Enhancing Intracellular Uptake of Ivermectin through Liposomal Encapsulation
Meryem Kocas, Fumiyoshi Yamashita, Tansel Comoglu, Qiyue Zhang
AAPS PharmSciTech, doi:10.1208/s12249-025-03113-8
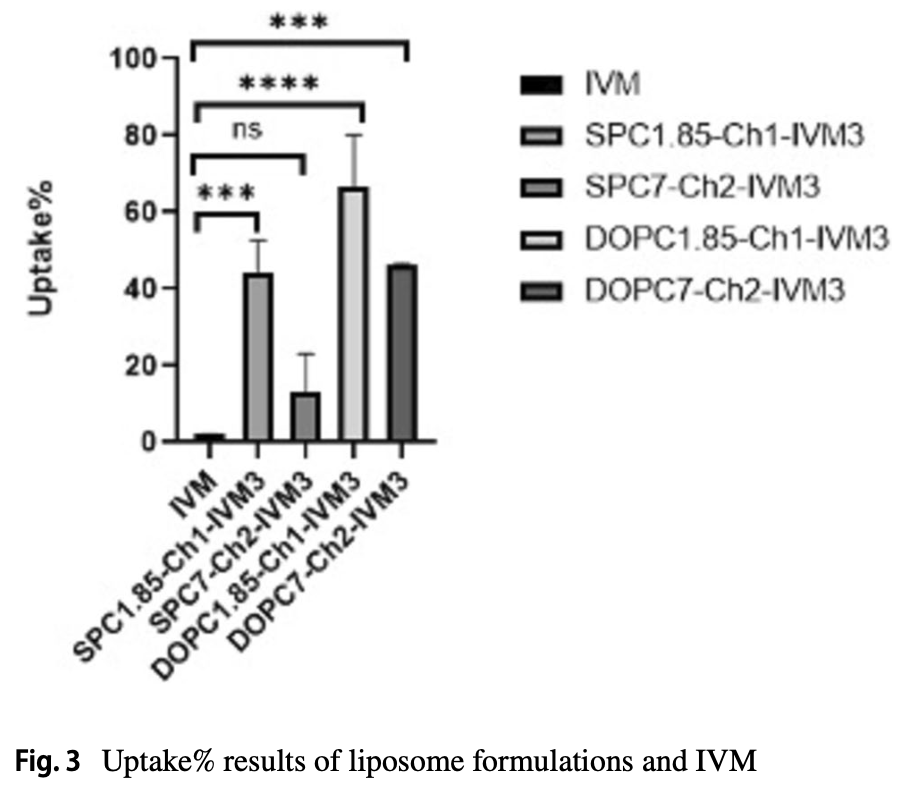
Ivermectin (IVM), an antiparasitic drug approved by the Food and Drug Administration (FDA), is widely used to treat several neglected tropical diseases, including onchocerciasis, helminthiases, and scabies. Additionally, IVM has shown potential as a potent inhibitor of certain RNA viruses, such as SARS-CoV-2. However, IVM is highly hydrophobic, essentially insoluble in water, which limits its bioavailability and therapeutic effectiveness. The use of liposomes as drug carriers offers several advantages, including enhanced solubility for lipophilic drugs, passive targeting of immune system cells, sustained release, and improved tissue penetration. To address the limitations of IVM, including its poor solubility and bioavailability, liposomal formulations were developed using a combination of soyphosphatidylcholine (SPC), dioleylphosphatidylcholine (DOPC), cholesterol (Ch), and diethylphosphate (DCP) in two distinct molar ratios (1.85:1:0.15 and 7:2:1) via the ethanol injection method. The physicochemical properties of the placebo and IVM-loaded liposomes were extensively characterized in our earlier study, including the particle size, polydispersity index, and zeta potential. The present work adds a deeper level of investigation into how to effect cellular uptake and cytotoxicity in vitro of both free IVM and IVM-loaded liposomes in Vero E6 cells. The half-maximal cytotoxic concentrations (CC 50 ) for free IVM and IVM-loaded liposomes were 10 μM and > 110 μM, respectively and the cellular uptake of IVM-loaded liposomes ranged from 13 to 60%, whereas free IVM showed a significantly lower uptake of only 2%. These results demonstrate that liposomal encapsulation effectively enhances IVM's cellular uptake while reducing its cytotoxicity, thus offering a promising strategy for improving the effectiveness of IVM.
Supplementary Information The online version contains supplementary material available at https:// doi . org/ 10. 1208/ s12249-025-03113-8. Author Contributions Meryem Kocas (ORCID: 0000-0002-4165-6191): The author contributed to the conception or design of the study; acquisition, analysis and interpretation of data for the study. She has also drafted or critically revised the manuscript for important intellectual content; and has agreed to be responsible for final approval of the version to be published and for all aspects of the publication. Fumiyoshi Yamashita (ORCID: 0000-0002-3503-8696): The author contributed to the conception or design of the study; acquisition, analysis and interpretation of data for the study. He has also drafted or critically revised the manuscript for important intellectual content; and has agreed to be responsible for final approval of the version to be published and for all aspects of the publication. Tansel Comoglu (ORCID: 0000-0002-4221-5814): The author contributed to the conception or design of the study; acquisition, analysis and interpretation of data for the study. She has also drafted or critically revised the manuscript for important intellectual content; and has agreed to be responsible for final approval of the version to be published and for all aspects of the publication. Qiyue Zhang (ORCID: 0000-0001-8440-2071): The author contributed to the conception or design of the study; acquisition, analysis and interpretation of data for the..
References
Andar, Hood, Vreeland, Devoe, Swaan, Microfluidic preparation of liposomes to determine particle size influence on cellular uptake mechanisms, Pharm Res
Arisoy, Kocas, Comoglu, Guderer, Banerjee, Development of ACE2 loaded decoy liposomes and their effect on SARS-CoV-2 for Covid-19 treatment, Pharm Dev Technol
Bassissi, Lespine, Alvinerie, Assessment of a liposomal formulation of ivermectin in rabbit after a single subcutaneous administration, Parasitol Res
Briuglia, Rotella, Mcfarlane, Lamprou, Influence of cholesterol on liposome stability and on in vitro drug release, Drug Deliv Transl Res
Calvagno, Paolino, Cosco, Iannone, Castelli, Effects of lipid composition and preparation conditions on physical-chemical properties, technological parameters and in vitro biological activity of gemcitabine-loaded liposomes, Curr Drug Deliv
Chantarasrivong, Higuchi, Tsuda, Yamane, Hashida et al., Sialyl LewisX mimic-decorated liposomes for anti-angiogenic everolimus delivery to E-selectin expressing endothelial cells, RSC Adv
Chen, Kubo, Ivermectin and its target molecules: shared and unique modulation mechanisms of ion channels and receptors by ivermectin, J Physiol
Coelho, Ferreira, Alves, Cordeiro, Fonseca et al., Drug delivery systems: Advanced technologies potentially applicable in personalized treatments, Epma J
Croci, Bottaro, Chan, Watanabe, Pezzullo et al., Liposomal systems as nanocarriers for the antiviral agent ivermectin, Int J Biomater
Delandre, Gendrot, Jardot, Bideau, Boxberger et al., Antiviral activity of repurposing ivermectin against a panel of 30 clinical SARS-CoV-2 strains belonging to 14 variants, Pharmaceuticals
Digiacomo, Cardarelli, Pozzi, Palchetti, Digman et al., An apolipoprotein-enriched biomolecular corona switches the cellular uptake mechanism and trafficking pathway of lipid nanoparticles, Nanoscale
Du, Sun, Ethanol injection method for liposome preparation, Methods Mol Biol
Düzgüneş, Nir, Mechanisms and kinetics of liposome-cell interactions, Adv Drug Deliv Rev
El Maghraby, Arafa, Liposomes for enhanced cellular uptake of anticancer agents, Curr Drug Deliv
Eloy, De Souza, Petrilli, Barcellos, Lee et al., Liposomes as carriers of hydrophilic small molecule drugs: strategies to enhance encapsulation and delivery, Colloids Surf, B
Feng, Wang, Cai, Bai, Zhu, Ivermectin accelerates autophagic death of glioma cells by inhibiting glycolysis through blocking GLUT4 mediated JAK/STAT signaling pathway activation, Environ Toxicol
Friedrich, Pfeifer, Binder, Aigner, Barbosa et al., Selection and validation of siRNAs preventing uptake and replication of SARS-CoV-2, Front Bioeng Biotechnol
Fujita, Plianchaisuk, Deguchi, Ito, Nao et al., Virological characteristics of a SARS-CoV-2-related bat coronavirus, EBioMedicine
Gandek, Van Der Koog, Nagelkerke, A comparison of cellular uptake mechanisms, delivery efficacy, and intracellular fate between liposomes and extracellular vesicles, Adv Healthcare Mater
Gardikis, Hatziantoniou, Madalina, Fessas, Signorelli et al., New drug delivery nanosystem combining liposomal and dendrimeric technology (liposomal locked-in dendrimers) for cancer therapy, J Pharm Sci
Gong, Wang, Leong, Determination of cellular uptake and endocytic pathways, Bio Protoc
Gouda, Sakr, Nasr, Sammour, Ethanol injection technique for liposomes formulation: An insight into development, influencing factors, challenges and applications, J Drug Deliv Sci Technol
Horowitz, Barenholz, Gabizon, In vitro cytotoxicity of liposome-encapsulated doxorubicin: dependence on liposome composition and drug release, Biochim Biophys Acta (BBA) Biomembr
Hou, Sun, Pan, Gu, Effects of phytosterol butyrate ester on the characteristics of soybean phosphatidylcholine liposomes, J Oleo Sci
Jaafar-Maalej, Diab, Andrieu, Elaissari, Fessi, Ethanol injection method for hydrophilic and lipophilic drug-loaded liposome preparation, J Liposome Res
Jitobaom, Boonarkart, Manopwisedjaroen, Punyadee, Borwornpinyo et al., Favipiravir and ivermectin show in vitro synergistic antiviral activity against SARS-CoV-2, Acta Virol
Johnson-Arbor, Ivermectin: a mini-review, Clin Toxicol
Kapoor, Lee, Tyner, Liposomal drug product development and quality: current us experience and perspective, AAPS J
Kinobe, Owens, A systematic review of experimental evidence for antiviral effects of ivermectin and an in silico analysis of ivermectin's possible mode of action against SARS-CoV-2, Fundam Clin Pharmacol
Kocas, Comoglu, Ozkul, Development and in vitro antiviral activity of ivermectin liposomes as a potential drug carrier system, Arch Pharm
Kulkarni, Shaw, Chapter 10 -Microscopy Techniques
Kullenberg, Degerstedt, Calitz, Pavlović, Balgoma et al., In vitro cell toxicity and intracellular uptake of doxorubicin exposed as a solution or liposomes: implications for treatment of hepatocellular carcinoma, Cells
Lai, Cao, Ou-Yang, Tsai, Lin et al., Different methods of detaching adherent cells and their effects on the cell surface expression of Fas receptor and Fas ligand, Sci Rep
Lakkaraju, Rahman, Dubinsky, Low-density lipoprotein receptor-related protein mediates the endocytosis of anionic liposomes in neurons* 210, J Biol Chem
Large, Abdelmessih, Fink, Auguste, Liposome composition in drug delivery design, synthesis, characterization, and clinical application, Adv Drug Deliv Rev
Lee, Son, Na, Yi, Koo et al., The effects of doxorubicin-loaded liposomes on viability, stem cell surface marker expression and secretion of vascular endothelial growth factor of three-dimensional stem cell spheroids, Exp Ther Med
Liu, Chen, Zhang, A Review of liposomes as a drug delivery system: current status of approved products, regulatory environments, and future perspectives, Molecules
Lombardo, Kiselev, Methods of liposomes preparation: formation and control factors of versatile nanocarriers for biomedical and nanomedicine application, Pharmaceutics
Miles, Cullen, Kenaan, Gu, Andrews et al., Unravelling the interactions between small molecules and liposomal bilayers via molecular dynamics and thermodynamic modelling, Int J Pharm
Montizaan, Yang, Reker-Smit, Salvati, Comparison of the uptake mechanisms of zwitterionic and negatively charged liposomes by HeLa cells, Nanomedicine: Nanotechnol Biol Med
Nakhaei, Margiana, Bokov, Abdelbasset, Kouhbanani et al., Liposomes: structure, biomedical applications, and stability parameters with emphasis on cholesterol, Front Bioeng Biotechnol
Nsairat, Khater, Sayed, Odeh, Bawab et al., Liposomes: structure, composition, types, and clinical applications, Heliyon
Omura, Crump, Ivermectin: panacea for resource-poor communities?, Trends Parasitol
Rolim, Santos, Chaves, Gonçalves, Freitas-Neto et al., Preformulation study of ivermectin raw material, J Therm Anal Calorim
Saddiqi, Kadir, Abdullah, Bakar Zakaria, Banke, Preparation, characterization and in vitro cytotoxicity evaluation of free and liposome-encapsulated tylosin, Open-Nano
Sakai-Kato, Yoshida, Izutsu, Effect of surface charge on the size-dependent cellular internalization of liposomes, Chem Phys Lipids
Samad, Sultana, Aqil, Liposomal drug delivery systems: an update review, Curr Drug Deliv
Schulz, Neodo, Coulibaly, Keiser, Development and validation of a LC-MS/MS method for ivermectin quantification in dried blood spots: application to a pharmacokinetic study in Trichuris trichiura-infected adults, Anal Methods
Shah, Nguyen, Patel, Cote, Al-Fatease et al., Liposomes produced by microfluidics and extrusion: A comparison for scale-up purposes, Nanomedicine: Nanotechnol Biol Med
Shailesh, Neelam, Sandeep, Gupta, Liposomes: a review, J Pharm Res
Soni, Saini, Formulation design and optimization of cationic-charged liposomes of brimonidine tartrate for effective ocular drug delivery by design of experiment (DoE) approach, Drug Dev Ind Pharm
Strachan, Dyett, Nasa, Valery, Conn, Toxicity and cellular uptake of lipid nanoparticles of different structure and composition, J Colloid Interface Sci
Syama, Jakubek, Chen, Zaifman, Tam et al., Development of lipid nanoparticles and liposomes reference materials (II): cytotoxic profiles, Sci Rep
Tang, Hu, Wang, Yao, Zhang et al., Ivermectin, a potential anticancer drug derived from an antiparasitic drug, Pharmacol Res
Ugwu, Conradie, Anticancer properties of complexes derived from bidentate ligands, J Inorg Biochem
Zhang, Chen, Swaroop, Xu, Wang et al., Heparan sulfate assists SARS-CoV-2 in cell entry and can be targeted by approved drugs in vitro, Cell Discov
Zhang, Ni, Zhang, Xu, Gao et al., Ivermectin confers its cytotoxic effects by inducing AMPK/mTOR-mediated autophagy and DNA damage, Chemosphere
Zhaorigetu, Rodriguez-Aguayo, Sood, Lopez-Berestein, Walton, Delivery of negatively charged liposomes into the atherosclerotic plaque of apolipoprotein E-deficient mouse aortic tissue, J Liposome Res
Zhou, Wu, Ning, Wu, Xu et al., Ivermectin has new application in inhibiting colorectal cancer cell growth, Front Pharmacol
Zhu, Li, Hou, Liu, Liu et al., Preparation and quality evaluation of ivermectin liposome, J Northwest A & F Univ-Nat Sci Ed
Çağdaş, Sezer, Bucak, Liposomes as potential drug carrier systems for drug delivery, Appl Nanotechnol Drug Deliv
DOI record:
{
"DOI": "10.1208/s12249-025-03113-8",
"ISSN": [
"1530-9932"
],
"URL": "http://dx.doi.org/10.1208/s12249-025-03113-8",
"abstract": "<jats:title>Abstract</jats:title>\n <jats:p>Ivermectin (IVM), an antiparasitic drug approved by the Food and Drug Administration (FDA), is widely used to treat several neglected tropical diseases, including onchocerciasis, helminthiases, and scabies. Additionally, IVM has shown potential as a potent inhibitor of certain RNA viruses, such as SARS-CoV-2. However, IVM is highly hydrophobic, essentially insoluble in water, which limits its bioavailability and therapeutic effectiveness. The use of liposomes as drug carriers offers several advantages, including enhanced solubility for lipophilic drugs, passive targeting of immune system cells, sustained release, and improved tissue penetration. To address the limitations of IVM, including its poor solubility and bioavailability, liposomal formulations were developed using a combination of soyphosphatidylcholine (SPC), dioleylphosphatidylcholine (DOPC), cholesterol (Ch), and diethylphosphate (DCP) in two distinct molar ratios (1.85:1:0.15 and 7:2:1) via the ethanol injection method. The physicochemical properties of the placebo and IVM-loaded liposomes were extensively characterized in our earlier study, including the particle size, polydispersity index, and zeta potential. The present work adds a deeper level of investigation into how to effect cellular uptake and cytotoxicity <jats:italic>in vitro</jats:italic> of both free IVM and IVM-loaded liposomes in Vero E6 cells. The half-maximal cytotoxic concentrations (CC<jats:sub>50</jats:sub>) for free IVM and IVM-loaded liposomes were 10 μM and > 110 μM, respectively and the cellular uptake of IVM-loaded liposomes ranged from 13 to 60%, whereas free IVM showed a significantly lower uptake of only 2%. These results demonstrate that liposomal encapsulation effectively enhances IVM’s cellular uptake while reducing its cytotoxicity, thus offering a promising strategy for improving the effectiveness of IVM.</jats:p>\n <jats:p>\n <jats:bold>Graphical Abstract</jats:bold>\n </jats:p>",
"alternative-id": [
"3113"
],
"article-number": "123",
"assertion": [
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Received",
"name": "received",
"order": 1,
"value": "19 November 2024"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Accepted",
"name": "accepted",
"order": 2,
"value": "10 April 2025"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "First Online",
"name": "first_online",
"order": 3,
"value": "2 May 2025"
},
{
"group": {
"label": "Declarations",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 1
},
{
"group": {
"label": "Conflict of interest",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 2,
"value": "The authors declare no conflict of interest."
}
],
"author": [
{
"ORCID": "https://orcid.org/0000-0002-4165-6191",
"affiliation": [],
"authenticated-orcid": false,
"family": "Kocas",
"given": "Meryem",
"sequence": "first"
},
{
"ORCID": "https://orcid.org/0000-0002-3503-8696",
"affiliation": [],
"authenticated-orcid": false,
"family": "Yamashita",
"given": "Fumiyoshi",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0002-4221-5814",
"affiliation": [],
"authenticated-orcid": false,
"family": "Comoglu",
"given": "Tansel",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0001-8440-2071",
"affiliation": [],
"authenticated-orcid": false,
"family": "Zhang",
"given": "Qiyue",
"sequence": "additional"
}
],
"container-title": "AAPS PharmSciTech",
"container-title-short": "AAPS PharmSciTech",
"content-domain": {
"crossmark-restriction": false,
"domain": [
"link.springer.com"
]
},
"created": {
"date-parts": [
[
2025,
5,
2
]
],
"date-time": "2025-05-02T16:07:57Z",
"timestamp": 1746202077000
},
"deposited": {
"date-parts": [
[
2025,
5,
2
]
],
"date-time": "2025-05-02T17:02:50Z",
"timestamp": 1746205370000
},
"funder": [
{
"name": "Social Science University of Ankara"
}
],
"indexed": {
"date-parts": [
[
2025,
5,
3
]
],
"date-time": "2025-05-03T04:09:38Z",
"timestamp": 1746245378192,
"version": "3.40.4"
},
"is-referenced-by-count": 0,
"issue": "5",
"issued": {
"date-parts": [
[
2025,
5,
2
]
]
},
"journal-issue": {
"issue": "5",
"published-online": {
"date-parts": [
[
2025,
6
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2025,
5,
2
]
],
"date-time": "2025-05-02T00:00:00Z",
"timestamp": 1746144000000
}
},
{
"URL": "https://creativecommons.org/licenses/by/4.0",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2025,
5,
2
]
],
"date-time": "2025-05-02T00:00:00Z",
"timestamp": 1746144000000
}
}
],
"link": [
{
"URL": "https://link.springer.com/content/pdf/10.1208/s12249-025-03113-8.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/article/10.1208/s12249-025-03113-8/fulltext.html",
"content-type": "text/html",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/content/pdf/10.1208/s12249-025-03113-8.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "297",
"original-title": [],
"prefix": "10.1208",
"published": {
"date-parts": [
[
2025,
5,
2
]
]
},
"published-online": {
"date-parts": [
[
2025,
5,
2
]
]
},
"publisher": "Springer Science and Business Media LLC",
"reference": [
{
"DOI": "10.1016/j.pt.2014.07.005",
"author": "S Omura",
"doi-asserted-by": "publisher",
"first-page": "445",
"issue": "9",
"journal-title": "Trends Parasitol",
"key": "3113_CR1",
"unstructured": "Omura S, Crump A. Ivermectin: panacea for resource-poor communities? Trends Parasitol. 2014;30(9):445–55.",
"volume": "30",
"year": "2014"
},
{
"DOI": "10.1016/j.phrs.2020.105207",
"author": "M Tang",
"doi-asserted-by": "publisher",
"first-page": "105207",
"journal-title": "Pharmacol Res",
"key": "3113_CR2",
"unstructured": "Tang M, Hu X, Wang Y, Yao X, Zhang W, Yu C, et al. Ivermectin, a potential anticancer drug derived from an antiparasitic drug. Pharmacol Res. 2021;163:105207.",
"volume": "163",
"year": "2021"
},
{
"DOI": "10.1113/JP275236",
"author": "IS Chen",
"doi-asserted-by": "publisher",
"first-page": "1833",
"issue": "10",
"journal-title": "J Physiol",
"key": "3113_CR3",
"unstructured": "Chen IS, Kubo Y. Ivermectin and its target molecules: shared and unique modulation mechanisms of ion channels and receptors by ivermectin. J Physiol. 2018;596(10):1833–45.",
"volume": "596",
"year": "2018"
},
{
"DOI": "10.1080/15563650.2022.2043338",
"author": "K Johnson-Arbor",
"doi-asserted-by": "publisher",
"first-page": "571",
"issue": "5",
"journal-title": "Clin Toxicol",
"key": "3113_CR4",
"unstructured": "Johnson-Arbor K. Ivermectin: a mini-review. Clin Toxicol. 2022;60(5):571–5.",
"volume": "60",
"year": "2022"
},
{
"DOI": "10.1007/s00436-005-0073-z",
"author": "F Bassissi",
"doi-asserted-by": "publisher",
"first-page": "244",
"issue": "3",
"journal-title": "Parasitol Res",
"key": "3113_CR5",
"unstructured": "Bassissi F, Lespine A, Alvinerie M. Assessment of a liposomal formulation of ivermectin in rabbit after a single subcutaneous administration. Parasitol Res. 2006;98(3):244–9.",
"volume": "98",
"year": "2006"
},
{
"DOI": "10.1155/2016/8043983",
"author": "R Croci",
"doi-asserted-by": "publisher",
"first-page": "8043983",
"journal-title": "Int J Biomater",
"key": "3113_CR6",
"unstructured": "Croci R, Bottaro E, Chan KWK, Watanabe S, Pezzullo M, Mastrangelo E, et al. Liposomal systems as nanocarriers for the antiviral agent ivermectin. Int J Biomater. 2016;2016:8043983.",
"volume": "2016",
"year": "2016"
},
{
"author": "X Zhu",
"first-page": "24",
"issue": "4",
"journal-title": "J Northwest A & F Univ- Nat Sci Ed",
"key": "3113_CR7",
"unstructured": "Zhu X, Li Y, Hou B, Liu L, Liu A, Xiong Y, et al. Preparation and quality evaluation of ivermectin liposome. J Northwest A & F Univ- Nat Sci Ed. 2010;38(4):24–30.",
"volume": "38",
"year": "2010"
},
{
"DOI": "10.1016/j.heliyon.2022.e09394",
"author": "H Nsairat",
"doi-asserted-by": "publisher",
"first-page": "e09394",
"issue": "5",
"journal-title": "Heliyon",
"key": "3113_CR8",
"unstructured": "Nsairat H, Khater D, Sayed U, Odeh F, Al Bawab A, Alshaer W. Liposomes: structure, composition, types, and clinical applications. Heliyon. 2022;8(5):e09394.",
"volume": "8",
"year": "2022"
},
{
"author": "S Shailesh",
"first-page": "1163",
"issue": "7",
"journal-title": "J Pharm Res",
"key": "3113_CR9",
"unstructured": "Shailesh S, Neelam S, Sandeep K, Gupta G. Liposomes: a review. J Pharm Res. 2009;2(7):1163–7.",
"volume": "2",
"year": "2009"
},
{
"DOI": "10.1080/10837450.2022.2042557",
"author": "S Arisoy",
"doi-asserted-by": "publisher",
"first-page": "1",
"journal-title": "Pharm Dev Technol",
"key": "3113_CR10",
"unstructured": "Arisoy S, Kocas M, Comoglu T, Guderer I, Banerjee S. Development of ACE2 loaded decoy liposomes and their effect on SARS-CoV-2 for Covid-19 treatment. Pharm Dev Technol. 2022;27:1–11.",
"volume": "27",
"year": "2022"
},
{
"DOI": "10.1007/s13167-010-0001-x",
"author": "JF Coelho",
"doi-asserted-by": "publisher",
"first-page": "164",
"issue": "1",
"journal-title": "Epma J",
"key": "3113_CR11",
"unstructured": "Coelho JF, Ferreira PC, Alves P, Cordeiro R, Fonseca AC, Góis JR, et al. Drug delivery systems: Advanced technologies potentially applicable in personalized treatments. Epma J. 2010;1(1):164–209.",
"volume": "1",
"year": "2010"
},
{
"author": "M Çağdaş",
"first-page": "1",
"journal-title": "Appl Nanotechnol Drug Deliv",
"key": "3113_CR12",
"unstructured": "Çağdaş M, Sezer AD, Bucak S. Liposomes as potential drug carrier systems for drug delivery. Appl Nanotechnol Drug Deliv. 2014;1:1–50.",
"volume": "1",
"year": "2014"
},
{
"DOI": "10.3389/fbioe.2021.705886",
"author": "P Nakhaei",
"doi-asserted-by": "publisher",
"first-page": "705886",
"journal-title": "Front Bioeng Biotechnol",
"key": "3113_CR13",
"unstructured": "Nakhaei P, Margiana R, Bokov DO, Abdelbasset WK, Jadidi Kouhbanani MA, Varma RS, et al. Liposomes: structure, biomedical applications, and stability parameters with emphasis on cholesterol. Front Bioeng Biotechnol. 2021;9:705886.",
"volume": "9",
"year": "2021"
},
{
"DOI": "10.1016/j.colsurfb.2014.09.029",
"author": "JO Eloy",
"doi-asserted-by": "publisher",
"first-page": "345",
"journal-title": "Colloids Surf, B",
"key": "3113_CR14",
"unstructured": "Eloy JO, de Souza MC, Petrilli R, Barcellos JPA, Lee RJ, Marchetti JM. Liposomes as carriers of hydrophilic small molecule drugs: strategies to enhance encapsulation and delivery. Colloids Surf, B. 2014;123:345–63.",
"volume": "123",
"year": "2014"
},
{
"DOI": "10.3390/pharmaceutics14030543",
"author": "D Lombardo",
"doi-asserted-by": "publisher",
"first-page": "543",
"issue": "3",
"journal-title": "Pharmaceutics",
"key": "3113_CR15",
"unstructured": "Lombardo D, Kiselev MA. Methods of liposomes preparation: formation and control factors of versatile nanocarriers for biomedical and nanomedicine application. Pharmaceutics. 2022;14(3):543.",
"volume": "14",
"year": "2022"
},
{
"DOI": "10.1208/s12248-017-0049-9",
"author": "M Kapoor",
"doi-asserted-by": "publisher",
"first-page": "632",
"issue": "3",
"journal-title": "AAPS J",
"key": "3113_CR16",
"unstructured": "Kapoor M, Lee SL, Tyner KM. Liposomal drug product development and quality: current us experience and perspective. AAPS J. 2017;19(3):632–41.",
"volume": "19",
"year": "2017"
},
{
"DOI": "10.2174/156720107782151269",
"author": "A Samad",
"doi-asserted-by": "publisher",
"first-page": "297",
"issue": "4",
"journal-title": "Curr Drug Deliv",
"key": "3113_CR17",
"unstructured": "Samad A, Sultana Y, Aqil M. Liposomal drug delivery systems: an update review. Curr Drug Deliv. 2007;4(4):297–305.",
"volume": "4",
"year": "2007"
},
{
"DOI": "10.3390/molecules27041372",
"author": "P Liu",
"doi-asserted-by": "publisher",
"first-page": "1372",
"issue": "4",
"journal-title": "Molecules.",
"key": "3113_CR18",
"unstructured": "Liu P, Chen G, Zhang J. A Review of liposomes as a drug delivery system: current status of approved products, regulatory environments, and future perspectives. Molecules. 2022;27(4):1372.",
"volume": "27",
"year": "2022"
},
{
"DOI": "10.1007/978-1-0716-2954-3_5",
"author": "G Du",
"doi-asserted-by": "publisher",
"first-page": "65",
"journal-title": "Methods Mol Biol",
"key": "3113_CR19",
"unstructured": "Du G, Sun X. Ethanol injection method for liposome preparation. Methods Mol Biol. 2023;2622:65–70.",
"volume": "2622",
"year": "2023"
},
{
"DOI": "10.3109/08982100903347923",
"author": "C Jaafar-Maalej",
"doi-asserted-by": "publisher",
"first-page": "228",
"issue": "3",
"journal-title": "J Liposome Res",
"key": "3113_CR20",
"unstructured": "Jaafar-Maalej C, Diab R, Andrieu V, Elaissari A, Fessi H. Ethanol injection method for hydrophilic and lipophilic drug-loaded liposome preparation. J Liposome Res. 2010;20(3):228–43.",
"volume": "20",
"year": "2010"
},
{
"DOI": "10.1016/j.jddst.2020.102174",
"author": "A Gouda",
"doi-asserted-by": "publisher",
"first-page": "102174",
"journal-title": "J Drug Deliv Sci Technol",
"key": "3113_CR21",
"unstructured": "Gouda A, Sakr OS, Nasr M, Sammour O. Ethanol injection technique for liposomes formulation: An insight into development, influencing factors, challenges and applications. J Drug Deliv Sci Technol. 2021;61:102174.",
"volume": "61",
"year": "2021"
},
{
"DOI": "10.2174/156720107779314749",
"author": "M Grazia Calvagno",
"doi-asserted-by": "publisher",
"first-page": "89",
"issue": "1",
"journal-title": "Curr Drug Deliv",
"key": "3113_CR22",
"unstructured": "Grazia Calvagno M, Celia C, Paolino D, Cosco D, Iannone M, Castelli F, et al. Effects of lipid composition and preparation conditions on physical-chemical properties, technological parameters and in vitro biological activity of gemcitabine-loaded liposomes. Curr Drug Deliv. 2007;4(1):89–101.",
"volume": "4",
"year": "2007"
},
{
"DOI": "10.1016/j.addr.2021.113851",
"author": "DE Large",
"doi-asserted-by": "publisher",
"first-page": "113851",
"journal-title": "Adv Drug Deliv Rev",
"key": "3113_CR23",
"unstructured": "Large DE, Abdelmessih RG, Fink EA, Auguste DT. Liposome composition in drug delivery design, synthesis, characterization, and clinical application. Adv Drug Deliv Rev. 2021;176:113851.",
"volume": "176",
"year": "2021"
},
{
"DOI": "10.1016/S0169-409X(99)00037-X",
"author": "N Düzgüneş",
"doi-asserted-by": "publisher",
"first-page": "3",
"issue": "1",
"journal-title": "Adv Drug Deliv Rev",
"key": "3113_CR24",
"unstructured": "Düzgüneş N, Nir S. Mechanisms and kinetics of liposome–cell interactions. Adv Drug Deliv Rev. 1999;40(1):3–18.",
"volume": "40",
"year": "1999"
},
{
"DOI": "10.1016/j.jcis.2020.05.002",
"author": "JB Strachan",
"doi-asserted-by": "publisher",
"first-page": "241",
"journal-title": "J Colloid Interface Sci",
"key": "3113_CR25",
"unstructured": "Strachan JB, Dyett BP, Nasa Z, Valery C, Conn CE. Toxicity and cellular uptake of lipid nanoparticles of different structure and composition. J Colloid Interface Sci. 2020;576:241–51.",
"volume": "576",
"year": "2020"
},
{
"DOI": "10.1002/ardp.202300708",
"author": "M Kocas",
"doi-asserted-by": "publisher",
"first-page": "2300708",
"issue": "8",
"journal-title": "Arch Pharm",
"key": "3113_CR26",
"unstructured": "Kocas M, Comoglu T, Ozkul A. Development and in vitro antiviral activity of ivermectin liposomes as a potential drug carrier system. Arch Pharm. 2024;357(8):2300708.",
"volume": "357",
"year": "2024"
},
{
"DOI": "10.3390/ph15040445",
"author": "O Delandre",
"doi-asserted-by": "publisher",
"first-page": "445",
"issue": "4",
"journal-title": "Pharmaceuticals",
"key": "3113_CR27",
"unstructured": "Delandre O, Gendrot M, Jardot P, Le Bideau M, Boxberger M, Boschi C, et al. Antiviral activity of repurposing ivermectin against a panel of 30 clinical SARS-CoV-2 strains belonging to 14 variants. Pharmaceuticals. 2022;15(4):445.",
"volume": "15",
"year": "2022"
},
{
"DOI": "10.1016/j.ebiom.2024.105181",
"author": "S Fujita",
"doi-asserted-by": "publisher",
"first-page": "105181",
"journal-title": "EBioMedicine",
"key": "3113_CR28",
"unstructured": "Fujita S, Plianchaisuk A, Deguchi S, Ito H, Nao N, Wang L, et al. Virological characteristics of a SARS-CoV-2-related bat coronavirus, BANAL-20-236. EBioMedicine. 2024;104:105181.",
"volume": "104",
"year": "2024"
},
{
"DOI": "10.1016/B978-0-12-801024-2.00010-8",
"doi-asserted-by": "crossref",
"key": "3113_CR29",
"unstructured": "Kulkarni VS, Shaw C. Chapter 10 - Microscopy Techniques. In: Kulkarni VS, Shaw C, editors. Essential Chemistry for Formulators of Semisolid and Liquid Dosages. Boston: Academic Press; 2016. p. 183-92."
},
{
"DOI": "10.1039/C8AY00828K",
"author": "JD Schulz",
"doi-asserted-by": "publisher",
"first-page": "2901",
"issue": "24",
"journal-title": "Anal Methods",
"key": "3113_CR30",
"unstructured": "Schulz JD, Neodo A, Coulibaly JT, Keiser J. Development and validation of a LC-MS/MS method for ivermectin quantification in dried blood spots: application to a pharmacokinetic study in Trichuris trichiura-infected adults. Anal Methods. 2018;10(24):2901–9.",
"volume": "10",
"year": "2018"
},
{
"DOI": "10.1111/fcp.12644",
"author": "RT Kinobe",
"doi-asserted-by": "publisher",
"first-page": "260",
"issue": "2",
"journal-title": "Fundam Clin Pharmacol",
"key": "3113_CR31",
"unstructured": "Kinobe RT, Owens L. A systematic review of experimental evidence for antiviral effects of ivermectin and an in silico analysis of ivermectin’s possible mode of action against SARS-CoV-2. Fundam Clin Pharmacol. 2021;35(2):260–76.",
"volume": "35",
"year": "2021"
},
{
"DOI": "10.1038/s41598-022-23013-2",
"author": "K Syama",
"doi-asserted-by": "publisher",
"first-page": "18071",
"issue": "1",
"journal-title": "Sci Rep",
"key": "3113_CR32",
"unstructured": "Syama K, Jakubek ZJ, Chen S, Zaifman J, Tam YYC, Zou S. Development of lipid nanoparticles and liposomes reference materials (II): cytotoxic profiles. Sci Rep. 2022;12(1):18071.",
"volume": "12",
"year": "2022"
},
{
"DOI": "10.3389/av.2023.12265",
"author": "K Jitobaom",
"doi-asserted-by": "publisher",
"first-page": "12265",
"journal-title": "Acta Virol",
"key": "3113_CR33",
"unstructured": "Jitobaom K, Boonarkart C, Manopwisedjaroen S, Punyadee N, Borwornpinyo S, Thitithanyanont A, et al. Favipiravir and ivermectin show in vitro synergistic antiviral activity against SARS-CoV-2. Acta Virol. 2023;67:12265.",
"volume": "67",
"year": "2023"
},
{
"author": "H Lee",
"first-page": "4950",
"issue": "6",
"journal-title": "Exp Ther Med",
"key": "3113_CR34",
"unstructured": "Lee H, Son J, Na CB, Yi G, Koo H, Park JB. The effects of doxorubicin-loaded liposomes on viability, stem cell surface marker expression and secretion of vascular endothelial growth factor of three-dimensional stem cell spheroids. Exp Ther Med. 2018;15(6):4950–60.",
"volume": "15",
"year": "2018"
},
{
"DOI": "10.1039/C9RA01943J",
"author": "C Chantarasrivong",
"doi-asserted-by": "publisher",
"first-page": "20518",
"journal-title": "RSC Adv",
"key": "3113_CR35",
"unstructured": "Chantarasrivong C, Higuchi Y, Tsuda M, Yamane Y, Hashida M, Konishi M, et al. Sialyl LewisX mimic-decorated liposomes for anti-angiogenic everolimus delivery to E-selectin expressing endothelial cells. RSC Adv. 2019;9:20518–27.",
"volume": "9",
"year": "2019"
},
{
"DOI": "10.1016/j.jinorgbio.2023.112268",
"author": "DI Ugwu",
"doi-asserted-by": "publisher",
"first-page": "112268",
"journal-title": "J Inorg Biochem",
"key": "3113_CR36",
"unstructured": "Ugwu DI, Conradie J. Anticancer properties of complexes derived from bidentate ligands. J Inorg Biochem. 2023;246:112268.",
"volume": "246",
"year": "2023"
},
{
"DOI": "10.3389/fbioe.2022.801870",
"author": "M Friedrich",
"doi-asserted-by": "publisher",
"first-page": "801870",
"journal-title": "Front Bioeng Biotechnol",
"key": "3113_CR37",
"unstructured": "Friedrich M, Pfeifer G, Binder S, Aigner A, Vollmer Barbosa P, Makert GR, et al. Selection and validation of siRNAs preventing uptake and replication of SARS-CoV-2. Front Bioeng Biotechnol. 2022;10:801870.",
"volume": "10",
"year": "2022"
},
{
"DOI": "10.3389/fphar.2021.717529",
"author": "S Zhou",
"doi-asserted-by": "publisher",
"first-page": "717529",
"journal-title": "Front Pharmacol",
"key": "3113_CR38",
"unstructured": "Zhou S, Wu H, Ning W, Wu X, Xu X, Ma Y, et al. Ivermectin has new application in inhibiting colorectal cancer cell growth. Front Pharmacol. 2021;12:717529.",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.3390/cells10071717",
"author": "F Kullenberg",
"doi-asserted-by": "publisher",
"first-page": "1717",
"issue": "7",
"journal-title": "Cells",
"key": "3113_CR39",
"unstructured": "Kullenberg F, Degerstedt O, Calitz C, Pavlović N, Balgoma D, Gråsjö J, et al. In vitro cell toxicity and intracellular uptake of doxorubicin exposed as a solution or liposomes: implications for treatment of hepatocellular carcinoma. Cells. 2021;10(7):1717.",
"volume": "10",
"year": "2021"
},
{
"DOI": "10.1038/s41598-022-09605-y",
"author": "T-Y Lai",
"doi-asserted-by": "publisher",
"first-page": "5713",
"issue": "1",
"journal-title": "Sci Rep",
"key": "3113_CR40",
"unstructured": "Lai T-Y, Cao J, Ou-Yang P, Tsai C-Y, Lin C-W, Chen C-C, et al. Different methods of detaching adherent cells and their effects on the cell surface expression of Fas receptor and Fas ligand. Sci Rep. 2022;12(1):5713.",
"volume": "12",
"year": "2022"
},
{
"DOI": "10.1038/s41421-020-00222-5",
"author": "Q Zhang",
"doi-asserted-by": "publisher",
"first-page": "80",
"issue": "1",
"journal-title": "Cell Discov",
"key": "3113_CR41",
"unstructured": "Zhang Q, Chen CZ, Swaroop M, Xu M, Wang L, Lee J, et al. Heparan sulfate assists SARS-CoV-2 in cell entry and can be targeted by approved drugs in vitro. Cell Discov. 2020;6(1):80.",
"volume": "6",
"year": "2020"
},
{
"DOI": "10.21769/BioProtoc.3169",
"author": "J Gong",
"doi-asserted-by": "publisher",
"first-page": "e3169",
"issue": "4",
"journal-title": "Bio Protoc",
"key": "3113_CR42",
"unstructured": "Gong J, Wang HX, Leong KW. Determination of cellular uptake and endocytic pathways. Bio Protoc. 2019;9(4):e3169.",
"volume": "9",
"year": "2019"
},
{
"DOI": "10.1080/03639045.2022.2070198",
"author": "PK Soni",
"doi-asserted-by": "publisher",
"first-page": "1847",
"issue": "11",
"journal-title": "Drug Dev Ind Pharm",
"key": "3113_CR43",
"unstructured": "Soni PK, Saini TR. Formulation design and optimization of cationic-charged liposomes of brimonidine tartrate for effective ocular drug delivery by design of experiment (DoE) approach. Drug Dev Ind Pharm. 2021;47(11):1847–66.",
"volume": "47",
"year": "2021"
},
{
"DOI": "10.1016/j.nano.2019.02.019",
"author": "VM Shah",
"doi-asserted-by": "publisher",
"first-page": "146",
"journal-title": "Nanomedicine: Nanotechnol Biol Med",
"key": "3113_CR44",
"unstructured": "Shah VM, Nguyen DX, Patel P, Cote B, Al-Fatease A, Pham Y, et al. Liposomes produced by microfluidics and extrusion: A comparison for scale-up purposes. Nanomedicine: Nanotechnol Biol Med. 2019;18:146–56.",
"volume": "18",
"year": "2019"
},
{
"DOI": "10.1016/j.ijpharm.2024.124367",
"author": "CM Miles",
"doi-asserted-by": "publisher",
"first-page": "124367",
"journal-title": "Int J Pharm",
"key": "3113_CR45",
"unstructured": "Miles CM, Cullen S, Kenaan H, Gu W, Andrews GP, Sosso GC, et al. Unravelling the interactions between small molecules and liposomal bilayers via molecular dynamics and thermodynamic modelling. Int J Pharm. 2024;660:124367.",
"volume": "660",
"year": "2024"
},
{
"DOI": "10.1007/s13346-015-0220-8",
"author": "ML Briuglia",
"doi-asserted-by": "publisher",
"first-page": "231",
"issue": "3",
"journal-title": "Drug Deliv Transl Res",
"key": "3113_CR46",
"unstructured": "Briuglia ML, Rotella C, McFarlane A, Lamprou DA. Influence of cholesterol on liposome stability and on in vitro drug release. Drug Deliv Transl Res. 2015;5(3):231–42.",
"volume": "5",
"year": "2015"
},
{
"DOI": "10.1007/978-1-60327-198-1_6",
"doi-asserted-by": "publisher",
"key": "3113_CR47",
"unstructured": "Clogston JD, Patri AK. Zeta potential measurement. Characterization of nanoparticles intended for drug delivery. 2011;63–70. https://doi.org/10.1007/978-1-60327-198-1_6."
},
{
"DOI": "10.1016/j.chemosphere.2020.127448",
"author": "P Zhang",
"doi-asserted-by": "publisher",
"first-page": "127448",
"journal-title": "Chemosphere",
"key": "3113_CR48",
"unstructured": "Zhang P, Ni H, Zhang Y, Xu W, Gao J, Cheng J, et al. Ivermectin confers its cytotoxic effects by inducing AMPK/mTOR-mediated autophagy and DNA damage. Chemosphere. 2020;259:127448.",
"volume": "259",
"year": "2020"
},
{
"DOI": "10.2174/1567201817666200708113131",
"author": "MG El Maghraby",
"doi-asserted-by": "publisher",
"first-page": "861",
"issue": "10",
"journal-title": "Curr Drug Deliv",
"key": "3113_CR49",
"unstructured": "El Maghraby MG, Arafa FM. Liposomes for enhanced cellular uptake of anticancer agents. Curr Drug Deliv. 2020;17(10):861–73.",
"volume": "17",
"year": "2020"
},
{
"DOI": "10.1016/0005-2736(92)90084-Y",
"author": "AT Horowitz",
"doi-asserted-by": "publisher",
"first-page": "203",
"issue": "2",
"journal-title": "Biochim Biophys Acta (BBA) Biomembr",
"key": "3113_CR50",
"unstructured": "Horowitz AT, Barenholz Y, Gabizon AA. In vitro cytotoxicity of liposome-encapsulated doxorubicin: dependence on liposome composition and drug release. Biochim Biophys Acta (BBA) Biomembr. 1992;1109(2):203–9.",
"volume": "1109",
"year": "1992"
},
{
"DOI": "10.1007/s10973-014-3691-9",
"author": "LA Rolim",
"doi-asserted-by": "publisher",
"first-page": "807",
"issue": "1",
"journal-title": "J Therm Anal Calorim",
"key": "3113_CR51",
"unstructured": "Rolim LA, dos Santos FCM, Chaves LL, Gonçalves MLCM, Freitas-Neto JL, da Silva do Nascimento AL, et al. Preformulation study of ivermectin raw material. J Therm Anal Calorim. 2015;120(1):807–16.",
"volume": "120",
"year": "2015"
},
{
"DOI": "10.5650/jos.ess21033",
"author": "L Hou",
"doi-asserted-by": "publisher",
"first-page": "1295",
"issue": "9",
"journal-title": "J Oleo Sci",
"key": "3113_CR52",
"unstructured": "Hou L, Sun X, Pan L, Gu K. Effects of phytosterol butyrate ester on the characteristics of soybean phosphatidylcholine liposomes. J Oleo Sci. 2021;70(9):1295–306.",
"volume": "70",
"year": "2021"
},
{
"DOI": "10.1002/jps.22121",
"author": "K Gardikis",
"doi-asserted-by": "publisher",
"first-page": "3561",
"journal-title": "J Pharm Sci",
"key": "3113_CR53",
"unstructured": "Gardikis K, Hatziantoniou S, Madalina B, Fessas D, Signorelli M, Felekis T, et al. New drug delivery nanosystem combining liposomal and dendrimeric technology (liposomal locked-in dendrimers) for cancer therapy. J Pharm Sci. 2010;99:3561–71.",
"volume": "99",
"year": "2010"
},
{
"DOI": "10.1002/adhm.202300319",
"author": "TB Gandek",
"doi-asserted-by": "publisher",
"first-page": "2300319",
"issue": "25",
"journal-title": "Adv Healthcare Mater",
"key": "3113_CR54",
"unstructured": "Gandek TB, van der Koog L, Nagelkerke A. A comparison of cellular uptake mechanisms, delivery efficacy, and intracellular fate between liposomes and extracellular vesicles. Adv Healthcare Mater. 2023;12(25):2300319.",
"volume": "12",
"year": "2023"
},
{
"DOI": "10.22270/jddt.v0i0.843",
"doi-asserted-by": "publisher",
"key": "3113_CR55",
"unstructured": "Vishvakarma P, Sharma S. LIPOSOMES: AN OVERVIEW. Journal of Drug Delivery and Therapeutics. 2014. https://doi.org/10.22270/jddt.v0i0.843."
},
{
"DOI": "10.1016/S0076-6879(03)72013-8",
"doi-asserted-by": "publisher",
"key": "3113_CR56",
"unstructured": "Nir S, Nieva JL. Uptake of Liposomes by Cells: Experimental procedures and modeling. methods in enzymology. 372: Academic Press; 2003;235–48. https://doi.org/10.1016/S0076-6879(03)72013-8."
},
{
"DOI": "10.1074/jbc.M111764200",
"author": "A Lakkaraju",
"doi-asserted-by": "publisher",
"first-page": "15085",
"issue": "17",
"journal-title": "J Biol Chem",
"key": "3113_CR57",
"unstructured": "Lakkaraju A, Rahman Y-E, Dubinsky JM. Low-density lipoprotein receptor-related protein mediates the endocytosis of anionic liposomes in neurons* 210. J Biol Chem. 2002;277(17):15085–92.",
"volume": "277",
"year": "2002"
},
{
"DOI": "10.1016/j.nano.2020.102300",
"author": "D Montizaan",
"doi-asserted-by": "publisher",
"first-page": "102300",
"journal-title": "Nanomedicine: Nanotechnol Biol Med",
"key": "3113_CR58",
"unstructured": "Montizaan D, Yang K, Reker-Smit C, Salvati A. Comparison of the uptake mechanisms of zwitterionic and negatively charged liposomes by HeLa cells. Nanomedicine: Nanotechnol Biol Med. 2020;30:102300.",
"volume": "30",
"year": "2020"
},
{
"DOI": "10.1007/s11095-013-1171-8",
"author": "AU Andar",
"doi-asserted-by": "publisher",
"first-page": "401",
"issue": "2",
"journal-title": "Pharm Res",
"key": "3113_CR59",
"unstructured": "Andar AU, Hood RR, Vreeland WN, DeVoe DL, Swaan PW. Microfluidic preparation of liposomes to determine particle size influence on cellular uptake mechanisms. Pharm Res. 2014;31(2):401–13.",
"volume": "31",
"year": "2014"
},
{
"DOI": "10.1016/j.chemphyslip.2019.01.004",
"author": "K Sakai-Kato",
"doi-asserted-by": "publisher",
"first-page": "104726",
"journal-title": "Chem Phys Lipids",
"key": "3113_CR60",
"unstructured": "Sakai-Kato K, Yoshida K, Izutsu K. Effect of surface charge on the size-dependent cellular internalization of liposomes. Chem Phys Lipids. 2019;224:104726.",
"volume": "224",
"year": "2019"
},
{
"DOI": "10.3109/08982104.2013.863208",
"author": "S Zhaorigetu",
"doi-asserted-by": "publisher",
"first-page": "182",
"issue": "3",
"journal-title": "J Liposome Res",
"key": "3113_CR61",
"unstructured": "Zhaorigetu S, Rodriguez-Aguayo C, Sood AK, Lopez-Berestein G, Walton BL. Delivery of negatively charged liposomes into the atherosclerotic plaque of apolipoprotein E-deficient mouse aortic tissue. J Liposome Res. 2014;24(3):182–90.",
"volume": "24",
"year": "2014"
},
{
"DOI": "10.1039/C7NR06437C",
"author": "L Digiacomo",
"doi-asserted-by": "publisher",
"first-page": "17254",
"issue": "44",
"journal-title": "Nanoscale",
"key": "3113_CR62",
"unstructured": "Digiacomo L, Cardarelli F, Pozzi D, Palchetti S, Digman M, Gratton E, et al. An apolipoprotein-enriched biomolecular corona switches the cellular uptake mechanism and trafficking pathway of lipid nanoparticles. Nanoscale. 2017;9(44):17254–62.",
"volume": "9",
"year": "2017"
},
{
"DOI": "10.1002/tox.23440",
"author": "Y Feng",
"doi-asserted-by": "publisher",
"first-page": "754",
"issue": "4",
"journal-title": "Environ Toxicol",
"key": "3113_CR63",
"unstructured": "Feng Y, Wang J, Cai B, Bai X, Zhu Y. Ivermectin accelerates autophagic death of glioma cells by inhibiting glycolysis through blocking GLUT4 mediated JAK/STAT signaling pathway activation. Environ Toxicol. 2022;37(4):754–64.",
"volume": "37",
"year": "2022"
},
{
"DOI": "10.1016/j.onano.2022.100108",
"author": "ME Saddiqi",
"doi-asserted-by": "publisher",
"first-page": "100108",
"journal-title": "OpenNano",
"key": "3113_CR64",
"unstructured": "Saddiqi ME, Abdul Kadir A, Abdullah FFJ, Abu Bakar Zakaria MZ, Banke IS. Preparation, characterization and in vitro cytotoxicity evaluation of free and liposome-encapsulated tylosin. OpenNano. 2022;8:100108.",
"volume": "8",
"year": "2022"
}
],
"reference-count": 64,
"references-count": 64,
"relation": {},
"resource": {
"primary": {
"URL": "https://link.springer.com/10.1208/s12249-025-03113-8"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Enhancing Intracellular Uptake of Ivermectin through Liposomal Encapsulation",
"type": "journal-article",
"update-policy": "https://doi.org/10.1007/springer_crossmark_policy",
"volume": "26"
}