Ivermectin compared with placebo in the clinical course in Mexican patients with asymptomatic and mild COVID-19: a randomized clinical trial
Carmen De La Rocha, Marco A Cid-López, Blanca I Venegas-López, Sandra C Gómez-Méndez, Adriana Sánchez-Ortiz, Alma M Pérez-Ríos, Ricardo A Llamas-Velázquez, Aidé I Meza-Acuña, Bárbara Vargas-Íñiguez, Daniela Rosales-Galván, Alejandra Tavares-Váldez, Nizdali Luna-Gudiño, Cinthia V Hernández-Puente, Jovana Milenkovic, Cecilia Iglesias-Palomares, Miriam Méndez-Del Villar, Gerardo A Gutiérrez-Dieck, Carlos G Valderrábano-Roldán, Jennefer Mercado-Cerda, Jocelyn G Robles-Bojórquez, Arieh R Mercado-Sesma
BMC Infectious Diseases, doi:10.1186/s12879-022-07890-6
Background: Despite the development and application of vaccines against Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) around the world, the scientific community is still trying to find some therapies to avoid or ameliorate the fatal evolution of the Coronavirus disease 2019 . Since the publication of the potential use of ivermectin as a treatment against the disease, a pleiad of information about it has been published. However, the evidence is not strong or weak enough to conclude its usefulness in the clinical evolution of patients infected with SARS-CoV-2. We evaluate the efficacy and safety of ivermectin in the treatment of Mexican patients with asymptomatic and mild COVID-19 in a three-day administration in comparison to placebo.
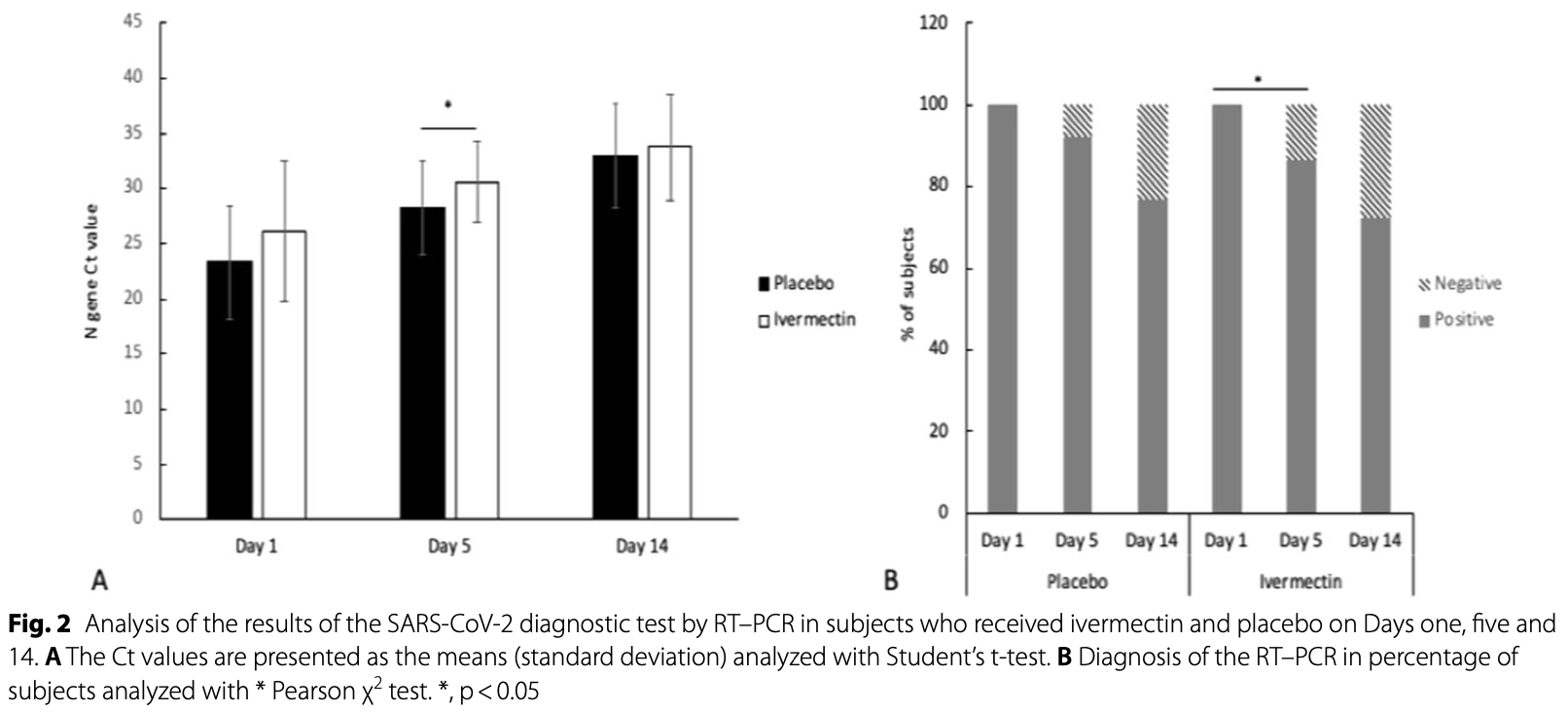
Methods: A randomized, double-blind, placebo-controlled trial was carried out in 66 adults with asymptomatic and mild COVID-19. Patients were randomly assigned 1:1 ratio to ivermectin plus acetaminophen or placebo plus acetaminophen. The primary endpoint was the proportion of subjects without a disease progression to severity according to COVID-19 guidelines by the National Institutes of Health (NIH) since randomization to 14 days. Results: None of the participants presented progression to a severe state in either group. Viral load was measured on Days 1, 5, and 14. No significant differences were observed in baseline or 14-day between groups (p = 0.720 and 0.362, respectively). However, on Day 5, a significant difference in viral load was observed between groups (p = 0.039). The frequency of symptoms was similar between groups, and no significant differences were observed. The most frequent symptom was cough. One severe adverse event associated with SARS-CoV-2 infection was observed in the ivermectin group.
Supplementary Information The online version contains supplementary material available at https:// doi. org/ 10. 1186/ s12879-022-07890-6. Additional file 1. CONSORT checklist.
Additional file 2. Supplementary tables. Author contributions dlRC, MSAR and CLM: Conceptualization, methodology, formal analysis, investigation, writing original draft visualization, and validation. VLB and GMSC: writing review and editing. HPCV, MJ, SOA, PRAM, LVRA, MAAI, VIB, IPC, and RGD: resources, formal analysis, software and methodology. TVA, LGN, MVM GDGA, VRCG, MCJ and RBJG: writing review, conceptualization, funding acquisition and supervision. All authors contributed to the interpretation of the results, provided critical revisions. All authors read and approved the final manuscript.
Funding Investigación Biomédica para el Desarrollo de Fármacos S.A. de C.V. covered all the expenses generated during the study. This trial was supported Investigación Biomédica para el Desarrollo de Fármacos S.A. de C.V. The funding source participated in study design, data collection, data analysis, data interpretation, or writing of the report, since most of the team are affiliated, but no commercial purposes are intended with this trial results.
Declarations Ethics approval and consent to participate The protocol was approved by the local ethics, biosafety and investigation committees and the Mexican health ministry COFEPRIS: 203301410A0055. The study is registered on ClinicalTrials.gov: NCT04407507...
References
Ahmed, Karim, Ross, A five-day course of ivermectin for the treatment of COVID-19 may reduce the duration of illness, Int J Infect Dis
Caly, Druce, Catton, Jans, Wagstaff, The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro, Antiviral Res
Canga, Prieto, Liébana, Martínez, Vega et al., The pharmacokinetics and interactions of ivermectin in humans-a minireview, AAPS J
Chaccour, Casellas, Blanco-Di Matteo, The effect of early treatment with ivermectin on viral load, symptoms and humoral response in patients with non-severe COVID-19: a pilot, double-blind, placebocontrolled, randomized clinical trial, EClinicalMedicine
Crump, Ivermectin: enigmatic multifaceted 'wonder' drug continues to surprise and exceed expectations, J Antibiot
Garg, Paliwal, Gupta, Encephalopathy in patients with COVID-19: a review, J Med Virol
Hariyanto, Halim, Gunawan, Kurniawan, Ivermectin and outcomes from Covid-19 pneumonia: a systematic review and metaanalysis of randomized clinical trial studies, Rev Med Virol,
doi:10.1002/rmv.2265Heidary, Gharebaghi, Ivermectin: a systematic review from antiviral effects to COVID-19 complementary regimen, J Antibiot
Huang, Wang, Li, Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China, Lancet
Kim, An, Kim, Hwang, Comparative efficacy and safety of pharmacological interventions for the treatment of COVID-19: a systematic review and network meta-analysis, PLoS Med
Liu, Yan, Wan, Viral dynamics in mild and severe cases of COVID-19, Lancet Infect Dis
López-Medina, López, Hurtado, Effect of ivermectin on time to resolution of symptoms among adults with mild COVID-19: a randomized clinical trial, JAMA
Merck, Co, Stromectrol, FDA approved package insert
Mohan, Tiwari, Suri, Ivermectin in mild and moderate COVID-19 (RIVET-COV): a randomized, placebo-controlled trial, Prepr Res Sq,
doi:10.21203/rs.3.rs-191648/v1Okumuş, Demirtürk, Çetinkaya, Evaluation of the effectiveness and safety of adding ivermectin to treatment in severe COVID-19 patients, BMC Infect Dis
Popp, Stegemann, Metzendorf, Gould, Kranke et al., Ivermectin for preventing and treating COVID-19, Cochrane Database System Rev
Pott-Junior, Paoliello, Miguel, Use of ivermectin in the treatment of Covid-19: a pilot trial, Toxicol Rep
Samaha, Mouawia, Fawaz, Effects of a single dose of ivermectin on viral and clinical outcomes in asymptomatic SARS-CoV-2 infected subjects: a pilot clinical trial in lebanon, Viruses
Schmith, Zhou, Lohmer, The approved dose of ivermectin alone is not the ideal dose for the treatment of COVID-19, Clin Pharmacol Ther
Shahbaznejad, Davoudi, Eslami, Effects of ivermectin in patients with COVID-19: a multicenter, double-blind, randomized, controlled clinical trial, Clin Ther,
doi:10.1016/j.clinthera.2021.04.007Wölfel, Corman, Guggemos, Virological assessment of hospitalized patients with COVID-2019, Nature
Yang, Atkinson, Wang, The broad spectrum antiviral ivermectin targets the host nuclear transport importin α/β1 heterodimer, Antiviral Res
Ye, Ren, Lv, Encephalitis as a clinical manifestation of COVID-19, Brain Behav Immun
DOI record:
{
"DOI": "10.1186/s12879-022-07890-6",
"ISSN": [
"1471-2334"
],
"URL": "http://dx.doi.org/10.1186/s12879-022-07890-6",
"abstract": "<jats:title>Abstract</jats:title><jats:sec>\n <jats:title>Background</jats:title>\n <jats:p>Despite the development and application of vaccines against Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) around the world, the scientific community is still trying to find some therapies to avoid or ameliorate the fatal evolution of the Coronavirus disease 2019 (COVID-19). Since the publication of the potential use of ivermectin as a treatment against the disease, a pleiad of information about it has been published. However, the evidence is not strong or weak enough to conclude its usefulness in the clinical evolution of patients infected with SARS-CoV-2. We evaluate the efficacy and safety of ivermectin in the treatment of Mexican patients with asymptomatic and mild COVID-19 in a three-day administration in comparison to placebo.</jats:p>\n </jats:sec><jats:sec>\n <jats:title>Methods</jats:title>\n <jats:p>A randomized, double-blind, placebo-controlled trial was carried out in 66 adults with asymptomatic and mild COVID-19. Patients were randomly assigned 1:1 ratio to ivermectin plus acetaminophen or placebo plus acetaminophen. The primary endpoint was the proportion of subjects without a disease progression to severity according to COVID-19 guidelines by the National Institutes of Health (NIH) since randomization to 14 days.</jats:p>\n </jats:sec><jats:sec>\n <jats:title>Results</jats:title>\n <jats:p>None of the participants presented progression to a severe state in either group. Viral load was measured on Days 1, 5, and 14. No significant differences were observed in baseline or 14-day between groups (p = 0.720 and 0.362, respectively). However, on Day 5, a significant difference in viral load was observed between groups (p = 0.039). The frequency of symptoms was similar between groups, and no significant differences were observed. The most frequent symptom was cough. One severe adverse event associated with SARS-CoV-2 infection was observed in the ivermectin group.</jats:p>\n </jats:sec><jats:sec>\n <jats:title>Conclusions</jats:title>\n <jats:p>At standard doses, ivermectin is not effective to prevent progression to a severe state or reducing symptoms in adults with asymptomatic and mild COVID-19.</jats:p>\n <jats:p><jats:italic>Trial registration</jats:italic> The study was registered with ClinicalTrial.gov (NCT04407507) on May 29, 2020.</jats:p>\n </jats:sec>",
"alternative-id": [
"7890"
],
"article-number": "917",
"assertion": [
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Received",
"name": "received",
"order": 1,
"value": "13 May 2022"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Accepted",
"name": "accepted",
"order": 2,
"value": "21 November 2022"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "First Online",
"name": "first_online",
"order": 3,
"value": "8 December 2022"
},
{
"group": {
"label": "Declarations",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 1
},
{
"group": {
"label": "Ethics approval and consent to participate",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 2,
"value": "The protocol was approved by the local ethics, biosafety and investigation committees and the Mexican health ministry COFEPRIS: 203301410A0055. The study is registered on ClinicalTrials.gov: NCT04407507. Patients gave their written consent prior the participation on the study and informed consent was obtained from all the participants."
},
{
"group": {
"label": "Consent for publication",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 3,
"value": "Not applicable."
},
{
"group": {
"label": "Competing interests",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 4,
"value": "The authors declare that they have no competing interests."
}
],
"author": [
{
"affiliation": [],
"family": "de la Rocha",
"given": "Carmen",
"sequence": "first"
},
{
"affiliation": [],
"family": "Cid-López",
"given": "Marco A.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Venegas-López",
"given": "Blanca I.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gómez-Méndez",
"given": "Sandra C.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sánchez-Ortiz",
"given": "Adriana",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Pérez-Ríos",
"given": "Alma M.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Llamas-Velázquez",
"given": "Ricardo A.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Meza-Acuña",
"given": "Aidé I.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Vargas-Íñiguez",
"given": "Bárbara",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rosales-Galván",
"given": "Daniela",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tavares-Váldez",
"given": "Alejandra",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Luna-Gudiño",
"given": "Nizdali",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hernández-Puente",
"given": "Cinthia V.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Milenkovic",
"given": "Jovana",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Iglesias-Palomares",
"given": "Cecilia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Méndez-del Villar",
"given": "Miriam",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gutiérrez-Dieck",
"given": "Gerardo A.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Valderrábano-Roldán",
"given": "Carlos G.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mercado-Cerda",
"given": "Jennefer",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Robles-Bojórquez",
"given": "Jocelyn G.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-9025-9328",
"affiliation": [],
"authenticated-orcid": false,
"family": "Mercado-Sesma",
"given": "Arieh R.",
"sequence": "additional"
}
],
"clinical-trial-number": [
{
"clinical-trial-number": "nct04407507",
"registry": "10.18810/clinical-trials-gov"
}
],
"container-title": "BMC Infectious Diseases",
"container-title-short": "BMC Infect Dis",
"content-domain": {
"crossmark-restriction": false,
"domain": [
"link.springer.com"
]
},
"created": {
"date-parts": [
[
2022,
12,
8
]
],
"date-time": "2022-12-08T12:02:59Z",
"timestamp": 1670500979000
},
"deposited": {
"date-parts": [
[
2022,
12,
8
]
],
"date-time": "2022-12-08T12:03:55Z",
"timestamp": 1670501035000
},
"indexed": {
"date-parts": [
[
2022,
12,
9
]
],
"date-time": "2022-12-09T06:02:43Z",
"timestamp": 1670565763566
},
"is-referenced-by-count": 0,
"issue": "1",
"issued": {
"date-parts": [
[
2022,
12,
8
]
]
},
"journal-issue": {
"issue": "1",
"published-online": {
"date-parts": [
[
2022,
12
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
12,
8
]
],
"date-time": "2022-12-08T00:00:00Z",
"timestamp": 1670457600000
}
},
{
"URL": "https://creativecommons.org/licenses/by/4.0",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
12,
8
]
],
"date-time": "2022-12-08T00:00:00Z",
"timestamp": 1670457600000
}
}
],
"link": [
{
"URL": "https://link.springer.com/content/pdf/10.1186/s12879-022-07890-6.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/article/10.1186/s12879-022-07890-6/fulltext.html",
"content-type": "text/html",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/content/pdf/10.1186/s12879-022-07890-6.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "297",
"original-title": [],
"prefix": "10.1186",
"published": {
"date-parts": [
[
2022,
12,
8
]
]
},
"published-online": {
"date-parts": [
[
2022,
12,
8
]
]
},
"publisher": "Springer Science and Business Media LLC",
"reference": [
{
"DOI": "10.1016/S0140-6736(20)30183-5",
"author": "C Huang",
"doi-asserted-by": "publisher",
"first-page": "497",
"journal-title": "Lancet",
"key": "7890_CR1",
"unstructured": "Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506.",
"volume": "395",
"year": "2020"
},
{
"key": "7890_CR2",
"unstructured": "World Health Organization. Coronavirus disease (COVID-2019) situation report. 2021. https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19---20-july-2021."
},
{
"DOI": "10.1016/j.antiviral.2020.104787",
"author": "L Caly",
"doi-asserted-by": "publisher",
"first-page": "104787",
"journal-title": "Antiviral Res",
"key": "7890_CR3",
"unstructured": "Caly L, Druce JD, Catton MG, Jans DA, Wagstaff KM. The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antiviral Res. 2020;178:104787.",
"volume": "178",
"year": "2020"
},
{
"DOI": "10.1002/rmv.2265",
"author": "TI Hariyanto",
"doi-asserted-by": "publisher",
"journal-title": "Rev Med Virol",
"key": "7890_CR4",
"unstructured": "Hariyanto TI, Halim DA, Rosalind J, Gunawan C, Kurniawan A. Ivermectin and outcomes from Covid-19 pneumonia: a systematic review and meta-analysis of randomized clinical trial studies. Rev Med Virol. 2021. https://doi.org/10.1002/rmv.2265.",
"year": "2021"
},
{
"DOI": "10.1371/journal.pmed.1003501",
"author": "MS Kim",
"doi-asserted-by": "publisher",
"first-page": "e1003501",
"journal-title": "PLoS Med",
"key": "7890_CR5",
"unstructured": "Kim MS, An MH, Kim WJ, Hwang TH. Comparative efficacy and safety of pharmacological interventions for the treatment of COVID-19: a systematic review and network meta-analysis. PLoS Med. 2020;17:e1003501.",
"volume": "17",
"year": "2020"
},
{
"DOI": "10.1038/s41429-020-0336-z",
"author": "F Heidary",
"doi-asserted-by": "publisher",
"first-page": "593",
"journal-title": "J Antibiot",
"key": "7890_CR6",
"unstructured": "Heidary F, Gharebaghi R. Ivermectin: a systematic review from antiviral effects to COVID-19 complementary regimen. J Antibiot. 2020;73:593–602.",
"volume": "73",
"year": "2020"
},
{
"DOI": "10.1016/j.antiviral.2020.104760",
"author": "SNY Yang",
"doi-asserted-by": "publisher",
"journal-title": "Antiviral Res",
"key": "7890_CR7",
"unstructured": "Yang SNY, Atkinson SC, Wang C, et al. The broad spectrum antiviral ivermectin targets the host nuclear transport importin α/β1 heterodimer. Antiviral Res. 2020;177: 104760.",
"volume": "177",
"year": "2020"
},
{
"DOI": "10.1038/ja.2017.11",
"author": "A Crump",
"doi-asserted-by": "publisher",
"first-page": "495",
"journal-title": "J Antibiot",
"key": "7890_CR8",
"unstructured": "Crump A. Ivermectin: enigmatic multifaceted ‘wonder’ drug continues to surprise and exceed expectations. J Antibiot. 2017;70:495–505.",
"volume": "70",
"year": "2017"
},
{
"key": "7890_CR9",
"unstructured": "National Institutes of Health. COVID-19 Treatment Guidelines Panel. Coronavirus Disease 2019 (COVID-19) treatment guidelines. 2019. https://www.covid19treatmentguidelines.nih.gov/."
},
{
"DOI": "10.1038/s41586-020-2196-x",
"author": "R Wölfel",
"doi-asserted-by": "publisher",
"first-page": "465",
"journal-title": "Nature",
"key": "7890_CR10",
"unstructured": "Wölfel R, Corman VM, Guggemos W, et al. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020;581:465–9.",
"volume": "581",
"year": "2020"
},
{
"DOI": "10.1016/j.ijid.2020.11.191",
"author": "S Ahmed",
"doi-asserted-by": "publisher",
"first-page": "214",
"journal-title": "Int J Infect Dis",
"key": "7890_CR11",
"unstructured": "Ahmed S, Karim MM, Ross AG, et al. A five-day course of ivermectin for the treatment of COVID-19 may reduce the duration of illness. Int J Infect Dis. 2021;103:214–6.",
"volume": "103",
"year": "2021"
},
{
"DOI": "10.1016/j.toxrep.2021.03.003",
"author": "H Pott-Junior",
"doi-asserted-by": "publisher",
"first-page": "505",
"journal-title": "Toxicol Rep",
"key": "7890_CR12",
"unstructured": "Pott-Junior H, Bastos Paoliello MM, Miguel AQC, et al. Use of ivermectin in the treatment of Covid-19: a pilot trial. Toxicol Rep. 2021;8:505–10.",
"volume": "8",
"year": "2021"
},
{
"DOI": "10.1016/j.eclinm.2020.100720",
"author": "C Chaccour",
"doi-asserted-by": "publisher",
"journal-title": "EClinicalMedicine",
"key": "7890_CR13",
"unstructured": "Chaccour C, Casellas A, Blanco-Di Matteo A, et al. The effect of early treatment with ivermectin on viral load, symptoms and humoral response in patients with non-severe COVID-19: a pilot, double-blind, placebo-controlled, randomized clinical trial. EClinicalMedicine. 2021;32: 100720.",
"volume": "32",
"year": "2021"
},
{
"DOI": "10.3390/v13060989",
"author": "AA Samaha",
"doi-asserted-by": "publisher",
"first-page": "989",
"journal-title": "Viruses",
"key": "7890_CR14",
"unstructured": "Samaha AA, Mouawia H, Fawaz M, et al. Effects of a single dose of ivermectin on viral and clinical outcomes in asymptomatic SARS-CoV-2 infected subjects: a pilot clinical trial in lebanon. Viruses. 2021;13:989.",
"volume": "13",
"year": "2021"
},
{
"DOI": "10.21203/rs.3.rs-191648/v1",
"author": "A Mohan",
"doi-asserted-by": "publisher",
"journal-title": "Prepr Res Sq",
"key": "7890_CR15",
"unstructured": "Mohan A, Tiwari P, Suri T, et al. Ivermectin in mild and moderate COVID-19 (RIVET-COV): a randomized, placebo-controlled trial. Prepr Res Sq. 2021. https://doi.org/10.21203/rs.3.rs-191648/v1.",
"year": "2021"
},
{
"DOI": "10.1002/cpt.1889",
"author": "VD Schmith",
"doi-asserted-by": "publisher",
"first-page": "762",
"journal-title": "Clin Pharmacol Ther",
"key": "7890_CR16",
"unstructured": "Schmith VD, Zhou JJ, Lohmer LRL. The approved dose of ivermectin alone is not the ideal dose for the treatment of COVID-19. Clin Pharmacol Ther. 2020;108:762–5.",
"volume": "108",
"year": "2020"
},
{
"DOI": "10.1208/s12248-007-9000-9",
"author": "AG Canga",
"doi-asserted-by": "publisher",
"first-page": "42",
"journal-title": "AAPS J",
"key": "7890_CR17",
"unstructured": "Canga AG, Prieto AMS, Liébana MJD, Martínez NF, Vega MS, Vieitez JJG. The pharmacokinetics and interactions of ivermectin in humans–a mini-review. AAPS J. 2008;10:42–6.",
"volume": "10",
"year": "2008"
},
{
"DOI": "10.1016/S1473-3099(20)30232-2",
"author": "Y Liu",
"doi-asserted-by": "publisher",
"first-page": "656",
"journal-title": "Lancet Infect Dis",
"key": "7890_CR18",
"unstructured": "Liu Y, Yan L-M, Wan L, et al. Viral dynamics in mild and severe cases of COVID-19. Lancet Infect Dis. 2020;20:656–7.",
"volume": "20",
"year": "2020"
},
{
"DOI": "10.1101/2020.06.06.20124461",
"author": "JC Rajter",
"doi-asserted-by": "publisher",
"journal-title": "medRxiv",
"key": "7890_CR19",
"unstructured": "Rajter JC, Sherman MS, Fatteh N, Vogel F, Sacks J, Rajter J-J. ICON (Ivermectin in COvid Nineteen) study: use of ivermectin is associated with lower mortality in hospitalized patients with COVID19. medRxiv. 2020. https://doi.org/10.1101/2020.06.06.20124461.",
"year": "2020"
},
{
"DOI": "10.1186/s12879-021-06104-9",
"author": "N Okumuş",
"doi-asserted-by": "publisher",
"first-page": "411",
"journal-title": "BMC Infect Dis",
"key": "7890_CR20",
"unstructured": "Okumuş N, Demirtürk N, Çetinkaya RA, et al. Evaluation of the effectiveness and safety of adding ivermectin to treatment in severe COVID-19 patients. BMC Infect Dis. 2021;21:411.",
"volume": "21",
"year": "2021"
},
{
"key": "7890_CR21",
"unstructured": "Merck & Co. Stromectrol. FDA approved package insert 2009. 2009. https://www.accessdata.fda.gov/drugsatfda_docs/label/2009/050742s026lbl.pdf."
},
{
"DOI": "10.1016/j.clinthera.2021.04.007",
"author": "L Shahbaznejad",
"doi-asserted-by": "publisher",
"journal-title": "Clin Ther",
"key": "7890_CR22",
"unstructured": "Shahbaznejad L, Davoudi A, Eslami G, et al. Effects of ivermectin in patients with COVID-19: a multicenter, double-blind, randomized, controlled clinical trial. Clin Ther. 2021. https://doi.org/10.1016/j.clinthera.2021.04.007.",
"year": "2021"
},
{
"DOI": "10.1001/jama.2021.3071",
"author": "E López-Medina",
"doi-asserted-by": "publisher",
"first-page": "1426",
"journal-title": "JAMA",
"key": "7890_CR23",
"unstructured": "López-Medina E, López P, Hurtado IC, et al. Effect of ivermectin on time to resolution of symptoms among adults with mild COVID-19: a randomized clinical trial. JAMA. 2021;325:1426–35.",
"volume": "325",
"year": "2021"
},
{
"author": "M Popp",
"first-page": "CD015017",
"journal-title": "Cochrane Database System Rev",
"key": "7890_CR24",
"unstructured": "Popp M, Stegemann M, Metzendorf M-I, Gould S, Kranke P, Meybohm P, Skoetz N, Weibel S. Ivermectin for preventing and treating COVID-19. Cochrane Database System Rev. 2021;7:CD015017.",
"volume": "7",
"year": "2021"
},
{
"DOI": "10.1016/j.bbi.2020.04.017",
"author": "M Ye",
"doi-asserted-by": "publisher",
"first-page": "945",
"journal-title": "Brain Behav Immun",
"key": "7890_CR25",
"unstructured": "Ye M, Ren Y, Lv T. Encephalitis as a clinical manifestation of COVID-19. Brain Behav Immun. 2020;88:945–6.",
"volume": "88",
"year": "2020"
},
{
"DOI": "10.1002/jmv.26207",
"author": "RK Garg",
"doi-asserted-by": "publisher",
"first-page": "206",
"journal-title": "J Med Virol",
"key": "7890_CR26",
"unstructured": "Garg RK, Paliwal VK, Gupta A. Encephalopathy in patients with COVID-19: a review. J Med Virol. 2020;93:206–22.",
"volume": "93",
"year": "2020"
}
],
"reference-count": 26,
"references-count": 26,
"relation": {},
"resource": {
"primary": {
"URL": "https://bmcinfectdis.biomedcentral.com/articles/10.1186/s12879-022-07890-6"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Infectious Diseases"
],
"subtitle": [],
"title": "Ivermectin compared with placebo in the clinical course in Mexican patients with asymptomatic and mild COVID-19: a randomized clinical trial",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1007/springer_crossmark_policy",
"volume": "22"
}