The selective effect of Ivermectin on different human coronaviruses; in-vitro study
Amina Dakeh Shahin, Nofar Atari, Tal Meningher, Oran Erster, Dror Avni, Eli Schwartz, Michal Mandelboim
doi:10.21203/rs.3.rs-4180797/v1
Background: The outbreak of coronavirus disease COVID-19, caused by Severe Acute Respiratory Coronavirus-2 (SARS-CoV-2) has become an urgent public health concern worldwide. Although several clinical trials have pointed to new drugs with some anti-COVID-19 activity, we are far from having a safe and effective drug. In this study, we tested the effect of ivermectin on several coronaviruses (serotypes), including variants of SARS-CoV-2.
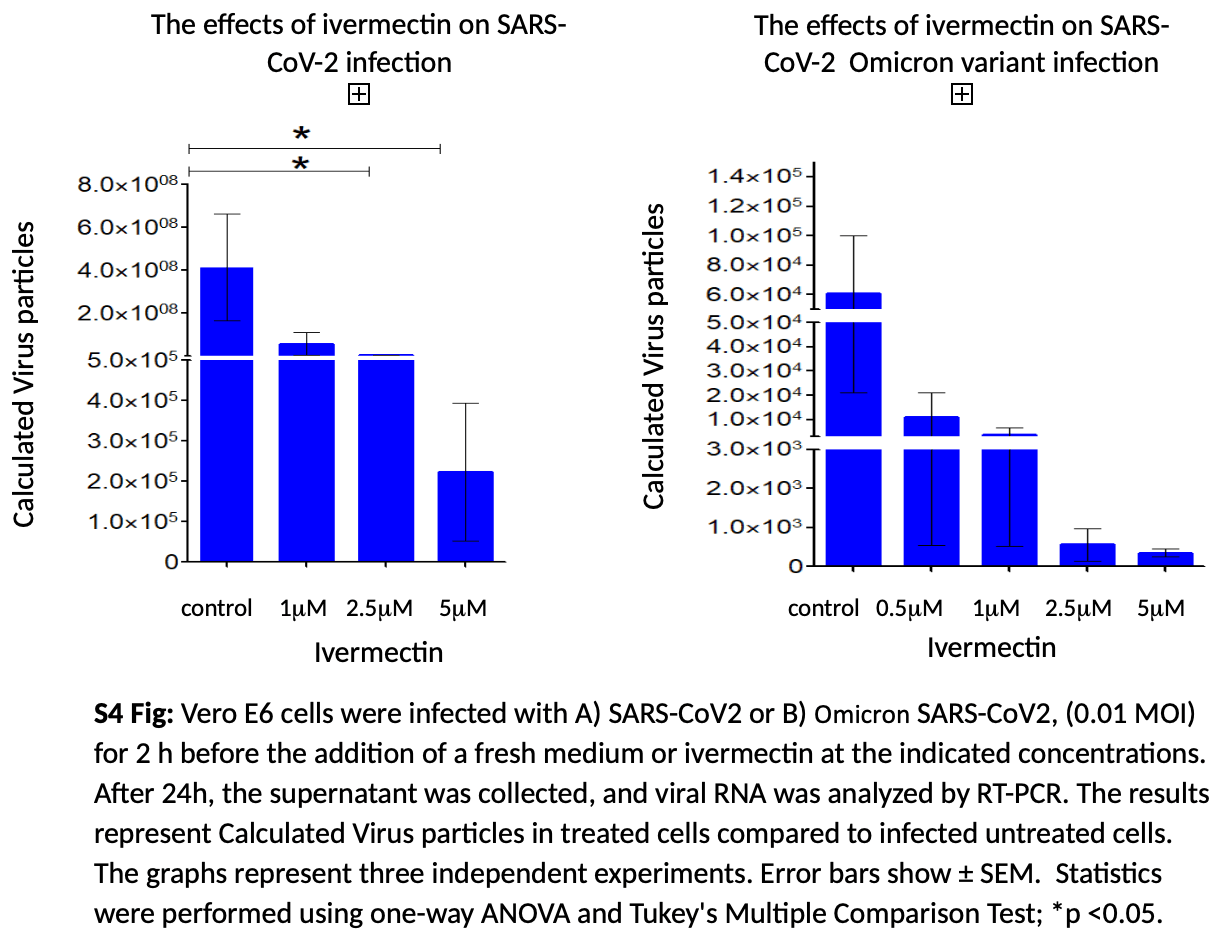
Methods: The effect of ivermectin was tested on cells infected with four different coronaviruses: NL63 (Alphacoronavirus genus.), OC43, SARS-CoV-2, and Omicron (all Betacoronavirus genus). Two hours post-infection, different doses of ivermectin were added to the cell culture. Results: There was no effect of even a high dose of ivermectin on NL63, however, we found a significant effect on OC43 PFU with a 40% inhibition at a dose of 5M. The impact of ivermectin on SARS-CoV-2 and on its Omicron variant was much more pronounced and at a dose of 5M there was inhibition of 90% and 95% respectively. Discussion: Although coronaviruses have been recognized as human pathogens for more than 50 years, no effective treatment strategy exists. Our current study did not demonstrate any effect of ivermectin on Alphacoronavirus but it had a specific impact on the Betacoronavirus genus with a mild impact on OC43 and a decidedly pronounced effect on SARS-CoV-2 including its Omicron variant. Ivermectin should be further studied as a single agent or as part of combined treatment against Coronaviruses.
Author Contributions: A.D.S., N. A., and T.M. performed the experiments. O. E. provided and calibrated the reagent for the RT-qPCR to all coronavirus variants. M. M., D. A., and E. S. conceived and planned the experiments, analyzed and interpreted the results supervised the project, and led the writing of the manuscript. All authors have read and agreed to the published version of the manuscript." Funding: Dakeh Shahin's fellowship was funded by The Meir and Edith Rosenfeld Foundation Institution Institutional Review Board Statement: All the experiments were done in cells in a culture with viruses that were also grown and isolated from cell culture. First isolation of Viruses were from positive nasopharyngeal swab samples. Since the virus was isolated from the swab media that was added to the cells in culture, and in practice no human tissue or material was used, according to the Institutional Review Board committee patient's consent was not required. This was approved by the Institutional Review Board, approval number 7875-20-SM
Conflicts of Interest: The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Supplementary Files This is a list of supplementary les associated with this preprint. Click to download. supplementary.docx
References
Altschul, Gapped BLAST and PSI-BLAST: a new generation of protein database search programs, Nucleic Acids Res,
doi:10.1093/nar/25.17.3389Alvisi, Importin α/β-dependent nuclear transport of human parvovirus B19 nonstructural protein 1 is essential for viral replication, Antiviral research,
doi:10.1016/j.antiviral.2023.105588Bennett, Zhao, Bosard, Imperiale, Role of a nuclear localization signal on the minor capsid proteins VP2 and VP3 in BKPyV nuclear entry, Virology,
doi:10.1016/j.virol.2014.10.013Biber, The effect of ivermectin on the viral load and culture viability in early treatment of nonhospitalized patients with mild COVID-19 -a double-blind, randomized placebo-controlled trial, International journal of infectious diseases : IJID : official publication of the International Society for Infectious Diseases,
doi:10.1016/j.ijid.2022.07.003Brucková, Mcintosh, Kapikian, Chanock, The Adaptation of Two Human Coronavirus Strains (OC38 and OC43) to Growth in Cell Monolayers, Proceedings of the Society for Experimental Biology and Medicine,
doi:10.3181/00379727-135-35068Bryant, Ivermectin for Prevention and Treatment of COVID-19 Infection: A Systematic Review, Meta-analysis, and Trial Sequential Analysis to Inform Clinical Guidelines, American journal of therapeutics,
doi:10.1097/mjt.0000000000001402Chaouat, Anti-human ACE2 antibody neutralizes and inhibits virus production of SARS-CoV-2 variants of concern, iScience,
doi:10.1016/j.isci.2022.104935Cobos-Campos, Potential use of ivermectin for the treatment and prophylaxis of SARS-CoV-2 infection, Current research in translational medicine,
doi:10.1016/j.retram.2021.103309Corman, Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR, Euro surveillance : bulletin Europeen sur les maladies transmissibles = European communicable disease bulletin,
doi:10.2807/1560-7917.es.2020.25.3.2000045De La Rocha, Ivermectin compared with placebo in the clinical course in Mexican patients with asymptomatic and mild COVID-19: a randomized clinical trial, BMC infectious diseases,
doi:10.1186/s12879-022-07890-6Eweas, Alhossary, Abdel-Moneim, Molecular Docking Reveals Ivermectin and Remdesivir as Potential Repurposed Drugs Against SARS-CoV-2, Front Microbiol,
doi:10.3389/fmicb.2020.592908Hajjar, Memish, Mcintosh, Middle East Respiratory Syndrome Coronavirus (MERS-CoV): a perpetual challenge, Annals of Saudi medicine,
doi:10.5144/0256-4947.2013.427Herzog, Drosten, Müller, Plaque assay for human coronavirus NL63 using human colon carcinoma cells, Virology journal,
doi:10.1186/1743-422x-5-138Hill, Mirchandani, Pilkington, Ivermectin for COVID-19: Addressing Potential Bias and Medical Fraud, Open forum infectious diseases,
doi:10.1093/ofid/ofab645ofab645Jochmans, Leyssen, Neyts, A novel method for high-throughput screening to quantify antiviral activity against viruses that induce limited CPE, Journal of virological methods,
doi:10.1016/j.jviromet.2012.04.011Kim, An, Kim, Hwang, Comparative efficacy and safety of pharmacological interventions for the treatment of COVID-19: A systematic review and network meta-analysis, PLoS medicine,
doi:10.1371/journal.pmed.1003501Kim, Antiviral effects of human placenta hydrolysate (Laennec(®)) against SARS-CoV-2 in vitro and in the ferret model, Journal of microbiology,
doi:10.1007/s12275-021-1367-2King, Tessier, Dodge, Weinberg, Mymryk, Inhibition of Human Adenovirus Replication by the Importin α/β1 Nuclear Import Inhibitor Ivermectin, Journal of virology,
doi:10.1128/jvi.00710-20Kow, Merchant, Mustafa, Hasan, The association between the use of ivermectin and mortality in patients with COVID-19: a meta-analysis, Pharmacological reports,
doi:10.1007/s43440-021-00245-zLehrer, Rheinsten, Ivermectin Docks to the SARS-CoV-2 Spike Receptor-binding Domain Attached to ACE2, Vivo,
doi:10.21873/invivo.12134Li, Ivermectin effectively inhibits hepatitis E virus replication, requiring the host nuclear transport protein importin α1, Archives of virology,
doi:10.1007/s00705-021-05096-wLin, Identification of residues in the receptor-binding domain (RBD) of the spike protein of human coronavirus NL63 that are critical for the RBD-ACE2 receptor interaction, The Journal of general virology,
doi:10.1099/vir.0.83331-0Lustig, Neutralizing Response against Variants after SARS-CoV-2 Infection and One Dose of BNT162b2, N Engl J Med,
doi:10.1056/NEJMc2104036Malin, Suárez, Priesner, Fätkenheuer, Rybniker, Remdesivir against COVID-19 and Other Viral Diseases, Clinical microbiology reviews,
doi:10.1128/cmr.00162-20Momekov, Momekova, Ivermectin as a potential COVID-19 treatment from the pharmacokinetic point of view: antiviral levels are not likely attainable with known dosing regimens, Biotechnology & Biotechnological Equipment,
doi:10.1080/13102818.2020.1775118Najjar-Debbiny, Effectiveness of Paxlovid in Reducing Severe COVID-19 and Mortality in High Risk Patients. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America,
doi:10.1093/cid/ciac443Patil, Verma, Masand, Prospective mode of action of Ivermectin: SARS-CoV-2, European journal of medicinal chemistry reports,
doi:10.1016/j.ejmcr.2021.100018Raza, Ivermectin Inhibits Bovine Herpesvirus 1 DNA Polymerase Nuclear Import and Interferes With Viral Replication, Microorganisms
Rozenberg, Evaluation of the relationship between quantitative PCR results and cell culturing of SARS2-CoV with respect to symptoms onset and Viral loada systematic review, medRxiv,
doi:10.1101/2021.08.23.21262162Siedner, Ivermectin for the Treatment of COVID-19 Disease: Too Good to Pass Up or Too Good to Be True?, Open forum infectious diseases,
doi:10.1093/ofid/ofab318Siemieniuk, Drug treatments for covid-19: living systematic review and network meta-analysis, Bmj,
doi:10.1136/bmj.m2980Singh, Singh, Singh, Misra, Molnupiravir in COVID-19: A systematic review of literature, Diabetes & metabolic syndrome,
doi:10.1016/j.dsx.2021.102329Suputtamongkol, Ivermectin Accelerates Circulating Nonstructural Protein 1 (NS1) Clearance in Adult Dengue Patients: A Combined Phase 2/3 Randomized Double-blinded Placebo Controlled Trial, Clinical Infectious Diseases,
doi:10.1093/cid/ciaa1332Tamura, Nei, Kumar, Prospects for inferring very large phylogenies by using the neighbor-joining method, Proc Natl Acad Sci U S A,
doi:10.1073/pnas.0404206101Tamura, Stecher, Kumar, MEGA11: Molecular Evolutionary Genetics Analysis Version 11, Molecular Biology and Evolution,
doi:10.1093/molbev/msab120Wagstaff, Rawlinson, Hearps, Jans, An AlphaScreen®-based assay for high-throughput screening for specific inhibitors of nuclear import, Journal of biomolecular screening,
doi:10.1177/1087057110390360Wu, Li, Peng, Li, Crystal structure of NL63 respiratory coronavirus receptor-binding domain complexed with its human receptor, Proc Natl Acad Sci U S A,
doi:10.1073/pnas.0908837106Yesilbag, Toker, Ates, Ivermectin also inhibits the replication of bovine respiratory viruses (BRSV, BPIV-3, BoHV-1, BCoV and BVDV) in vitro, Virus research,
doi:10.1016/j.virusres.2021.198384Zaidi, Dehgani-Mobaraki, The mechanisms of action of ivermectin against SARS-CoV-2-an extensive review, The Journal of Antibiotics,
doi:10.1038/s41429-021-00491-6Zein, Sulistiyana, Raffaelo, Pranata, Ivermectin and mortality in patients with COVID-19: A systematic review, meta-analysis, and meta-regression of randomized controlled trials, Diabetes & metabolic syndrome,
doi:10.1016/j.dsx.2021.102186