It is now known that both DNA and RNA viruses can direct their proteins to nuclear bodies, causing alterations in their composition, dynamics, and functions. Although many of these mechanisms are still under investigation, it is well known that the interaction between viral and nuclear body proteins is necessary for the success of the viral infection cycle. In this review, we concisely describe the interaction between viral and nuclear body proteins. Furthermore, we focus on the role of the nucleolus in RNA virus infections. Finally, we discuss the possible implications of the interaction of viral proteins on cellular transcription and the formation/degradation of non-coding RNAs.
Conflicts of Interest: The authors declare no conflicts of interest.
References
Ahmad, 5-Fluorouracil in Combination with Deoxyribonucleosides and Deoxyribose as Possible Therapeutic Options for the Coronavirus, COVID-19 Infection, Med. Hypotheses,
doi:10.1016/j.mehy.2020.109754Amrane, Jaubert, Bedrat, Rundstadler, Recordon-Pinson et al., Deciphering RNA G-Quadruplex Function during the Early Steps of HIV-1 Infection, Nucleic Acids Res,
doi:10.1093/nar/gkac1030Angelov, Bondarenko, Almagro, Menoni, Mongélard et al., Nucleolin Is a Histone Chaperone with FACT-like Activity and Assists Remodeling of Nucleosomes, EMBO J,
doi:10.1038/sj.emboj.7601046Artusi, Perrone, Lago, Raffa, Di Iorio et al., Visualization of DNA G-Quadruplexes in Herpes Simplex Virus 1-Infected Cells, Nucleic Acids Res,
doi:10.1093/nar/gkw968Aubert, O'donohue, Lebaron, Gleizes, Pre-Ribosomal RNA Processing in Human Cells: From Mechanisms to Congenital Diseases, Biomolecules,
doi:10.3390/biom8040123Balaban, Sztacho, Antiga, Miladinović, Harata et al., PIP2-Effector Protein MPRIP Regulates RNA Polymerase II Condensation and Transcription, Biomolecules,
doi:10.3390/biom13030426Belachew, Gao, Byrd, Raney, Hepatitis C Virus Nonstructural Protein NS3 Unfolds Viral G-Quadruplex RNA Structures, J. Biol. Chem,
doi:10.1016/j.jbc.2022.102486Bergeron, Fafard-Couture, Scott, Small Nucleolar RNAs: Continuing Identification of Novel Members and Increasing Diversity of Their Molecular Mechanisms of Action, Biochem. Soc. Trans,
doi:10.1042/BST20191046Bergstrand, O'brien, Farnebo, The Cajal Body Protein WRAP53β Prepares the Scene for Repair of DNA Double-Strand Breaks by Regulating Local Ubiquitination, Front. Mol. Biosci,
doi:10.3389/fmolb.2019.00051Carotenuto, Pecoraro, Palma, Russo, Russo, Therapeutic Approaches Targeting Nucleolus in Cancer, Cells,
doi:10.3390/cells8091090Chen, Huang, Nucleolar Components Involved in Ribosome Biogenesis Cycle between the Nucleolus and Nucleoplasm in Interphase Cells, J. Cell Biol,
doi:10.1083/jcb.153.1.169Chung, Ravichandran, Park, Lee, Kim et al., G-Quadruplexes Formed by Varicella-Zoster Virus Reiteration Sequences Suppress Expression of Glycoprotein C and Regulate Viral Cell-to-Cell Spread, PLoS Pathog,
doi:10.1371/journal.ppat.1011095Correll, Rudloff, Schmit, Ball, Karpova et al., Crossing Boundaries of Light Microscopy Resolution Discerns Novel Assemblies in the Nucleolus, Histochem. Cell Biol,
doi:10.1007/s00418-024-02297-7Coyaud, Ranadheera, Cheng, Gonçalves, Dyakov et al., Global Interactomics Uncovers Extensive Organellar Targeting by Zika Virus, Mol. Cell Proteom,
doi:10.1074/mcp.TIR118.000800Decle-Carrasco, Rodríguez-Piña, Rodríguez-Zapata, Castano, Current Research on Viral Proteins That Interact with Fibrillarin, Mol. Biol. Rep,
doi:10.1007/s11033-023-08343-2Deffrasnes, Marsh, Foo, Rootes, Gould et al., Genome-Wide siRNA Screening at Biosafety Level 4 Reveals a Crucial Role for Fibrillarin in Henipavirus Infection, PLoS Pathog,
doi:10.1371/journal.ppat.1005478Desterro, Keegan, Lafarga, Berciano, O'connell et al., Dynamic Association of RNA-Editing Enzymes with the Nucleolus, J. Cell Sci,
doi:10.1242/jcs.00371Deutzmann, Sullivan, Dhanasekaran, Li, Chen et al., Nuclear to Cytoplasmic Transport Is a Druggable Dependency in MYC-Driven Hepatocellular Carcinoma, Nat. Commun,
doi:10.1038/s41467-024-45128-yDing, Wu, Sun, Yan, Bai et al., Androgen Receptor Transactivates KSHV Noncoding RNA PAN to Promote Lytic Replication-Mediated Oncogenesis: A Mechanism of Sex Disparity in KS, PLOS Pathog,
doi:10.1371/journal.ppat.1009947Dobbyn, O'keefe, Analysis of Snu13p Mutations Reveals Differential Interactions with the U4 snRNA and U3 snoRNA, RNA,
doi:10.1261/rna.5970404Dove, You, Reed, Emmett, Brooks et al., Changes in Nucleolar Morphology and Proteins during Infection with the Coronavirus Infectious Bronchitis Virus, Cell. Microbiol,
doi:10.1111/j.1462-5822.2006.00698.xDumelie, Chen, Miller, Attarwala, Gross et al., Biomolecular Condensates Create Phospholipid-Enriched Microenvironments, Nat. Chem. Biol,
doi:10.1038/s41589-023-01474-4Falaleeva, Welden, Duncan, Stamm, C/D-Box snoRNAs Form Methylating and Non-Methylating Ribonucleoprotein Complexes: Old Dogs Show New Tricks, Bioessays,
doi:10.1002/bies.201600264Fan, Nadar, Chen, Weng, Lin et al., Small Noncoding RNA Modulates Japanese Encephalitis Virus Replication and Translation in Trans, Virol. J,
doi:10.1186/1743-422X-8-492Fleta-Soriano, Martinez, Hinkelmann, Gerth, Washausen et al., The Myxobacterial Metabolite Ratjadone a Inhibits HIV Infection by Blocking the Rev/CRM1-Mediated Nuclear Export Pathway, Microb. Cell Factories,
doi:10.1186/1475-2859-13-17Forte, Indovina, Montagnaro, Costa, Iannuzzi et al., The Oncolytic Caprine Herpesvirus 1 (CpHV-1) Induces Apoptosis and Synergizes with Cisplatin in Mesothelioma Cell Lines: A New Potential Virotherapy Approach, Viruses,
doi:10.3390/v13122458Fortes, Lamond, Ortín, Influenza Virus NS1 Protein Alters the Subnuclear Localization of Cellular Splicing Components, J. Gen. Virol,
doi:10.1099/0022-1317-76-4-1001Gautier, Bergès, Tollervey, Hurt, Nucleolar KKE/D Repeat Proteins Nop56p and Nop58p Interact with Nop1p and Are Required for Ribosome Biogenesis, Mol. Cell Biol,
doi:10.1128/MCB.17.12.7088Gemmill, Nelson, Badmalia, Pereira, Kerr et al., The 3' Terminal Region of Zika Virus RNA Contains a Conserved G-Quadruplex and Is Unfolded by Human DDX17, Biochem. Cell Biol
Ginn, La Montagna, Wu, Shi, Diverse Roles of Long Non-coding RNAs in Viral Diseases, Rev. Med. Virol,
doi:10.1002/rmv.2198Hoboth, Sztacho, Hozák, Nuclear Patterns of Phosphatidylinositol 4,5-and 3,4-Bisphosphate Revealed by Super-Resolution Microscopy Differ between the Consecutive Stages of RNA Polymerase II Transcription, FEBS J,
doi:10.1111/febs.17136Hoppe, Beech, Dimmock, Leppard, Interaction of the Adenovirus Type 5 E4 Orf3 Protein with Promyelocytic Leukemia Protein Isoform II Is Required for ND10 Disruption, J. Virol,
doi:10.1128/JVI.80.6.3042-3049.2006Huang, Du, Wen, Lu, Zhao, snoRNAs: Functions and Mechanisms in Biological Processes, and Roles in Tumor Pathophysiology, Cell Death Discov,
doi:10.1038/s41420-022-01056-8Hutzinger, Feederle, Mrazek, Schiefermeier, Balwierz et al., Expression and Processing of a Small Nucleolar RNA from the Epstein-Barr Virus Genome, PLoS Pathog,
doi:10.1371/journal.ppat.1000547Höfler, Lukat, Blankenfeldt, Carlomagno, Eukaryotic Box C/D Methylation Machinery Has Two Non-Symmetric Protein Assembly Sites, Sci. Rep,
doi:10.1038/s41598-021-97030-yHöfler, Lukat, Blankenfeldt, Carlomagno, High-Resolution Structure of Eukaryotic Fibrillarin Interacting with Nop56 Amino-Terminal Domain, RNA,
doi:10.1261/rna.077396.120Iarovaia, Ioudinkova, Velichko, Razin, Manipulation of Cellular Processes via Nucleolus Hijaking in the Course of Viral Infection in Mammals, Cells,
doi:10.3390/cells10071597Jamal, Alharbi, Ahmad, Identification of Doxorubicin as a Potential Therapeutic against SARS-CoV-2 (COVID-19) Protease: A Molecular Docking and Dynamics Simulation Studies, J. Biomol. Struct. Dyn,
doi:10.1080/07391102.2021.1905551Jarboui, Bidoia, Woods, Roe, Wynne et al., Nucleolar Protein Trafficking in Response to HIV-1 Tat: Rewiring the Nucleolus, PLoS ONE,
doi:10.1371/journal.pone.0048702Jesús-González, Palacios-Rápalo, Reyes-Ruiz, Osuna-Ramos, Cordero-Rivera et al., The Nuclear Pore Complex Is a Key Target of Viral Proteases to Promote Viral Replication, Viruses,
doi:10.3390/v13040706Jesús-González, Palacios-Rápalo, Reyes-Ruiz, Osuna-Ramos, Farfán-Morales et al., Nucleo-Cytoplasmic Transport of ZIKV Non-Structural 3 Protein Is Mediated by Importin-α/β and Exportin CRM-1, J. Virol,
doi:10.1128/jvi.01773-22Jiao, Liu, Yu, Pan, Zhang et al., Ribosome Biogenesis in Disease: New Players and Therapeutic Targets, Signal Transduct. Target. Ther,
doi:10.1038/s41392-022-01285-4Jorquera, Mathew, Pickens, Williams, Luczo et al., KPT-335), a Selective Inhibitor of Nuclear Export, Reduces Respiratory Syncytial Virus Replication In Vitro, J. Virol,
doi:10.1128/JVI.01684-18Kaptein, De Burghgraeve, Froeyen, Pastorino, Alen et al., A Derivate of the Antibiotic Doxorubicin Is a Selective Inhibitor of Dengue and Yellow Fever Virus Replication In Vitro, Antimicrob. Agents Chemother,
doi:10.1128/AAC.00686-10Kashyap, Murray, Walker, Chang, Tamir et al., a Novel Selective of Nuclear Export, Reduces SARS-CoV-2 Infection and Protects the Respiratory System in Vivo, Antiviral Res,
doi:10.1016/j.antiviral.2021.105115Kim, Macfarlane, Kalinina, Rakitina, Ryabov et al., Interaction of a Plant Virus-Encoded Protein with the Major Nucleolar Protein Fibrillarin Is Required for Systemic Virus Infection, Proc. Natl. Acad. Sci,
doi:10.1073/pnas.0704632104Klump, Perez, Patrick, Adams-Boone, Cohen et al., TCAB1 Prevents Nucleolar Accumulation of the Telomerase RNA to Facilitate Telomerase Assembly, Cell Rep,
doi:10.1016/j.celrep.2023.112577Kobayashi, Fujimoto, Sato, Hayashi, Burma et al., Nucleolin Participates in DNA Double-Strand Break-Induced Damage Response through MDC1-Dependent Pathway, PLoS ONE,
doi:10.1371/journal.pone.0049245Lafarga, Hervás, Santa-Cruz, Villegas, Crespo et al., Accessory Body" of Cajal in the Neuronal Nucleus. A Light and Electron Microscopic Approach, Anat. Embryol,
doi:10.1007/BF00317942Lafontaine, Riback, Bascetin, Brangwynne, The Nucleolus as a Multiphase Liquid Condensate, Nat. Rev. Mol. Cell Biol,
doi:10.1038/s41580-020-0272-6Lafontaine, Tollervey, Nop58p Is a Common Component of the Box C+D snoRNPs That Is Required for snoRNA Stability, RNA,
doi:10.1017/S135583829998192XLau, Kerr, Camiolo, Nightingale, Gu et al., Human Cytomegalovirus RNA2.7 Is Required for Upregulating Multiple Cellular Genes to Promote Cell Motility and Viral Spread Late in Lytic Infection, J. Virol,
doi:10.1128/JVI.00698-21Lechertier, Grob, Hernandez-Verdun, Roussel, Fibrillarin and Nop56 Interact before Being Co-Assembled in Box C/D snoRNPs, Exp. Cell Res,
doi:10.1016/j.yexcr.2009.01.016Lettin, Erbay, Blair, Viruses and Cajal Bodies: A Critical Cellular Target in Virus Infection?, Viruses,
doi:10.3390/v15122311Li, Macnaughton, Gao, Lai, RNA-Templated Replication of Hepatitis Delta Virus: Genomic and Antigenomic RNAs Associate with Different Nuclear Bodies, J. Virol,
doi:10.1128/JVI.02650-05Lindström, Jurada, Bursac, Orsolic, Bartek et al., Nucleolus as an Emerging Hub in Maintenance of Genome Stability and Cancer Pathogenesis, Oncogene,
doi:10.1038/s41388-017-0121-zLogan, Lett, Hebert, The Cajal Body Protein Coilin Is a Regulator of the miR-210 hypoxamiR and Influences MIR210HG Alternative Splicing, J. Cell Sci,
doi:10.1242/jcs.258575Logan, Mclaurin, Hebert, Synergistic Interactions between Cajal Bodies and the miRNA Processing Machinery, Mol. Biol. Cell,
doi:10.1091/mbc.E20-02-0144Love, Sweitzer, Hanover, Reconstitution of HIV-1 Rev Nuclear Export: Independent Requirements for Nuclear Import and Export, Proc. Natl. Acad. Sci,
doi:10.1073/pnas.95.18.10608Lung, Tong, To, Emerging Roles of Small Epstein-Barr Virus Derived Non-Coding RNAs in Epithelial Malignancy, Int. J. Mol. Sci,
doi:10.3390/ijms140917378Lv, Cui, Chen, Zhou, Zhang, G-Quadruplex Ligands Inhibit Chikungunya Virus Replication, J. Med. Virol,
doi:10.1002/jmv.27622Lykke-Andersen, Ardal, Hollensen, Damgaard, Jensen et al., D snoRNP Autoregulation by a Cis-Acting snoRNA in the NOP56 Pre-mRNA, Mol. Cell,
doi:10.1016/j.molcel.2018.08.017Ma, Li, Dong, Yang, Zhou et al., PML Body Component Sp100A Restricts Wild-Type Herpes Simplex Virus 1 Infection, J. Virol,
doi:10.1128/jvi.00279-22Marmier-Gourrier, Cléry, Senty-Ségault, Charpentier, Schlotter et al., A Structural, Phylogenetic, and Functional Study of 15.5-kD/Snu13 Protein Binding on U3 Small Nucleolar RNA, RNA,
doi:10.1261/rna.2130503Mattick, Amaral, Carninci, Carpenter, Chang et al., Long Non-Coding RNAs: Definitions, Functions, Challenges and Recommendations, Nat. Rev. Mol. Cell Biol,
doi:10.1038/s41580-022-00566-8Melén, Tynell, Fagerlund, Roussel, Hernandez-Verdun et al., Influenza A H3N2 Subtype Virus NS1 Protein Targets into the Nucleus and Binds Primarily via Its C-Terminal NLS2/NoLS to Nucleolin and Fibrillarin, Virol. J,
doi:10.1186/1743-422X-9-167Michieletto, Lusic, Marenduzzo, Orlandini, Physical Principles of Retroviral Integration in the Human Genome, Nat. Commun,
doi:10.1038/s41467-019-08333-8Mitrea, Cika, Guy, Ban, Banerjee et al., Nucleophosmin Integrates within the Nucleolus via Multi-Modal Interactions with Proteins Displaying R-Rich Linear Motifs and rRNA, eLife,
doi:10.7554/eLife.13571Murayama, Harada, Shibata, Kuroda, Hayakawa et al., Influenza A Virus Non-Structural Protein 1 (NS1) Interacts with Cellular Multifunctional Protein Nucleolin during Infection, Biochem. Biophys. Res. Commun,
doi:10.1016/j.bbrc.2007.08.091Naipauer, García Solá, Salyakina, Rosario, Williams et al., A Non-Coding RNA Network Involved in KSHV Tumorigenesis, Front. Oncol,
doi:10.3389/fonc.2021.687629Nakano, Watanabe, HTLV-1 Rex Tunes the Cellular Environment Favorable for Viral Replication, Viruses,
doi:10.3390/v8030058Norseen, Johnson, Lieberman, Role for G-Quadruplex RNA Binding by Epstein-Barr Virus Nuclear Antigen 1 in DNA Replication and Metaphase Chromosome Attachment, J. Virol,
doi:10.1128/JVI.00747-09Okuwaki, The Structure and Functions of NPM1/Nucleophsmin/B23, a Multifunctional Nucleolar Acidic Protein, J. Biochem,
doi:10.1093/jb/mvm222Okuwaki, Tsujimoto, Nagata, The RNA Binding Activity of a Ribosome Biogenesis Factor, Nucleophosmin/B23, Is Modulated by Phosphorylation with a Cell Cycle-Dependent Kinase and by Association with Its Subtype, MBoC,
doi:10.1091/mbc.02-03-0036Olson, Dundr, Nucleolus: Structure and Function
Palacios-Rápalo, Farfan-Morales, Cordero-Rivera, Jesús-González, Reyes-Ruiz et al., An Ivermectin-Atorvastatin Combination Impairs Nuclear Transport Inhibiting Dengue Infection in Vitro and in Vivo, iScience,
doi:10.1016/j.isci.2023.108294Peltonen, Colis, Liu, Jäämaa, Zhang et al., Small Molecule BMH-Compounds That Inhibit RNA Polymerase I and Cause Nucleolar Stress, Mol. Cancer Ther,
doi:10.1158/1535-7163.MCT-14-0256Pereira-Santana, Gamboa-Tuz, Zhao, Schranz, Vinuesa et al., Fibrillarin Evolution through the Tree of Life: Comparative Genomics and Microsynteny Network Analyses Provide New Insights into the Evolutionary History of Fibrillarin, PLoS Comput. Biol,
doi:10.1371/journal.pcbi.1008318Perwitasari, Johnson, Yan, Howerth, Shacham et al., a Novel Selective Inhibitor of Nuclear Export, Reduces Influenza a Virus Replication In Vitro and In Vivo, J. Virol,
doi:10.1128/JVI.01774-14Potapova, Unruh, Conkright-Fincham, Banks, Florens et al., Distinct States of Nucleolar Stress Induced by Anticancer Drugs, eLife,
doi:10.7554/eLife.88799Qin, Zhao, Liu, Zhang, Yang et al., RNA G-Quadruplex Formed in SARS-CoV-2 Used for COVID-19 Treatment in Animal Models, Cell Discov,
doi:10.1038/s41421-022-00450-xRattay, Hufbauer, Hoboth, Sztacho, Akgül, Viruses and Phospholipids: Friends and Foes during Infection, J Med. Virol,
doi:10.1002/jmv.28658Rausch, Grice, HIV Rev Assembly on the Rev Response Element (RRE): A Structural Perspective, Viruses,
doi:10.3390/v7062760Ravichandran, Kim, Bansal, Ghosh, Hur et al., Genome-Wide Analysis of Regulatory G-Quadruplexes Affecting Gene Expression in Human Cytomegalovirus, PLoS Pathog,
doi:10.1371/journal.ppat.1007334Rawlinson, Zhao, Rozario, Rootes, Mcmillan et al., Viral Regulation of Host Cell Biology by Hijacking of the Nucleolar DNA-Damage Response, Nat. Commun,
doi:10.1038/s41467-018-05354-7Raychaudhuri, Fontanes, Barat, Dasgupta, Activation of Ribosomal RNA Transcription by Hepatitis C Virus Involves Upstream Binding Factor Phosphorylation Via Induction of Cyclin D1, Cancer Res,
doi:10.1158/0008-5472.CAN-08-3468Rossetto, Pari, Kshv, PAN RNA Associates with Demethylases UTX and JMJD3 to Activate Lytic Replication through a Physical Interaction with the Virus Genome, PLoS Pathog,
doi:10.1371/journal.ppat.1002680Rossetto, Pari, PAN's Labyrinth: Molecular Biology of Kaposi's Sarcoma-Associated Herpesvirus (KSHV) PAN RNA, a Multifunctional Long Noncoding RNA, Viruses,
doi:10.3390/v6114212Sakthivel, Brown-Suedel, Bouchier-Hayes, Chapter Seven-The Role of the Nucleolus in Regulating the Cell Cycle and the DNA Damage Response
Salvetti, Couté, Epstein, Arata, Kraut et al., Nuclear Functions of Nucleolin through Global Proteomics and Interactomic Approaches, J. Proteome Res,
doi:10.1021/acs.jproteome.6b00126Sawyer, Sturgill, Sung, Hager, Dundr, Cajal Body Function in Genome Organization and Transcriptome Diversity, Bioessays,
doi:10.1002/bies.201600144Scherer, Schilling, Stamminger, The Human CMV IE1 Protein: An Offender of PML Nuclear Bodies, Adv. Anat. Embryol. Cell Biol,
doi:10.1007/978-3-319-53168-7_4Schuessler, Funk, Lazear, Cooper, Torres et al., West Nile Virus Noncoding Subgenomic RNA Contributes to Viral Evasion of the Type I Interferon-Mediated Antiviral Response, J. Virol,
doi:10.1128/JVI.00207-12Shubina, Musinova, Sheval, Nucleolar Methyltransferase Fibrillarin: Evolution of Structure and Functions, Biochemistry,
doi:10.1134/S0006297916090030Shubina, Musinova, Sheval, Proliferation, Cancer, and Aging-Novel Functions of the Nucleolar Methyltransferase Fibrillarin?, Cell Biol. Int,
doi:10.1002/cbin.11044Simões, Martins, Ferreira, Early Intranuclear Replication of African Swine Fever Virus Genome Modifies the Landscape of the Host Cell Nucleus, Virus Res,
doi:10.1016/j.virusres.2015.07.006Sirri, Hernandez-Verdun, Roussel, Cyclin-Dependent Kinases Govern Formation and Maintenance of the Nucleolus, J. Cell Biol,
doi:10.1083/jcb.200201024Sztacho, Sobol, Balaban, Lopes, Hozák, Nuclear Phosphoinositides and Phase Separation: Important Players in Nuclear Compartmentalization, Adv. Biol. Regul,
doi:10.1016/j.jbior.2018.09.009Tai, Liu, Pan, Wong, Tai et al., Chemovirotherapeutic Treatment Using Camptothecin Enhances Oncolytic Measles Virus-Mediated Killing of Breast Cancer Cells, Sci. Rep,
doi:10.1038/s41598-019-43047-3Tang, Vijayakumar, Zhang, Fullwood, Super-Enhancers, Phase-Separated Condensates, and 3D Genome Organization in Cancer, Cancers,
doi:10.3390/cancers14122866Terrell, Le, Paul, Brinton, Wilson et al., Structure of an RNA G-Quadruplex from the West Nile Virus Genome, Nat. Commun,
doi:10.1038/s41467-024-49761-5Tokunaga, Miyamoto, Suzuki, Otani, Inuki et al., Novel Anti-Flavivirus Drugs Targeting the Nucleolar Distribution of Core Protein, Virology,
doi:10.1016/j.virol.2019.11.015Wang, The Opening of Pandora's Box: An Emerging Role of Long Noncoding RNA in Viral Infections, Front. Immunol,
doi:10.3389/fimmu.2018.03138White, Erbay, Blair, The Cajal Body Protein P80-Coilin Forms a Complex with the Adenovirus L4-22K Protein and Facilitates the Nuclear Export of Adenovirus mRNA, mBio,
doi:10.1128/mbio.01459-23Wu, Chu, Antiviral Screen Identifies EV71 Inhibitors and Reveals Camptothecin-Target, DNA Topoisomerase 1 as a Novel EV71 Host Factor, Antivir. Res,
doi:10.1016/j.antiviral.2017.04.008Xu, Hurley, A First-in-Class Clinical G-Quadruplex-Targeting Drug. The Bench-to-Bedside Translation of the Fluoroquinolone QQ58 to CX-5461 (Pidnarulex), Bioorganic Med. Chem. Lett,
doi:10.1016/j.bmcl.2022.129016Yan, Du, Wang, Li, Non-Structural Protein 1 of H3N2 Influenza A Virus Induces Nucleolar Stress via Interaction with Nucleolin, Sci. Rep,
doi:10.1038/s41598-017-18087-2Yang, Wang, Zhao, Sun, Yang et al., A Redox Mechanism Underlying Nucleolar Stress Sensing by Nucleophosmin, Nat. Commun,
doi:10.1038/ncomms13599Yao, Xu, Wang, Shan, Luan et al., Nascent Pre-rRNA Sorting via Phase Separation Drives the Assembly of Dense Fibrillar Components in the Human Nucleolus, Mol. Cell,
doi:10.1016/j.molcel.2019.08.014Yetming, Lupey-Green, Biryukov, Hughes, Marendy et al., The BHLF1 Locus of Epstein-Barr Virus Contributes to Viral Latency and B-Cell Immortalization, J. Virol,
doi:10.1128/JVI.01215-20Yin, Yin, Wang, Shen, Dai et al., Antiviral and Virucidal Activities of Camptothecin on Fowl Adenovirus Serotype 4 by Blocking Virus Replication, Front. Cell. Infect. Microbiol,
doi:10.3389/fcimb.2022.823820Young, Jensen, Burger, Pintel, Lorson, Minute Virus of Mice NS1 Interacts with the SMN Protein, and They Colocalize in Novel Nuclear Bodies Induced by Parvovirus Infection, J. Virol,
doi:10.1128/JVI.76.8.3892-3904.2002Zatsepina, Rousselet, Chan, Olson, Jordan et al., The Nucleolar Phosphoprotein B23 Redistributes in Part to the Spindle Poles during Mitosis, J. Cell Sci,
doi:10.1242/jcs.112.4.455Zhai, Comai, A Kinase Activity Associated with Simian Virus 40 Large T Antigen Phosphorylates Upstream Binding Factor (UBF) and Promotes Formation of a Stable Initiation Complex between UBF and SL1, Mol. Cell Biol,
doi:10.1128/MCB.19.4.2791Zhai, Tuan, Comai, SV40 Large T Antigen Binds to the TBP-TAF(I) Complex SL1 and Coactivates Ribosomal RNA Transcription, Genes Dev,
doi:10.1101/gad.11.12.1605Zhou, Yuan, Zhang, Dai, Du et al., Inhibition of Japanese Encephalitis Virus Proliferation by Long Non-Coding RNA SUSAJ1 in PK-15 Cells, Virol. J,
doi:10.1186/s12985-021-01492-5Zou, Xiong, Deng, Engelhardt, Yan et al., Kaposi's Sarcoma-Associated Herpesvirus Noncoding Polyadenylated Nuclear RNA Interacts with Virus-and Host Cell-Encoded Proteins and Suppresses Expression of Genes Involved in Immune Modulation, J. Virol,
doi:10.1128/JVI.05886-11DOI record:
{
"DOI": "10.3390/cells13181591",
"ISSN": [
"2073-4409"
],
"URL": "http://dx.doi.org/10.3390/cells13181591",
"abstract": "<jats:p>Nuclear bodies are structures in eukaryotic cells that lack a plasma membrane and are considered protein condensates, DNA, or RNA molecules. Known nuclear bodies include the nucleolus, Cajal bodies, and promyelocytic leukemia nuclear bodies. These bodies are involved in the concentration, exclusion, sequestration, assembly, modification, and recycling of specific components involved in the regulation of ribosome biogenesis, RNA transcription, and RNA processing. Additionally, nuclear bodies have been shown to participate in cellular processes such as the regulation of transcription of the cell cycle, mitosis, apoptosis, and the cellular stress response. The dynamics and functions of these bodies depend on the state of the cell. It is now known that both DNA and RNA viruses can direct their proteins to nuclear bodies, causing alterations in their composition, dynamics, and functions. Although many of these mechanisms are still under investigation, it is well known that the interaction between viral and nuclear body proteins is necessary for the success of the viral infection cycle. In this review, we concisely describe the interaction between viral and nuclear body proteins. Furthermore, we focus on the role of the nucleolus in RNA virus infections. Finally, we discuss the possible implications of the interaction of viral proteins on cellular transcription and the formation/degradation of non-coding RNAs.</jats:p>",
"alternative-id": [
"cells13181591"
],
"author": [
{
"affiliation": [
{
"name": "Laboratorio de Virología Perinatal y Diseño Molecular de Antígenos y Biomarcadores, Departamento de Inmunobioquímica, Instituto Nacional de Perinatología, Mexico City 11000, Mexico"
},
{
"name": "Posgrado en Biología Experimental, Departamento de Ciencias Biológicas y de la Salud (DCBS), Universidad Autónoma Metropolitana-Iztapalapa, Mexico City 09310, Mexico"
}
],
"family": "Ulloa-Aguilar",
"given": "José Manuel",
"sequence": "first"
},
{
"ORCID": "http://orcid.org/0009-0007-4214-2735",
"affiliation": [
{
"name": "Laboratorio de Virología Perinatal y Diseño Molecular de Antígenos y Biomarcadores, Departamento de Inmunobioquímica, Instituto Nacional de Perinatología, Mexico City 11000, Mexico"
},
{
"name": "Laboratorio de Microbiología Molecular, Departamento de Microbiología, Escuela Nacional de Ciencias Biologícas, Instituto Politécnico Nacional, Mexico City 11340, Mexico"
}
],
"authenticated-orcid": false,
"family": "Herrera Moro Huitron",
"given": "Luis",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Laboratorio de Virología Perinatal y Diseño Molecular de Antígenos y Biomarcadores, Departamento de Inmunobioquímica, Instituto Nacional de Perinatología, Mexico City 11000, Mexico"
},
{
"name": "Laboratorio de Microbiología Molecular, Departamento de Microbiología, Escuela Nacional de Ciencias Biologícas, Instituto Politécnico Nacional, Mexico City 11340, Mexico"
}
],
"family": "Benítez-Zeferino",
"given": "Rocío Yazmin",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-4350-9507",
"affiliation": [
{
"name": "Laboratorio de Microbiología Molecular, Departamento de Microbiología, Escuela Nacional de Ciencias Biologícas, Instituto Politécnico Nacional, Mexico City 11340, Mexico"
}
],
"authenticated-orcid": false,
"family": "Cerna-Cortes",
"given": "Jorge Francisco",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Departamento de Biomedicina Molecular, Centro de Investigación y de Estudios Avanzados del Instituto Politécnico Nacional (CINVESTAV-IPN), Mexico City 07360, Mexico"
}
],
"family": "García-Cordero",
"given": "Julio",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-0291-2620",
"affiliation": [
{
"name": "Laboratorio de Nutrigenética y Nutrigenómica, Instituto Nacional de Medicina Genómica (INMEGEN), Mexico City 14610, Mexico"
}
],
"authenticated-orcid": false,
"family": "León-Reyes",
"given": "Guadalupe",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Laboratorio de Virología Perinatal y Diseño Molecular de Antígenos y Biomarcadores, Departamento de Inmunobioquímica, Instituto Nacional de Perinatología, Mexico City 11000, Mexico"
}
],
"family": "Guzman-Bautista",
"given": "Edgar Rodrigo",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-9787-5588",
"affiliation": [
{
"name": "Departamento de Ciencias Naturales, Universidad Autonoma Metropolitana (UAM), Unidad Cuajimalpa, Mexico City 05348, Mexico"
}
],
"authenticated-orcid": false,
"family": "Farfan-Morales",
"given": "Carlos Noe",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-2379-8591",
"affiliation": [
{
"name": "Centro Médico Nacional “Adolfo Ruiz Cortines”, Instituto Mexicano del Seguro Social (IMSS), Veracruz 91897, Mexico"
}
],
"authenticated-orcid": false,
"family": "Reyes-Ruiz",
"given": "José Manuel",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Departamento de Ciencias de la Salud, Universidad Autónoma Metropolitana, Unidad Iztapalapa, Mexico City 09310, Mexico"
}
],
"family": "Miranda-Labra",
"given": "Roxana U.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-1415-6260",
"affiliation": [
{
"name": "Unidad de Investigación Biomédica de Zacatecas, Instituto Mexicano del Seguro Social, Zacatecas 98000, Mexico"
}
],
"authenticated-orcid": false,
"family": "De Jesús-González",
"given": "Luis Adrián",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-5726-5953",
"affiliation": [
{
"name": "Laboratorio de Virología Perinatal y Diseño Molecular de Antígenos y Biomarcadores, Departamento de Inmunobioquímica, Instituto Nacional de Perinatología, Mexico City 11000, Mexico"
}
],
"authenticated-orcid": false,
"family": "León-Juárez",
"given": "Moises",
"sequence": "additional"
}
],
"container-title": "Cells",
"container-title-short": "Cells",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2024,
9,
23
]
],
"date-time": "2024-09-23T07:36:38Z",
"timestamp": 1727076998000
},
"deposited": {
"date-parts": [
[
2024,
9,
23
]
],
"date-time": "2024-09-23T08:38:02Z",
"timestamp": 1727080682000
},
"indexed": {
"date-parts": [
[
2024,
9,
24
]
],
"date-time": "2024-09-24T04:01:57Z",
"timestamp": 1727150517829
},
"is-referenced-by-count": 0,
"issue": "18",
"issued": {
"date-parts": [
[
2024,
9,
21
]
]
},
"journal-issue": {
"issue": "18",
"published-online": {
"date-parts": [
[
2024,
9
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
9,
21
]
],
"date-time": "2024-09-21T00:00:00Z",
"timestamp": 1726876800000
}
}
],
"link": [
{
"URL": "https://www.mdpi.com/2073-4409/13/18/1591/pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "1968",
"original-title": [],
"page": "1591",
"prefix": "10.3390",
"published": {
"date-parts": [
[
2024,
9,
21
]
]
},
"published-online": {
"date-parts": [
[
2024,
9,
21
]
]
},
"publisher": "MDPI AG",
"reference": [
{
"DOI": "10.3390/v9120356",
"doi-asserted-by": "crossref",
"key": "ref_1",
"unstructured": "Kvansakul, M. (2017). Viral Infection and Apoptosis. Viruses, 9."
},
{
"DOI": "10.1101/cshperspect.a033001",
"doi-asserted-by": "crossref",
"key": "ref_2",
"unstructured": "Stern-Ginossar, N., Thompson, S.R., Mathews, M.B., and Mohr, I. (2019). Translational Control in Virus-Infected Cells. Cold Spring Harb. Perspect. Biol., 11."
},
{
"DOI": "10.1111/cmi.12465",
"article-title": "The Nucleolar Interface of RNA Viruses",
"author": "Rawlinson",
"doi-asserted-by": "crossref",
"first-page": "1108",
"journal-title": "Cell. Microbiol.",
"key": "ref_3",
"volume": "17",
"year": "2015"
},
{
"DOI": "10.1038/s41467-018-05354-7",
"article-title": "Viral Regulation of Host Cell Biology by Hijacking of the Nucleolar DNA-Damage Response",
"author": "Rawlinson",
"doi-asserted-by": "crossref",
"first-page": "3057",
"journal-title": "Nat. Commun.",
"key": "ref_4",
"volume": "9",
"year": "2018"
},
{
"DOI": "10.1002/jmv.28658",
"article-title": "Viruses and Phospholipids: Friends and Foes during Infection",
"author": "Rattay",
"doi-asserted-by": "crossref",
"first-page": "e28658",
"journal-title": "J Med. Virol.",
"key": "ref_5",
"volume": "95",
"year": "2023"
},
{
"DOI": "10.1007/978-94-007-4899-6_7",
"article-title": "Viral Integration and Consequences on Host Gene Expression",
"author": "Desfarges",
"doi-asserted-by": "crossref",
"first-page": "147",
"journal-title": "Viruses Essent. Agents Life",
"key": "ref_6",
"volume": "XVI",
"year": "2012"
},
{
"DOI": "10.1038/s41467-019-08333-8",
"article-title": "Physical Principles of Retroviral Integration in the Human Genome",
"author": "Michieletto",
"doi-asserted-by": "crossref",
"first-page": "575",
"journal-title": "Nat. Commun.",
"key": "ref_7",
"volume": "10",
"year": "2019"
},
{
"DOI": "10.3390/v5082019",
"article-title": "Viral Subversion of the Nuclear Pore Complex",
"author": "Mouland",
"doi-asserted-by": "crossref",
"first-page": "2019",
"journal-title": "Viruses",
"key": "ref_8",
"volume": "5",
"year": "2013"
},
{
"DOI": "10.1128/JVI.02650-05",
"article-title": "RNA-Templated Replication of Hepatitis Delta Virus: Genomic and Antigenomic RNAs Associate with Different Nuclear Bodies",
"author": "Li",
"doi-asserted-by": "crossref",
"first-page": "6478",
"journal-title": "J. Virol.",
"key": "ref_9",
"volume": "80",
"year": "2006"
},
{
"DOI": "10.1016/j.vaccine.2006.05.066",
"article-title": "Influenza A Virus and the Cell Nucleus",
"author": "Amorim",
"doi-asserted-by": "crossref",
"first-page": "6651",
"journal-title": "Vaccine",
"key": "ref_10",
"volume": "24",
"year": "2006"
},
{
"DOI": "10.1007/978-3-319-38882-3",
"doi-asserted-by": "crossref",
"key": "ref_11",
"unstructured": "Bazett-Jones, D., and Dellaire, G. (2016). The Nucleolus: Structure and Function. The Functional Nucleus, Springer."
},
{
"DOI": "10.1002/9780470015902.a0005975.pub3",
"doi-asserted-by": "crossref",
"key": "ref_12",
"unstructured": "Olson, M.O., and Dundr, M. (2015). Nucleolus: Structure and Function. eLS, John Wiley & Sons, Ltd."
},
{
"DOI": "10.1038/s41580-020-0272-6",
"article-title": "The Nucleolus as a Multiphase Liquid Condensate",
"author": "Lafontaine",
"doi-asserted-by": "crossref",
"first-page": "165",
"journal-title": "Nat. Rev. Mol. Cell Biol.",
"key": "ref_13",
"volume": "22",
"year": "2021"
},
{
"DOI": "10.1111/febs.17136",
"doi-asserted-by": "crossref",
"key": "ref_14",
"unstructured": "Hoboth, P., Sztacho, M., and Hozák, P. (2024). Nuclear Patterns of Phosphatidylinositol 4,5- and 3,4-Bisphosphate Revealed by Super-Resolution Microscopy Differ between the Consecutive Stages of RNA Polymerase II Transcription. FEBS J., online ahead of print."
},
{
"DOI": "10.1016/j.jbior.2018.09.009",
"article-title": "Nuclear Phosphoinositides and Phase Separation: Important Players in Nuclear Compartmentalization",
"author": "Sztacho",
"doi-asserted-by": "crossref",
"first-page": "111",
"journal-title": "Adv. Biol. Regul.",
"key": "ref_15",
"volume": "71",
"year": "2019"
},
{
"DOI": "10.1038/s41589-023-01474-4",
"article-title": "Biomolecular Condensates Create Phospholipid-Enriched Microenvironments",
"author": "Dumelie",
"doi-asserted-by": "crossref",
"first-page": "302",
"journal-title": "Nat. Chem. Biol.",
"key": "ref_16",
"volume": "20",
"year": "2024"
},
{
"DOI": "10.3390/biom13030426",
"doi-asserted-by": "crossref",
"key": "ref_17",
"unstructured": "Balaban, C., Sztacho, M., Antiga, L., Miladinović, A., Harata, M., and Hozák, P. (2023). PIP2-Effector Protein MPRIP Regulates RNA Polymerase II Condensation and Transcription. Biomolecules, 13."
},
{
"DOI": "10.1007/BF00317942",
"article-title": "The “Accessory Body” of Cajal in the Neuronal Nucleus. A Light and Electron Microscopic Approach",
"author": "Lafarga",
"doi-asserted-by": "crossref",
"first-page": "19",
"journal-title": "Anat. Embryol.",
"key": "ref_18",
"volume": "166",
"year": "1983"
},
{
"DOI": "10.1242/jcs.258575",
"article-title": "The Cajal Body Protein Coilin Is a Regulator of the miR-210 hypoxamiR and Influences MIR210HG Alternative Splicing",
"author": "Logan",
"doi-asserted-by": "crossref",
"first-page": "jcs258575",
"journal-title": "J. Cell Sci.",
"key": "ref_19",
"volume": "134",
"year": "2021"
},
{
"DOI": "10.3389/fmolb.2019.00051",
"doi-asserted-by": "crossref",
"key": "ref_20",
"unstructured": "Bergstrand, S., O’Brien, E.M., and Farnebo, M. (2019). The Cajal Body Protein WRAP53β Prepares the Scene for Repair of DNA Double-Strand Breaks by Regulating Local Ubiquitination. Front. Mol. Biosci., 6."
},
{
"DOI": "10.1091/mbc.E20-02-0144",
"article-title": "Synergistic Interactions between Cajal Bodies and the miRNA Processing Machinery",
"author": "Logan",
"doi-asserted-by": "crossref",
"first-page": "1561",
"journal-title": "Mol. Biol. Cell",
"key": "ref_21",
"volume": "31",
"year": "2020"
},
{
"DOI": "10.1002/bies.201600144",
"article-title": "Cajal Body Function in Genome Organization and Transcriptome Diversity",
"author": "Sawyer",
"doi-asserted-by": "crossref",
"first-page": "1197",
"journal-title": "Bioessays",
"key": "ref_22",
"volume": "38",
"year": "2016"
},
{
"DOI": "10.20944/preprints202310.1178.v1",
"doi-asserted-by": "crossref",
"key": "ref_23",
"unstructured": "Lettin, L., Erbay, B., and Blair, G.E. (2023). Viruses and Cajal Bodies: A Critical Cellular Target in Virus Infection?. Viruses, 15."
},
{
"DOI": "10.1128/JVI.76.8.3892-3904.2002",
"article-title": "Minute Virus of Mice NS1 Interacts with the SMN Protein, and They Colocalize in Novel Nuclear Bodies Induced by Parvovirus Infection",
"author": "Young",
"doi-asserted-by": "crossref",
"first-page": "3892",
"journal-title": "J. Virol.",
"key": "ref_24",
"volume": "76",
"year": "2002"
},
{
"DOI": "10.1016/j.virusres.2015.07.006",
"article-title": "Early Intranuclear Replication of African Swine Fever Virus Genome Modifies the Landscape of the Host Cell Nucleus",
"author": "Martins",
"doi-asserted-by": "crossref",
"first-page": "1",
"journal-title": "Virus Res.",
"key": "ref_25",
"volume": "210",
"year": "2015"
},
{
"DOI": "10.1099/0022-1317-76-4-1001",
"article-title": "Influenza Virus NS1 Protein Alters the Subnuclear Localization of Cellular Splicing Components",
"author": "Fortes",
"doi-asserted-by": "crossref",
"first-page": "1001",
"journal-title": "J. Gen. Virol.",
"key": "ref_26",
"volume": "76",
"year": "1995"
},
{
"DOI": "10.1074/mcp.TIR118.000800",
"article-title": "Global Interactomics Uncovers Extensive Organellar Targeting by Zika Virus",
"author": "Coyaud",
"doi-asserted-by": "crossref",
"first-page": "2242",
"journal-title": "Mol. Cell Proteom.",
"key": "ref_27",
"volume": "17",
"year": "2018"
},
{
"DOI": "10.1128/mbio.01459-23",
"doi-asserted-by": "crossref",
"key": "ref_28",
"unstructured": "White, L., Erbay, B., and Blair, G.E. (2023). The Cajal Body Protein P80-Coilin Forms a Complex with the Adenovirus L4-22K Protein and Facilitates the Nuclear Export of Adenovirus mRNA. mBio, 14."
},
{
"DOI": "10.1128/JVI.80.6.3042-3049.2006",
"article-title": "Interaction of the Adenovirus Type 5 E4 Orf3 Protein with Promyelocytic Leukemia Protein Isoform II Is Required for ND10 Disruption",
"author": "Hoppe",
"doi-asserted-by": "crossref",
"first-page": "3042",
"journal-title": "J. Virol.",
"key": "ref_29",
"volume": "80",
"year": "2006"
},
{
"DOI": "10.1128/jvi.00279-22",
"article-title": "PML Body Component Sp100A Restricts Wild-Type Herpes Simplex Virus 1 Infection",
"author": "Ma",
"doi-asserted-by": "crossref",
"first-page": "e0027922",
"journal-title": "J. Virol.",
"key": "ref_30",
"volume": "96",
"year": "2022"
},
{
"DOI": "10.1007/978-3-319-53168-7_4",
"article-title": "The Human CMV IE1 Protein: An Offender of PML Nuclear Bodies",
"author": "Scherer",
"doi-asserted-by": "crossref",
"first-page": "77",
"journal-title": "Adv. Anat. Embryol. Cell Biol.",
"key": "ref_31",
"volume": "223",
"year": "2017"
},
{
"DOI": "10.1016/j.ceb.2017.05.001",
"article-title": "Nuclear Bodies: News Insights into Structure and Function",
"author": "Fox",
"doi-asserted-by": "crossref",
"first-page": "94",
"journal-title": "Curr. Opin. Cell Biol.",
"key": "ref_32",
"volume": "46",
"year": "2017"
},
{
"DOI": "10.1111/febs.16117",
"article-title": "Nuclear Speckles: Dynamic Hubs of Gene Expression Regulation",
"doi-asserted-by": "crossref",
"first-page": "7234",
"journal-title": "FEBS J.",
"key": "ref_33",
"volume": "289",
"year": "2022"
},
{
"DOI": "10.3390/cancers14122866",
"doi-asserted-by": "crossref",
"key": "ref_34",
"unstructured": "Tang, S.C., Vijayakumar, U., Zhang, Y., and Fullwood, M.J. (2022). Super-Enhancers, Phase-Separated Condensates, and 3D Genome Organization in Cancer. Cancers, 14."
},
{
"DOI": "10.1083/jcb.153.1.169",
"article-title": "Nucleolar Components Involved in Ribosome Biogenesis Cycle between the Nucleolus and Nucleoplasm in Interphase Cells",
"author": "Chen",
"doi-asserted-by": "crossref",
"first-page": "169",
"journal-title": "J. Cell Biol.",
"key": "ref_35",
"volume": "153",
"year": "2001"
},
{
"DOI": "10.1038/s41392-022-01285-4",
"article-title": "Ribosome Biogenesis in Disease: New Players and Therapeutic Targets",
"author": "Jiao",
"doi-asserted-by": "crossref",
"first-page": "15",
"journal-title": "Signal Transduct. Target. Ther.",
"key": "ref_36",
"volume": "8",
"year": "2023"
},
{
"DOI": "10.3390/biom8040123",
"doi-asserted-by": "crossref",
"key": "ref_37",
"unstructured": "Aubert, M., O’Donohue, M.-F., Lebaron, S., and Gleizes, P.-E. (2018). Pre-Ribosomal RNA Processing in Human Cells: From Mechanisms to Congenital Diseases. Biomolecules, 8."
},
{
"DOI": "10.1016/j.molcel.2019.08.014",
"article-title": "Nascent Pre-rRNA Sorting via Phase Separation Drives the Assembly of Dense Fibrillar Components in the Human Nucleolus",
"author": "Yao",
"doi-asserted-by": "crossref",
"first-page": "767",
"journal-title": "Mol. Cell",
"key": "ref_38",
"volume": "76",
"year": "2019"
},
{
"DOI": "10.1016/j.celrep.2013.08.049",
"article-title": "The 5S RNP Couples P53 Homeostasis to Ribosome Biogenesis and Nucleolar Stress",
"author": "Sloan",
"doi-asserted-by": "crossref",
"first-page": "237",
"journal-title": "Cell Rep.",
"key": "ref_39",
"volume": "5",
"year": "2013"
},
{
"DOI": "10.1534/genetics.113.153197",
"article-title": "Ribosome Biogenesis in the Yeast Saccharomyces Cerevisiae",
"author": "Woolford",
"doi-asserted-by": "crossref",
"first-page": "643",
"journal-title": "Genetics",
"key": "ref_40",
"volume": "195",
"year": "2013"
},
{
"DOI": "10.1007/s00418-024-02297-7",
"article-title": "Crossing Boundaries of Light Microscopy Resolution Discerns Novel Assemblies in the Nucleolus",
"author": "Correll",
"doi-asserted-by": "crossref",
"first-page": "161",
"journal-title": "Histochem. Cell Biol.",
"key": "ref_41",
"volume": "162",
"year": "2024"
},
{
"DOI": "10.3389/fncel.2019.00156",
"doi-asserted-by": "crossref",
"key": "ref_42",
"unstructured": "Pfister, A.S. (2019). Emerging Role of the Nucleolar Stress Response in Autophagy. Front. Cell. Neurosci., 13."
},
{
"DOI": "10.1038/s41388-017-0121-z",
"article-title": "Nucleolus as an Emerging Hub in Maintenance of Genome Stability and Cancer Pathogenesis",
"author": "Jurada",
"doi-asserted-by": "crossref",
"first-page": "2351",
"journal-title": "Oncogene",
"key": "ref_43",
"volume": "37",
"year": "2018"
},
{
"DOI": "10.1016/bs.apcsb.2023.01.001",
"article-title": "Chapter Seven—The Role of the Nucleolus in Regulating the Cell Cycle and the DNA Damage Response",
"author": "Donev",
"doi-asserted-by": "crossref",
"first-page": "203",
"journal-title": "Advances in Protein Chemistry and Structural Biology",
"key": "ref_44",
"volume": "Volume 135",
"year": "2023"
},
{
"DOI": "10.1242/jcs.00371",
"article-title": "Dynamic Association of RNA-Editing Enzymes with the Nucleolus",
"author": "Desterro",
"doi-asserted-by": "crossref",
"first-page": "1805",
"journal-title": "J. Cell Sci.",
"key": "ref_45",
"volume": "116",
"year": "2003"
},
{
"DOI": "10.1016/j.celrep.2023.112577",
"article-title": "TCAB1 Prevents Nucleolar Accumulation of the Telomerase RNA to Facilitate Telomerase Assembly",
"author": "Klump",
"doi-asserted-by": "crossref",
"first-page": "112577",
"journal-title": "Cell Rep.",
"key": "ref_46",
"volume": "42",
"year": "2023"
},
{
"DOI": "10.1016/j.molcel.2010.09.024",
"article-title": "The Nucleolus under Stress",
"author": "Boulon",
"doi-asserted-by": "crossref",
"first-page": "216",
"journal-title": "Mol. Cell",
"key": "ref_47",
"volume": "40",
"year": "2010"
},
{
"DOI": "10.1093/jb/mvm222",
"article-title": "The Structure and Functions of NPM1/Nucleophsmin/B23, a Multifunctional Nucleolar Acidic Protein",
"author": "Okuwaki",
"doi-asserted-by": "crossref",
"first-page": "441",
"journal-title": "J. Biochem.",
"key": "ref_48",
"volume": "143",
"year": "2008"
},
{
"DOI": "10.1261/rna.077396.120",
"article-title": "High-Resolution Structure of Eukaryotic Fibrillarin Interacting with Nop56 Amino-Terminal Domain",
"author": "Lukat",
"doi-asserted-by": "crossref",
"first-page": "496",
"journal-title": "RNA",
"key": "ref_49",
"volume": "27",
"year": "2021"
},
{
"DOI": "10.1021/acs.jproteome.6b00126",
"article-title": "Nuclear Functions of Nucleolin through Global Proteomics and Interactomic Approaches",
"author": "Salvetti",
"doi-asserted-by": "crossref",
"first-page": "1659",
"journal-title": "J. Proteome Res.",
"key": "ref_50",
"volume": "15",
"year": "2016"
},
{
"DOI": "10.1128/MCB.17.12.7088",
"article-title": "Nucleolar KKE/D Repeat Proteins Nop56p and Nop58p Interact with Nop1p and Are Required for Ribosome Biogenesis",
"author": "Gautier",
"doi-asserted-by": "crossref",
"first-page": "7088",
"journal-title": "Mol. Cell Biol.",
"key": "ref_51",
"volume": "17",
"year": "1997"
},
{
"DOI": "10.1261/rna.5970404",
"article-title": "Analysis of Snu13p Mutations Reveals Differential Interactions with the U4 snRNA and U3 snoRNA",
"author": "Dobbyn",
"doi-asserted-by": "crossref",
"first-page": "308",
"journal-title": "RNA",
"key": "ref_52",
"volume": "10",
"year": "2004"
},
{
"DOI": "10.7554/eLife.13571",
"article-title": "Nucleophosmin Integrates within the Nucleolus via Multi-Modal Interactions with Proteins Displaying R-Rich Linear Motifs and rRNA",
"author": "Mitrea",
"doi-asserted-by": "crossref",
"first-page": "e13571",
"journal-title": "eLife",
"key": "ref_53",
"volume": "5",
"year": "2016"
},
{
"DOI": "10.1242/jcs.112.4.455",
"article-title": "The Nucleolar Phosphoprotein B23 Redistributes in Part to the Spindle Poles during Mitosis",
"author": "Zatsepina",
"doi-asserted-by": "crossref",
"first-page": "455",
"journal-title": "J. Cell Sci.",
"key": "ref_54",
"volume": "112",
"year": "1999"
},
{
"DOI": "10.1091/mbc.02-03-0036",
"article-title": "The RNA Binding Activity of a Ribosome Biogenesis Factor, Nucleophosmin/B23, Is Modulated by Phosphorylation with a Cell Cycle-Dependent Kinase and by Association with Its Subtype",
"author": "Okuwaki",
"doi-asserted-by": "crossref",
"first-page": "2016",
"journal-title": "MBoC",
"key": "ref_55",
"volume": "13",
"year": "2002"
},
{
"DOI": "10.1110/ps.8.4.905",
"article-title": "Nucleolar Protein B23 Has Molecular Chaperone Activities",
"author": "Szebeni",
"doi-asserted-by": "crossref",
"first-page": "905",
"journal-title": "Protein Sci.",
"key": "ref_56",
"volume": "8",
"year": "1999"
},
{
"DOI": "10.1002/cbin.11044",
"article-title": "Proliferation, Cancer, and Aging—Novel Functions of the Nucleolar Methyltransferase Fibrillarin?",
"author": "Shubina",
"doi-asserted-by": "crossref",
"first-page": "1463",
"journal-title": "Cell Biol. Int.",
"key": "ref_57",
"volume": "42",
"year": "2018"
},
{
"article-title": "Nucleolar Methyltransferase Fibrillarin: Evolution of Structure and Functions",
"author": "Shubina",
"first-page": "941",
"journal-title": "Biochemistry",
"key": "ref_58",
"volume": "81",
"year": "2016"
},
{
"DOI": "10.1016/j.yexcr.2009.01.016",
"article-title": "Fibrillarin and Nop56 Interact before Being Co-Assembled in Box C/D snoRNPs",
"author": "Lechertier",
"doi-asserted-by": "crossref",
"first-page": "928",
"journal-title": "Exp. Cell Res.",
"key": "ref_59",
"volume": "315",
"year": "2009"
},
{
"DOI": "10.1371/journal.pcbi.1008318",
"doi-asserted-by": "crossref",
"key": "ref_60",
"unstructured": "Pereira-Santana, A., Gamboa-Tuz, S.D., Zhao, T., Schranz, M.E., Vinuesa, P., Bayona, A., Rodríguez-Zapata, L.C., and Castano, E. (2020). Fibrillarin Evolution through the Tree of Life: Comparative Genomics and Microsynteny Network Analyses Provide New Insights into the Evolutionary History of Fibrillarin. PLoS Comput. Biol., 16."
},
{
"DOI": "10.1038/sj.emboj.7601046",
"article-title": "Nucleolin Is a Histone Chaperone with FACT-like Activity and Assists Remodeling of Nucleosomes",
"author": "Angelov",
"doi-asserted-by": "crossref",
"first-page": "1669",
"journal-title": "EMBO J.",
"key": "ref_61",
"volume": "25",
"year": "2006"
},
{
"DOI": "10.1371/journal.pone.0049245",
"doi-asserted-by": "crossref",
"key": "ref_62",
"unstructured": "Kobayashi, J., Fujimoto, H., Sato, J., Hayashi, I., Burma, S., Matsuura, S., Chen, D.J., and Komatsu, K. (2012). Nucleolin Participates in DNA Double-Strand Break-Induced Damage Response through MDC1-Dependent Pathway. PLoS ONE, 7."
},
{
"DOI": "10.1080/15476286.2021.2020455",
"article-title": "A Novel Role for Nucleolin in Splice Site Selection",
"author": "Shefer",
"doi-asserted-by": "crossref",
"first-page": "333",
"journal-title": "RNA Biol.",
"key": "ref_63",
"volume": "19",
"year": "2022"
},
{
"DOI": "10.3389/pore.2023.1610884",
"article-title": "The Roles of NOP56 in Cancer and SCA36",
"author": "Zhao",
"doi-asserted-by": "crossref",
"first-page": "1610884",
"journal-title": "Pathol. Oncol. Res.",
"key": "ref_64",
"volume": "29",
"year": "2023"
},
{
"DOI": "10.3390/biom10050783",
"doi-asserted-by": "crossref",
"key": "ref_65",
"unstructured": "Ojha, S., Malla, S., and Lyons, S.M. (2020). snoRNPs: Functions in Ribosome Biogenesis. Biomolecules, 10."
},
{
"DOI": "10.1042/BST20191046",
"article-title": "Small Nucleolar RNAs: Continuing Identification of Novel Members and Increasing Diversity of Their Molecular Mechanisms of Action",
"author": "Bergeron",
"doi-asserted-by": "crossref",
"first-page": "645",
"journal-title": "Biochem. Soc. Trans.",
"key": "ref_66",
"volume": "48",
"year": "2020"
},
{
"DOI": "10.1038/s41420-022-01056-8",
"article-title": "snoRNAs: Functions and Mechanisms in Biological Processes, and Roles in Tumor Pathophysiology",
"author": "Huang",
"doi-asserted-by": "crossref",
"first-page": "259",
"journal-title": "Cell Death Discov.",
"key": "ref_67",
"volume": "8",
"year": "2022"
},
{
"DOI": "10.1002/bies.201600264",
"doi-asserted-by": "crossref",
"key": "ref_68",
"unstructured": "Falaleeva, M., Welden, J.R., Duncan, M.J., and Stamm, S. (2017). C/D-Box snoRNAs Form Methylating and Non-Methylating Ribonucleoprotein Complexes: Old Dogs Show New Tricks. Bioessays, 39."
},
{
"DOI": "10.1016/j.molcel.2018.08.017",
"article-title": "Box C/D snoRNP Autoregulation by a Cis-Acting snoRNA in the NOP56 Pre-mRNA",
"author": "Ardal",
"doi-asserted-by": "crossref",
"first-page": "99",
"journal-title": "Mol. Cell",
"key": "ref_69",
"volume": "72",
"year": "2018"
},
{
"DOI": "10.1017/S135583829998192X",
"article-title": "Nop58p Is a Common Component of the Box C+D snoRNPs That Is Required for snoRNA Stability",
"author": "Lafontaine",
"doi-asserted-by": "crossref",
"first-page": "455",
"journal-title": "RNA",
"key": "ref_70",
"volume": "5",
"year": "1999"
},
{
"DOI": "10.1261/rna.2130503",
"article-title": "A Structural, Phylogenetic, and Functional Study of 15.5-kD/Snu13 Protein Binding on U3 Small Nucleolar RNA",
"author": "Charpentier",
"doi-asserted-by": "crossref",
"first-page": "821",
"journal-title": "RNA",
"key": "ref_71",
"volume": "9",
"year": "2003"
},
{
"DOI": "10.1038/s41598-021-97030-y",
"doi-asserted-by": "crossref",
"key": "ref_72",
"unstructured": "Höfler, S., Lukat, P., Blankenfeldt, W., and Carlomagno, T. (2021). Eukaryotic Box C/D Methylation Machinery Has Two Non-Symmetric Protein Assembly Sites. Sci. Rep., 11."
},
{
"DOI": "10.3389/fmicb.2022.1026887",
"doi-asserted-by": "crossref",
"key": "ref_73",
"unstructured": "Wang, X., Zhu, J., Zhang, D., and Liu, G. (2022). Ribosomal Control in RNA Virus-Infected Cells. Front. Microbiol., 13."
},
{
"DOI": "10.1128/JVI.72.9.7697-7702.1998",
"article-title": "The Nucleolus Is the Site of Borna Disease Virus RNA Transcription and Replication",
"author": "Pyper",
"doi-asserted-by": "crossref",
"first-page": "7697",
"journal-title": "J. Virol.",
"key": "ref_74",
"volume": "72",
"year": "1998"
},
{
"DOI": "10.1128/JVI.02046-13",
"article-title": "DNA Virus Replication Compartments",
"author": "Schmid",
"doi-asserted-by": "crossref",
"first-page": "1404",
"journal-title": "J. Virol.",
"key": "ref_75",
"volume": "88",
"year": "2014"
},
{
"DOI": "10.1007/s00705-001-0792-0",
"article-title": "The Nucleolus—A Gateway to Viral Infection?",
"author": "Hiscox",
"doi-asserted-by": "crossref",
"first-page": "1077",
"journal-title": "Arch. Virol.",
"key": "ref_76",
"volume": "147",
"year": "2002"
},
{
"DOI": "10.3390/cells10071597",
"doi-asserted-by": "crossref",
"key": "ref_77",
"unstructured": "Iarovaia, O.V., Ioudinkova, E.S., Velichko, A.K., and Razin, S.V. (2021). Manipulation of Cellular Processes via Nucleolus Hijaking in the Course of Viral Infection in Mammals. Cells, 10."
},
{
"DOI": "10.1073/pnas.0704632104",
"article-title": "Interaction of a Plant Virus-Encoded Protein with the Major Nucleolar Protein Fibrillarin Is Required for Systemic Virus Infection",
"author": "Kim",
"doi-asserted-by": "crossref",
"first-page": "11115",
"journal-title": "Proc. Natl. Acad. Sci. USA",
"key": "ref_78",
"volume": "104",
"year": "2007"
},
{
"DOI": "10.1007/s11033-023-08343-2",
"article-title": "Current Research on Viral Proteins That Interact with Fibrillarin",
"author": "Castano",
"doi-asserted-by": "crossref",
"first-page": "4631",
"journal-title": "Mol. Biol. Rep.",
"key": "ref_79",
"volume": "50",
"year": "2023"
},
{
"DOI": "10.1111/j.1462-5822.2006.00698.x",
"article-title": "Changes in Nucleolar Morphology and Proteins during Infection with the Coronavirus Infectious Bronchitis Virus",
"author": "Dove",
"doi-asserted-by": "crossref",
"first-page": "1147",
"journal-title": "Cell. Microbiol.",
"key": "ref_80",
"volume": "8",
"year": "2006"
},
{
"DOI": "10.1101/2022.06.07.495090",
"doi-asserted-by": "crossref",
"key": "ref_81",
"unstructured": "Mattola, S., Leclerc, S., Hakanen, S., Aho, V., Parrish, C.R., and Vihinen-Ranta, M. (2022). Parvovirus Infection Alters the Nucleolar Structure. bioRxiv."
},
{
"DOI": "10.3390/v7062760",
"article-title": "HIV Rev Assembly on the Rev Response Element (RRE): A Structural Perspective",
"author": "Rausch",
"doi-asserted-by": "crossref",
"first-page": "3053",
"journal-title": "Viruses",
"key": "ref_82",
"volume": "7",
"year": "2015"
},
{
"DOI": "10.1371/journal.pone.0048702",
"doi-asserted-by": "crossref",
"key": "ref_83",
"unstructured": "Jarboui, M.A., Bidoia, C., Woods, E., Roe, B., Wynne, K., Elia, G., Hall, W.W., and Gautier, V.W. (2012). Nucleolar Protein Trafficking in Response to HIV-1 Tat: Rewiring the Nucleolus. PLoS ONE, 7."
},
{
"DOI": "10.1073/pnas.95.18.10608",
"article-title": "Reconstitution of HIV-1 Rev Nuclear Export: Independent Requirements for Nuclear Import and Export",
"author": "Love",
"doi-asserted-by": "crossref",
"first-page": "10608",
"journal-title": "Proc. Natl. Acad. Sci. USA",
"key": "ref_84",
"volume": "95",
"year": "1998"
},
{
"DOI": "10.1371/journal.ppat.1005478",
"doi-asserted-by": "crossref",
"key": "ref_85",
"unstructured": "Deffrasnes, C., Marsh, G.A., Foo, C.H., Rootes, C.L., Gould, C.M., Grusovin, J., Monaghan, P., Lo, M.K., Tompkins, S.M., and Adams, T.E. (2016). Genome-Wide siRNA Screening at Biosafety Level 4 Reveals a Crucial Role for Fibrillarin in Henipavirus Infection. PLoS Pathog., 12."
},
{
"DOI": "10.1186/1743-422X-9-167",
"article-title": "Influenza A H3N2 Subtype Virus NS1 Protein Targets into the Nucleus and Binds Primarily via Its C-Terminal NLS2/NoLS to Nucleolin and Fibrillarin",
"author": "Tynell",
"doi-asserted-by": "crossref",
"first-page": "167",
"journal-title": "Virol. J.",
"key": "ref_86",
"volume": "9",
"year": "2012"
},
{
"DOI": "10.1016/j.bbrc.2007.08.091",
"article-title": "Influenza A Virus Non-Structural Protein 1 (NS1) Interacts with Cellular Multifunctional Protein Nucleolin during Infection",
"author": "Murayama",
"doi-asserted-by": "crossref",
"first-page": "880",
"journal-title": "Biochem. Biophys. Res. Commun.",
"key": "ref_87",
"volume": "362",
"year": "2007"
},
{
"DOI": "10.1038/s41598-017-18087-2",
"doi-asserted-by": "crossref",
"key": "ref_88",
"unstructured": "Yan, Y., Du, Y., Wang, G., and Li, K. (2017). Non-Structural Protein 1 of H3N2 Influenza A Virus Induces Nucleolar Stress via Interaction with Nucleolin. Sci. Rep., 7."
},
{
"DOI": "10.1371/journal.ppat.1000547",
"doi-asserted-by": "crossref",
"key": "ref_89",
"unstructured": "Hutzinger, R., Feederle, R., Mrazek, J., Schiefermeier, N., Balwierz, P.J., Zavolan, M., Polacek, N., Delecluse, H.-J., and Hüttenhofer, A. (2009). Expression and Processing of a Small Nucleolar RNA from the Epstein-Barr Virus Genome. PLoS Pathog., 5."
},
{
"DOI": "10.3390/ijms140917378",
"article-title": "Emerging Roles of Small Epstein-Barr Virus Derived Non-Coding RNAs in Epithelial Malignancy",
"author": "Lung",
"doi-asserted-by": "crossref",
"first-page": "17378",
"journal-title": "Int. J. Mol. Sci.",
"key": "ref_90",
"volume": "14",
"year": "2013"
},
{
"DOI": "10.1080/15476286.2021.1875184",
"article-title": "The Many Ways Epstein-Barr Virus Takes Advantage of the RNA Tool Kit",
"author": "Lee",
"doi-asserted-by": "crossref",
"first-page": "759",
"journal-title": "RNA Biol.",
"key": "ref_91",
"volume": "18",
"year": "2021"
},
{
"DOI": "10.1038/ncomms13599",
"article-title": "A Redox Mechanism Underlying Nucleolar Stress Sensing by Nucleophosmin",
"author": "Yang",
"doi-asserted-by": "crossref",
"first-page": "13599",
"journal-title": "Nat. Commun.",
"key": "ref_92",
"volume": "7",
"year": "2016"
},
{
"DOI": "10.1158/0008-5472.CAN-08-3468",
"article-title": "Activation of Ribosomal RNA Transcription by Hepatitis C Virus Involves Upstream Binding Factor Phosphorylation Via Induction of Cyclin D1",
"author": "Raychaudhuri",
"doi-asserted-by": "crossref",
"first-page": "2057",
"journal-title": "Cancer Res.",
"key": "ref_93",
"volume": "69",
"year": "2009"
},
{
"DOI": "10.1038/s41576-023-00662-1",
"article-title": "Non-Coding RNAs in Disease: From Mechanisms to Therapeutics",
"author": "Nemeth",
"doi-asserted-by": "crossref",
"first-page": "211",
"journal-title": "Nat. Rev. Genet.",
"key": "ref_94",
"volume": "25",
"year": "2024"
},
{
"DOI": "10.1038/s41580-022-00566-8",
"article-title": "Long Non-Coding RNAs: Definitions, Functions, Challenges and Recommendations",
"author": "Mattick",
"doi-asserted-by": "crossref",
"first-page": "430",
"journal-title": "Nat. Rev. Mol. Cell Biol.",
"key": "ref_95",
"volume": "24",
"year": "2023"
},
{
"DOI": "10.1515/jib-2019-0027",
"doi-asserted-by": "crossref",
"key": "ref_96",
"unstructured": "Zhang, P., Wu, W., Chen, Q., and Chen, M. (2019). Non-Coding RNAs and Their Integrated Networks. J. Integr. Bioinform., 16."
},
{
"DOI": "10.3389/fimmu.2018.03138",
"doi-asserted-by": "crossref",
"key": "ref_97",
"unstructured": "Wang, P. (2019). The Opening of Pandora’s Box: An Emerging Role of Long Noncoding RNA in Viral Infections. Front. Immunol., 9."
},
{
"DOI": "10.1002/rmv.2198",
"article-title": "Diverse Roles of Long Non-coding RNAs in Viral Diseases",
"author": "Ginn",
"doi-asserted-by": "crossref",
"first-page": "e2198",
"journal-title": "Rev. Med. Virol.",
"key": "ref_98",
"volume": "31",
"year": "2021"
},
{
"DOI": "10.3390/v6114212",
"article-title": "PAN’s Labyrinth: Molecular Biology of Kaposi’s Sarcoma-Associated Herpesvirus (KSHV) PAN RNA, a Multifunctional Long Noncoding RNA",
"author": "Rossetto",
"doi-asserted-by": "crossref",
"first-page": "4212",
"journal-title": "Viruses",
"key": "ref_99",
"volume": "6",
"year": "2014"
},
{
"DOI": "10.3389/fonc.2021.687629",
"doi-asserted-by": "crossref",
"key": "ref_100",
"unstructured": "Naipauer, J., García Solá, M.E., Salyakina, D., Rosario, S., Williams, S., Coso, O., Abba, M.C., Mesri, E.A., and Lacunza, E. (2021). A Non-Coding RNA Network Involved in KSHV Tumorigenesis. Front. Oncol., 11."
},
{
"DOI": "10.1371/journal.ppat.1002680",
"doi-asserted-by": "crossref",
"key": "ref_101",
"unstructured": "Rossetto, C.C., and Pari, G. (2012). KSHV PAN RNA Associates with Demethylases UTX and JMJD3 to Activate Lytic Replication through a Physical Interaction with the Virus Genome. PLoS Pathog., 8."
},
{
"DOI": "10.1186/s12985-021-01492-5",
"article-title": "Inhibition of Japanese Encephalitis Virus Proliferation by Long Non-Coding RNA SUSAJ1 in PK-15 Cells",
"author": "Zhou",
"doi-asserted-by": "crossref",
"first-page": "29",
"journal-title": "Virol. J.",
"key": "ref_102",
"volume": "18",
"year": "2021"
},
{
"DOI": "10.1128/JVI.00207-12",
"article-title": "West Nile Virus Noncoding Subgenomic RNA Contributes to Viral Evasion of the Type I Interferon-Mediated Antiviral Response",
"author": "Schuessler",
"doi-asserted-by": "crossref",
"first-page": "5708",
"journal-title": "J. Virol.",
"key": "ref_103",
"volume": "86",
"year": "2012"
},
{
"DOI": "10.1186/1743-422X-8-492",
"article-title": "Small Noncoding RNA Modulates Japanese Encephalitis Virus Replication and Translation in Trans",
"author": "Fan",
"doi-asserted-by": "crossref",
"first-page": "492",
"journal-title": "Virol. J.",
"key": "ref_104",
"volume": "8",
"year": "2011"
},
{
"DOI": "10.1128/JVI.01215-20",
"article-title": "The BHLF1 Locus of Epstein-Barr Virus Contributes to Viral Latency and B-Cell Immortalization",
"author": "Yetming",
"doi-asserted-by": "crossref",
"first-page": "e01215-20",
"journal-title": "J. Virol.",
"key": "ref_105",
"volume": "94",
"year": "2020"
},
{
"DOI": "10.1128/JVI.00698-21",
"article-title": "Human Cytomegalovirus RNA2.7 Is Required for Upregulating Multiple Cellular Genes to Promote Cell Motility and Viral Spread Late in Lytic Infection",
"author": "Lau",
"doi-asserted-by": "crossref",
"first-page": "e00698-21",
"journal-title": "J. Virol.",
"key": "ref_106",
"volume": "95",
"year": "2021"
},
{
"DOI": "10.1371/journal.ppat.1009947",
"doi-asserted-by": "crossref",
"key": "ref_107",
"unstructured": "Ding, M., Wu, J., Sun, R., Yan, L., Bai, L., Shi, J., Feng, H., Zhang, Y., Lan, K., and Wang, X. (2021). Androgen Receptor Transactivates KSHV Noncoding RNA PAN to Promote Lytic Replication–Mediated Oncogenesis: A Mechanism of Sex Disparity in KS. PLOS Pathog., 17."
},
{
"DOI": "10.3390/v11010033",
"doi-asserted-by": "crossref",
"key": "ref_108",
"unstructured": "Zou, W., Xiong, M., Deng, X., Engelhardt, J.F., Yan, Z., and Qiu, J. (2019). A Comprehensive RNA-Seq Analysis of Human Bocavirus 1 Transcripts in Infected Human Airway Epithelium. Viruses, 11."
},
{
"DOI": "10.1128/JVI.05886-11",
"article-title": "Kaposi’s Sarcoma-Associated Herpesvirus Noncoding Polyadenylated Nuclear RNA Interacts with Virus- and Host Cell-Encoded Proteins and Suppresses Expression of Genes Involved in Immune Modulation",
"author": "Rossetto",
"doi-asserted-by": "crossref",
"first-page": "13290",
"journal-title": "J. Virol.",
"key": "ref_109",
"volume": "85",
"year": "2011"
},
{
"DOI": "10.3390/v13040706",
"doi-asserted-by": "crossref",
"key": "ref_110",
"unstructured": "De Jesús-González, L.A., Palacios-Rápalo, S., Reyes-Ruiz, J.M., Osuna-Ramos, J.F., Cordero-Rivera, C.D., Farfan-Morales, C.N., Gutiérrez-Escolano, A.L., and del Ángel, R.M. (2021). The Nuclear Pore Complex Is a Key Target of Viral Proteases to Promote Viral Replication. Viruses, 13."
},
{
"DOI": "10.1016/j.virol.2019.11.015",
"article-title": "Novel Anti-Flavivirus Drugs Targeting the Nucleolar Distribution of Core Protein",
"author": "Tokunaga",
"doi-asserted-by": "crossref",
"first-page": "41",
"journal-title": "Virology",
"key": "ref_111",
"volume": "541",
"year": "2020"
},
{
"article-title": "Nucleo-Cytoplasmic Transport of ZIKV Non-Structural 3 Protein Is Mediated by Importin-α/β and Exportin CRM-1",
"author": "Cisneros",
"first-page": "e01773-22",
"journal-title": "J. Virol.",
"key": "ref_112",
"volume": "97",
"year": "2022"
},
{
"DOI": "10.1158/1535-7163.MCT-14-0256",
"article-title": "Small Molecule BMH-Compounds That Inhibit RNA Polymerase I and Cause Nucleolar Stress",
"author": "Peltonen",
"doi-asserted-by": "crossref",
"first-page": "2537",
"journal-title": "Mol. Cancer Ther.",
"key": "ref_113",
"volume": "13",
"year": "2014"
},
{
"DOI": "10.7554/eLife.88799",
"article-title": "Distinct States of Nucleolar Stress Induced by Anticancer Drugs",
"author": "Potapova",
"doi-asserted-by": "crossref",
"first-page": "RP88799",
"journal-title": "eLife",
"key": "ref_114",
"volume": "12",
"year": "2023"
},
{
"DOI": "10.3390/cells8091090",
"doi-asserted-by": "crossref",
"key": "ref_115",
"unstructured": "Carotenuto, P., Pecoraro, A., Palma, G., Russo, G., and Russo, A. (2019). Therapeutic Approaches Targeting Nucleolus in Cancer. Cells, 8."
},
{
"DOI": "10.1016/j.isci.2023.108294",
"article-title": "An Ivermectin—Atorvastatin Combination Impairs Nuclear Transport Inhibiting Dengue Infection in Vitro and in Vivo",
"doi-asserted-by": "crossref",
"first-page": "108294",
"journal-title": "iScience",
"key": "ref_116",
"volume": "26",
"year": "2023"
},
{
"DOI": "10.1038/s41467-024-45128-y",
"article-title": "Nuclear to Cytoplasmic Transport Is a Druggable Dependency in MYC-Driven Hepatocellular Carcinoma",
"author": "Deutzmann",
"doi-asserted-by": "crossref",
"first-page": "963",
"journal-title": "Nat. Commun.",
"key": "ref_117",
"volume": "15",
"year": "2024"
},
{
"DOI": "10.1016/j.bmcl.2022.129016",
"doi-asserted-by": "crossref",
"key": "ref_118",
"unstructured": "Xu, H., and Hurley, L.H. (2022). A First-in-Class Clinical G-Quadruplex-Targeting Drug. The Bench-to-Bedside Translation of the Fluoroquinolone QQ58 to CX-5461 (Pidnarulex). Bioorganic Med. Chem. Lett., 77."
},
{
"DOI": "10.1083/jcb.200201024",
"article-title": "Cyclin-Dependent Kinases Govern Formation and Maintenance of the Nucleolus",
"author": "Sirri",
"doi-asserted-by": "crossref",
"first-page": "969",
"journal-title": "J. Cell Biol.",
"key": "ref_119",
"volume": "156",
"year": "2002"
},
{
"DOI": "10.1101/gad.11.12.1605",
"article-title": "SV40 Large T Antigen Binds to the TBP-TAF(I) Complex SL1 and Coactivates Ribosomal RNA Transcription",
"author": "Zhai",
"doi-asserted-by": "crossref",
"first-page": "1605",
"journal-title": "Genes Dev.",
"key": "ref_120",
"volume": "11",
"year": "1997"
},
{
"DOI": "10.1128/MCB.19.4.2791",
"article-title": "A Kinase Activity Associated with Simian Virus 40 Large T Antigen Phosphorylates Upstream Binding Factor (UBF) and Promotes Formation of a Stable Initiation Complex between UBF and SL1",
"author": "Zhai",
"doi-asserted-by": "crossref",
"first-page": "2791",
"journal-title": "Mol. Cell Biol.",
"key": "ref_121",
"volume": "19",
"year": "1999"
},
{
"DOI": "10.3390/v13122458",
"doi-asserted-by": "crossref",
"key": "ref_122",
"unstructured": "Forte, I.M., Indovina, P., Montagnaro, S., Costa, A., Iannuzzi, C.A., Capone, F., Camerlingo, R., Malfitano, A.M., Pentimalli, F., and Ferrara, G. (2021). The Oncolytic Caprine Herpesvirus 1 (CpHV-1) Induces Apoptosis and Synergizes with Cisplatin in Mesothelioma Cell Lines: A New Potential Virotherapy Approach. Viruses, 13."
},
{
"DOI": "10.1016/j.mehy.2020.109754",
"article-title": "5-Fluorouracil in Combination with Deoxyribonucleosides and Deoxyribose as Possible Therapeutic Options for the Coronavirus, COVID-19 Infection",
"author": "Ahmad",
"doi-asserted-by": "crossref",
"first-page": "109754",
"journal-title": "Med. Hypotheses",
"key": "ref_123",
"volume": "142",
"year": "2020"
},
{
"DOI": "10.1016/j.antiviral.2017.04.008",
"article-title": "Antiviral Screen Identifies EV71 Inhibitors and Reveals Camptothecin-Target, DNA Topoisomerase 1 as a Novel EV71 Host Factor",
"author": "Wu",
"doi-asserted-by": "crossref",
"first-page": "122",
"journal-title": "Antivir. Res.",
"key": "ref_124",
"volume": "143",
"year": "2017"
},
{
"DOI": "10.3389/fcimb.2022.823820",
"doi-asserted-by": "crossref",
"key": "ref_125",
"unstructured": "Yin, D., Yin, L., Wang, J., Shen, X., Dai, Y., Zhao, R., Hu, X., Hou, H., Zhang, D., and Wang, G. (2022). Antiviral and Virucidal Activities of Camptothecin on Fowl Adenovirus Serotype 4 by Blocking Virus Replication. Front. Cell. Infect. Microbiol., 12."
},
{
"DOI": "10.1038/s41598-019-43047-3",
"doi-asserted-by": "crossref",
"key": "ref_126",
"unstructured": "Tai, C.-J., Liu, C.-H., Pan, Y.-C., Wong, S.H., Tai, C.-J., Richardson, C.D., and Lin, L.-T. (2019). Chemovirotherapeutic Treatment Using Camptothecin Enhances Oncolytic Measles Virus-Mediated Killing of Breast Cancer Cells. Sci. Rep., 9."
},
{
"DOI": "10.1128/AAC.00686-10",
"article-title": "A Derivate of the Antibiotic Doxorubicin Is a Selective Inhibitor of Dengue and Yellow Fever Virus Replication In Vitro",
"author": "Kaptein",
"doi-asserted-by": "crossref",
"first-page": "5269",
"journal-title": "Antimicrob. Agents Chemother.",
"key": "ref_127",
"volume": "54",
"year": "2010"
},
{
"DOI": "10.1080/07391102.2021.1905551",
"article-title": "Identification of Doxorubicin as a Potential Therapeutic against SARS-CoV-2 (COVID-19) Protease: A Molecular Docking and Dynamics Simulation Studies",
"author": "Alharbi",
"doi-asserted-by": "crossref",
"first-page": "7960",
"journal-title": "J. Biomol. Struct. Dyn.",
"key": "ref_128",
"volume": "40",
"year": "2022"
},
{
"DOI": "10.3390/v8030058",
"doi-asserted-by": "crossref",
"key": "ref_129",
"unstructured": "Nakano, K., and Watanabe, T. (2016). HTLV-1 Rex Tunes the Cellular Environment Favorable for Viral Replication. Viruses, 8."
},
{
"DOI": "10.1186/1475-2859-13-17",
"article-title": "The Myxobacterial Metabolite Ratjadone a Inhibits HIV Infection by Blocking the Rev/CRM1-Mediated Nuclear Export Pathway",
"author": "Martinez",
"doi-asserted-by": "crossref",
"first-page": "17",
"journal-title": "Microb. Cell Factories",
"key": "ref_130",
"volume": "13",
"year": "2014"
},
{
"DOI": "10.1128/JVI.01774-14",
"article-title": "Verdinexor, a Novel Selective Inhibitor of Nuclear Export, Reduces Influenza a Virus Replication In Vitro and In Vivo",
"author": "Perwitasari",
"doi-asserted-by": "crossref",
"first-page": "10228",
"journal-title": "J. Virol.",
"key": "ref_131",
"volume": "88",
"year": "2014"
},
{
"DOI": "10.1128/JVI.01684-18",
"article-title": "Verdinexor (KPT-335), a Selective Inhibitor of Nuclear Export, Reduces Respiratory Syncytial Virus Replication In Vitro",
"author": "Jorquera",
"doi-asserted-by": "crossref",
"first-page": "e01684-18",
"journal-title": "J. Virol.",
"key": "ref_132",
"volume": "93",
"year": "2019"
},
{
"DOI": "10.1016/j.antiviral.2021.105115",
"article-title": "Selinexor, a Novel Selective Inhibitor of Nuclear Export, Reduces SARS-CoV-2 Infection and Protects the Respiratory System in Vivo",
"author": "Kashyap",
"doi-asserted-by": "crossref",
"first-page": "105115",
"journal-title": "Antiviral Res.",
"key": "ref_133",
"volume": "192",
"year": "2021"
},
{
"DOI": "10.1128/JVI.00747-09",
"article-title": "Role for G-Quadruplex RNA Binding by Epstein-Barr Virus Nuclear Antigen 1 in DNA Replication and Metaphase Chromosome Attachment",
"author": "Norseen",
"doi-asserted-by": "crossref",
"first-page": "10336",
"journal-title": "J. Virol.",
"key": "ref_134",
"volume": "83",
"year": "2009"
},
{
"article-title": "Visualization of DNA G-Quadruplexes in Herpes Simplex Virus 1-Infected Cells",
"author": "Artusi",
"first-page": "10343",
"journal-title": "Nucleic Acids Res.",
"key": "ref_135",
"volume": "44",
"year": "2016"
},
{
"DOI": "10.1371/journal.ppat.1011095",
"doi-asserted-by": "crossref",
"key": "ref_136",
"unstructured": "Chung, W.-C., Ravichandran, S., Park, D., Lee, G.M., Kim, Y.-E., Choi, Y., Song, M.J., Kim, K.K., and Ahn, J.-H. (2023). G-Quadruplexes Formed by Varicella-Zoster Virus Reiteration Sequences Suppress Expression of Glycoprotein C and Regulate Viral Cell-to-Cell Spread. PLoS Pathog., 19."
},
{
"DOI": "10.1038/s41421-022-00450-x",
"article-title": "RNA G-Quadruplex Formed in SARS-CoV-2 Used for COVID-19 Treatment in Animal Models",
"author": "Qin",
"doi-asserted-by": "crossref",
"first-page": "86",
"journal-title": "Cell Discov.",
"key": "ref_137",
"volume": "8",
"year": "2022"
},
{
"DOI": "10.1016/j.jbc.2022.102486",
"doi-asserted-by": "crossref",
"key": "ref_138",
"unstructured": "Belachew, B., Gao, J., Byrd, A.K., and Raney, K.D. (2022). Hepatitis C Virus Nonstructural Protein NS3 Unfolds Viral G-Quadruplex RNA Structures. J. Biol. Chem., 298."
},
{
"DOI": "10.1371/journal.ppat.1007334",
"doi-asserted-by": "crossref",
"key": "ref_139",
"unstructured": "Ravichandran, S., Kim, Y.-E., Bansal, V., Ghosh, A., Hur, J., Subramani, V.K., Pradhan, S., Lee, M.K., Kim, K.K., and Ahn, J.-H. (2018). Genome-Wide Analysis of Regulatory G-Quadruplexes Affecting Gene Expression in Human Cytomegalovirus. PLoS Pathog., 14."
},
{
"DOI": "10.1002/jmv.27622",
"article-title": "G-Quadruplex Ligands Inhibit Chikungunya Virus Replication",
"author": "Lv",
"doi-asserted-by": "crossref",
"first-page": "2519",
"journal-title": "J. Med. Virol.",
"key": "ref_140",
"volume": "94",
"year": "2022"
},
{
"DOI": "10.1038/s41467-024-49761-5",
"article-title": "Structure of an RNA G-Quadruplex from the West Nile Virus Genome",
"author": "Terrell",
"doi-asserted-by": "crossref",
"first-page": "5428",
"journal-title": "Nat. Commun.",
"key": "ref_141",
"volume": "15",
"year": "2024"
},
{
"DOI": "10.1093/nar/gkac1030",
"article-title": "Deciphering RNA G-Quadruplex Function during the Early Steps of HIV-1 Infection",
"author": "Amrane",
"doi-asserted-by": "crossref",
"first-page": "12328",
"journal-title": "Nucleic Acids Res.",
"key": "ref_142",
"volume": "50",
"year": "2022"
},
{
"DOI": "10.1139/bcb-2023-0036",
"article-title": "The 3’ Terminal Region of Zika Virus RNA Contains a Conserved G-Quadruplex and Is Unfolded by Human DDX17",
"author": "Gemmill",
"doi-asserted-by": "crossref",
"first-page": "96",
"journal-title": "Biochem. Cell Biol.",
"key": "ref_143",
"volume": "102",
"year": "2024"
}
],
"reference-count": 143,
"references-count": 143,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.mdpi.com/2073-4409/13/18/1591"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "The Nucleolus and Its Interactions with Viral Proteins Required for Successful Infection",
"type": "journal-article",
"volume": "13"
}