Safety of inhaled ivermectin as a repurposed direct drug for treatment of COVID-19: A preclinical tolerance study
Suzan M. Mansour, Rehab N. Shamma, Kawkab A. Ahmed, Nirmeen A. Sabry, Gamal Esmat, Azza A. Mahmoud, Amr Maged
International Immunopharmacology, doi:10.1016/j.intimp.2021.108004
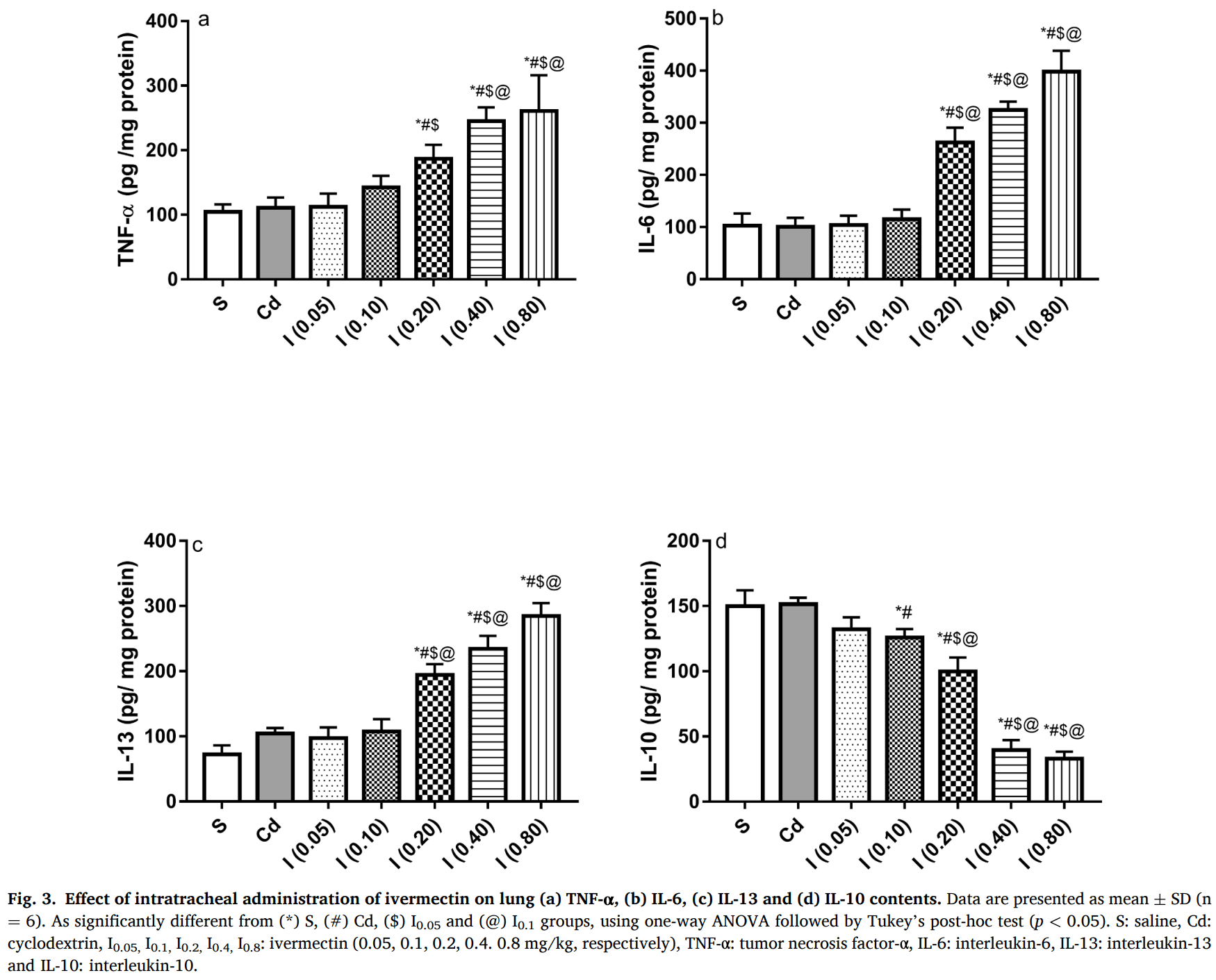
Introduction: SARS-CoV-2 replication in cell cultures has been shown to be inhibited by ivermectin. However, ivermectin's low aqueous solubility and bioavailability hinders its application in COVID-19 treatment. Also, it has been suggested that best outcomes for this medication can be achieved via direct administration to the lung. Objectives: This study aimed at evaluating the safety of a novel ivermectin inhalable formulation in rats as a preclinical step. Methods: Hydroxy propyl-β-cyclodextrin (HP-β-CD) was used to formulate readily soluble ivermectin lyophilized powder. Adult male rats were used to test lung toxicity for ivermectin-HP-β-CD formulations in doses of 0.05, 0.1, 0.2, 0.4 and 0.8 mg/kg for 3 successive days. Results: The X-ray diffraction for lyophilized ivermectin-HP-β-CD revealed its amorphous structure that increased drug aqueous solubility 127-fold and was rapidly dissolved within 5 s in saline. Pulmonary administration of ivermectin-HP-β-CD in doses of 0.2, 0.4 and 0.8 mg/kg showed dose-dependent increase in levels of TNF-α, IL-6, IL-13 and ICAM-1 as well as gene expression of MCP-1, protein expression of PIII-NP and serum levels of SP-D paralleled by reduction in IL-10. Moreover, lungs treated with ivermectin (0.2 mg/kg) revealed mild histopathological alterations, while severe pulmonary damage was seen in rats treated with ivermectin at doses of 0.4 and 0.8 mg/kg. However, ivermectin-HP-β-CD formulation administered in doses of 0.05 and 0.1 mg/kg revealed safety profiles.
Conclusion: The safety of inhaled ivermectin-HP-β-CD formulation is dose-dependent. Nevertheless, use of low doses (0.05 and 0.1 mg/kg) could be considered as a possible therapeutic regimen in COVID-19 cases.
References
Akram, Azhar, Shahzad, Latif, Khan, Pakistan Randomized and Observational Trial to Evaluate Coronavirus Treatment (PROTECT) of Hydroxychloroquine, Oseltamivir and Azithromycin to treat newly diagnosed patients with COVID-19 infection who have no comorbidities like diabetes mellitus: A structured summary of a study protocol for a randomized controlled trial, Trials
Al-Mazidi, Alotaibi, Nedjadi, Chaudhary, Alzoghaibi et al., Blocking of cytokines signalling attenuates evoked and spontaneous neuropathic pain behaviours in the paclitaxel rat model of chemotherapy-induced neuropathy, Eur. J. Pain
Ammar, Salama, Ghorab, Mahmoud, Formulation and biological evaluation of glimepiride-cyclodextrin-polymer systems, Int. J. Pharm
Arshad, Pertinez, Box, Tatham, Rajoli et al., Prioritization of Anti-SARS-Cov-2 Drug Repurposing Opportunities Based on Plasma and Target Site Concentrations Derived from their Established Human Pharmacokinetics, Clin. Pharmacol. Ther
Awasthi, Coalson, Yoder, Crouch, King, Deficiencies in lung surfactant proteins A and D are associated with lung infection in very premature neonatal baboons, Am. J. Respir. Crit. Care Med
Badawy, Ghorab, Adeyeye, Characterization and bioavailability of danazol-hydroxypropyl β-cyclodextrin coprecipitates, Int. J. Pharm
Bird, Morgan, Ramirez, Yong, Kovacs, Decreased pulmonary inflammation after ethanol exposure and burn injury in intercellular adhesion molecule-1 knockout mice, J. Burn Care Res
Bjermer, Lundgren, Hallgren, Hyaluronan and type III procollagen peptide concentrations in bronchoalveolar lavage fluid in idiopathic pulmonary fibrosis, Thorax
Brewster, Loftsson, Cyclodextrins as pharmaceutical solubilizers, Adv. Drug Deliv. Rev
Calabro, Tommasini, Donato, Raneri, Stancanelli et al., Effects of alpha-and beta-cyclodextrin complexation on the physico-chemical properties and antioxidant activity of some 3-hydroxyflavones, J. Pharm. Biomed. Anal. [Comparative Study
Caly, Druce, Catton, Jans, Wagstaff, The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro, Antiviral Res
Cataldo, Evrard, Noel, Foldart, Inventors; Use of cyclodextrin for treatment and prevention of bronchial inflammatory diseases
Chaccour, Abizanda, Irigoyen-Barrio, Casellas, Aldaz et al., Nebulized ivermectin for COVID-19 and other respiratory diseases, a proof of concept, dose-ranging study in rats, Sci. Rep
Chaccour, Hammann, Ramon-Garcia, Rabinovich, Ivermectin and COVID-19: Keeping Rigor in Times of Urgency, Am. J. Trop. Med. Hyg
Chiaramonte, Mentink-Kane, Jacobson, Cheever, Whitters et al., Regulation and function of the interleukin 13 receptor alpha 2 during a T helper cell type 2-dominant immune response, J. Exp. Med
Crump, Ivermectin: enigmatic multifaceted 'wonder' drug continues to surprise and exceed expectations, J. Antibiot
Doile, Fortunato, Schmucker, Schucko, Silva et al., Physicochemical properties and dissolution studies of dexamethasone acetate-beta-cyclodextrin inclusion complexes produced by different methods, AAPS PharmSciTech
Dolan, Ingham, Baombe, BET 1: Lopinavir-ritonavir and COVID-19, Emerg. Med. J
Eslami, Mousaviasl, Radmanesh, Jelvay, Bitaraf et al., The impact of sofosbuvir/daclatasvir or ribavirin in patients with severe COVID-19, J. Antimicrob. Chemother
Fernandes, Vieira, Veiga, Physicochemical characterization and in vitro dissolution behavior of nicardipine-cyclodextrins inclusion compounds, Eur. J. Pharm. Sci
Formiga, Leblanc, Souza, Farias, De Oliveira et al., Ivermectin: an award-winning drug with expected antiviral activity against COVID-19, J. Control. Release
Gonzalez Canga, Sahagun Prieto, Diez Liebana, Martinez, Sierra et al., The pharmacokinetics and interactions of ivermectin in humans-a mini-review, AAPS J
Guan, Zeng, Shi, Zheng, Fan et al., Aerosolization Performance, Antitussive Effect and Local Toxicity of Naringenin-Hydroxypropyl-beta-Cyclodextrin Inhalation Solution for Pulmonary Delivery, AAPS PharmSciTech
Guzzo, Furtek, Porras, Chen, Tipping et al., Safety, tolerability, and pharmacokinetics of escalating high doses of ivermectin in healthy adult subjects, J. Clin. Pharmacol
Hallahan, Geng, Shyr, Effects of intercellular adhesion molecule 1 (ICAM-1) null mutation on radiation-induced pulmonary fibrosis and respiratory insufficiency in mice, J. Natl Cancer Inst
Heidary, Gharebaghi, Ivermectin: a systematic review from antiviral effects to COVID-19 complementary regimen, J. Antibiot
Hilda, Das, TLR stimulation of human neutrophils lead to increased release of MCP-1, MIP-1alpha, IL-1beta, IL-8 and TNF during tuberculosis, Hum. Immunol
Inchem, Ivermectin, None
Jackson, Mckenna, Vitrification and crystallization of organic liquids confined to nanoscale pores, Chem. Mater
Jakubzick, Choi, Joshi, Keane, Kunkel et al., Therapeutic attenuation of pulmonary fibrosis via targeting of IL-4-and IL-13-responsive cells, J. Immunol
Javadzadeh, Yaqoubi, Therapeutic nanostructures for pulmonary drug delivery, Nanostructures for Drug Delivery
Jiang, Klein, Niederacher, Du, Marx et al., C/T polymorphism of the intercellular adhesion molecule-1 gene (exon 6, codon 469). A risk factor for coronary heart disease and myocardial infarction, Int. J. Cardiol
Juarez, Schcolnik-Cabrera, Duenas-Gonzalez, The multitargeted drug ivermectin: from an antiparasitic agent to a repositioned cancer drug, Am J Cancer Res
Kasgari, Moradi, Shabani, Babamahmoodi, Davoudi Badabi et al., Evaluation of the efficacy of sofosbuvir plus daclatasvir in combination with ribavirin for hospitalized COVID-19 patients with moderate disease compared with standard care: a single-centre, randomized controlled trial, J. Antimicrob. Chemother
Kaur, Shekhar, Sharma, Sarma, Prakash et al., Ivermectin as a potential drug for treatment of COVID-19: an in-sync review with clinical and computational attributes, Pharmacol. Rep
Ketkar, Yang, Wormser, Wang, Lack of efficacy of ivermectin for prevention of a lethal Zika virus infection in a murine system, Diagn. Microbiol. Infect. Dis
Korfhagen, Surfactant protein A (SP-A)-mediated bacterial clearance: SP-A and cystic fibrosis, Am. J. Respir. Cell Mol. Biol
Kuroki, Tsutahara, Shijubo, Takahashi, Shiratori et al., Elevated levels of lung surfactant protein A in sera from patients with idiopathic pulmonary fibrosis and pulmonary alveolar proteinosis, Am. Rev. Respir. Dis
Laskin, Malaviya, Laskin, Role of Macrophages in Acute Lung Injury and Chronic Fibrosis Induced by Pulmonary Toxicants, Toxicol. Sci
Lechner, Gantin, Seeger, Sarnecka, Portillo et al., Chemokines and cytokines in patients with an occult Onchocerca volvulus infection, Microbes Infect
Lin, Pearson, Chinchilli, Pietschmann, Luo et al., Polymorphisms of human SP-A, SP-B, and SP-D genes: association of SP-B Thr131Ile with ARDS, Clin. Genet
Loftsson, Jarho, Másson, Järvinen, Cyclodextrins in drug delivery, Expert Opinion on Drug Delivery
Lubrizol, Lyophilization of Pharmaceuticals: An Overview
Maged, Mahmoud, Ghorab, Hydroxypropyl-beta-cyclodextrin as cryoprotectant in nanoparticles prepared by nano-spray drying technique, J. Pharm. Sci. Emerg. Drugs,
doi:10.4172/2380-9477.1000121Maged, Mahmoud, Ghorab, Nano Spray Drying Technique as a Novel Approach To Formulate Stable Econazole Nitrate Nanosuspension Formulations for Ocular Use, Mol. Pharm
Mahmoud, Elkasabgy, Abdelkhalek, Design and characterization of emulsified spray dried alginate microparticles as a carrier for the dually acting drug roflumilast, Eur. J. Pharm. Sci
Mansour, El-Abhar, Soubh, MiR-200a inversely correlates with Hedgehog and TGF-beta canonical/non-canonical trajectories to orchestrate the anti-fibrotic effect of Tadalafil in a bleomycin-induced pulmonary fibrosis model, Inflammopharmacology
Markowska, Kaysiewicz, Markowska, Huczynski, Doxycycline, salinomycin, monensin and ivermectin repositioned as cancer drugs, Bioorg. Med. Chem. Lett
Marques, Hadgraft, Kellaway, Taylor, Studies of cyclodextrin inclusion complexes. IV. The pulmonary absorption of salbutamol from a complex with 2-hydroxypropyl-β-cyclodextrin in rabbits, Int. J. Pharm
Marshall, Bellingan, Webb, Puddicombe, Goldsack et al., Fibroproliferation occurs early in the acute respiratory distress syndrome and impacts on outcome, Am. J. Respir. Crit. Care Med
Martin, Physical pharmacy: physical chemical principles in the pharmaceutical sciences
Meini, Pagotto, Longo, Vendramin, Pecori et al., Role of Lopinavir/Ritonavir in the Treatment of Covid-19: A Review of Current Evidence, Guideline Recommendations, and Perspectives, J. Clin. Med
Meo, Klonoff, Akram, Efficacy of chloroquine and hydroxychloroquine in the treatment of COVID-19, Eur. Rev. Med. Pharmacol. Sci
Milani, Faghihi, Roulholamini, Najafabadi, Amini et al., Hydroxypropyl beta cyclodextrin: a water-replacement agent or a surfactant upon spray freeze-drying of IgG with enhanced stability and aerosolization, Drug Dev. Ind. Pharm
Mittal, Inhaled route and anti-inflammatory action of ivermectin: Do they hold promise in fighting against COVID-19?, Med. Hypotheses
Momekov, Momekova, Ivermectin as a potential COVID-19 treatment from the pharmacokinetic point of view: antiviral levels are not likely attainable with known dosing regimens, Biotechnol. Biotechnol. Equip
Montefort, Roche, Howarth, Djukanovic, Gratziou et al., Intercellular adhesion molecule-1 (ICAM-1) and endothelial leucocyte adhesion molecule-1 (ELAM-1) expression in the bronchial mucosa of normal and asthmatic subjects, Eur. Respir. J
Panee, Monocyte Chemoattractant Protein 1 (MCP-1) in obesity and diabetes, Cytokine
Phillips, Inhaled efficacious dose translation from rodent to human: A retrospective analysis of clinical standards for respiratory diseases, Pharmacol. Ther
Pilkington, Pepperrell, Hill, A review of the safety of favipiravir -a potential treatment in the COVID-19 pandemic?, J. Virus Erad
Rajewski, Stella, Pharmaceutical applications of cyclodextrins. 2. In vivo drug delivery, J. Pharm. Sci
Rosumeck, Nast, Dressler, Ivermectin and permethrin for treating scabies, Cochrane Database Syst. Rev
Roszer, Understanding the Mysterious M2 Macrophage through Activation Markers and Effector Mechanisms, Mediators Inflamm
Santanasto, Cvejkus, Wojczynski, Marron, Schupf et al., Circulating Procollagen type III N-terminal peptide (P3NP) and Physical Function in Adults from The Long Life Family Study, J. Gerontol. A Biol. Sci. Med. Sci
Saokham, Muankaew, Jansook, Loftsson, Solubility of Cyclodextrins and Drug/Cyclodextrin Complexes, Molecules
Saqrane, Mhammedi, Review on the global epidemiological situation and the efficacy of chloroquine and hydroxychloroquine for the treatment of COVID-19, New Microbes New Infect
Schmith, Zhou, Lohmer, The Approved Dose of Ivermectin Alone is not the Ideal Dose for the Treatment of COVID-19, Clin. Pharmacol. Ther
Semcheddine, Guissi, Liu, Wu, Wang, Effects of the preparation method on the formation of true nimodipine SBE-β-CD/HP-β-CD inclusion complexes and their dissolution rates enhancement, AAPS PharmSciTech
Sen Gupta, Biswal, Panda, Ray, Rana, Binding mechanism and structural insights into the identified protein target of COVID-19 and importinalpha with in-vitro effective drug ivermectin, J. Biomol. Struct. Dyn
Sen Gupta, Rana, Ivermectin, Famotidine, and Doxycycline: A Suggested Combinatorial Therapeutic for the Treatment of COVID-19, ACS Pharmacol Transl Sci
Serno, Geidobler, Winter, Protein stabilization by cyclodextrins in the liquid and dried state, Adv. Drug Deliv. Rev
Shao, Krishnamoorthy, Mitra, Cyclodextrins as nasal absorption promoters of insulin: mechanistic evaluations, Pharm. Res
Sharp, Product information for AusPAR Stromectol Ivermectin
Sharun, Dhama, Patel, Pathak, Tiwari et al., Ivermectin, a new candidate therapeutic against SARS-CoV-2/COVID-19, Ann Clin Microbiol Antimicrob
Shoukri, Ahmed, Shamma, In vitro and in vivo evaluation of nimesulide lyophilized orally disintegrating tablets, J. Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik eV
Soni, Wilson, O'dea, Yoshida, Katbeh et al., Alveolar macrophage-derived microvesicles mediate acute lung injury, Thorax
Su, Gu, Weng, Zhou, Li et al., Association of serum levels of laminin, type IV collagen, procollagen III N-terminal peptide, and hyaluronic acid with the progression of interstitial lung disease, Medicine
Sumagin, Lomakina, Sarelius, Leukocyte-endothelial cell interactions are linked to vascular permeability via ICAM-1-mediated signaling, Am. J. Physiol. Heart Circ. Physiol
Suvarna, Layton, Bancroft, Bancroft's theory and practice of histological techniques E-Book
Taneri, Güneri, Kata, Improvement in the physicochemical properties of ketoconazole through complexation with cyclodextrin derivatives, J Incl Macrocycl Chem
Tavornvipas, Tajiri, Hirayama, Arima, Uekama, Effects of hydrophilic cyclodextrins on aggregation of recombinant human growth hormone, Pharm. Res
Thibaut De Menonville, Rosignoli, Soares, Roquet, Bertino et al., Topical Treatment of Rosacea with Ivermectin Inhibits Gene Expression of Cathelicidin Innate Immune Mediators, LL-37 and KLK5, in Reconstructed and Ex Vivo Skin Models, Dermatol Ther (Heidelb)
Vass, Démuth, Farkas, Hirsch, Szabó et al., Continuous alternative to freeze drying: Manufacturing of cyclodextrin-based reconstitution powder from aqueous solution using scaled-up electrospinning, J. Control. Release
Vianna, Bentley, Ribeiro, Carvalho, Neto et al., Formation of cyclodextrin inclusion complexes with corticosteroids: their characterization and stability, Int. J. Pharm
Vitenberga, Pilmane, Inflammatory, anti-inflammatory and regulatory cytokines in relatively healthy lung tissue as an essential part of the local immune system, Biomed. Pap. Med. Fac. Univ. Palacky Olomouc Czech Repub
Wagstaff, Sivakumaran, Heaton, Harrich, Jans, Ivermectin is a specific inhibitor of importin alpha/beta-mediated nuclear import able to inhibit replication of HIV-1 and dengue virus, Biochem. J
Walters, Bhatnagar, Tchessalov, Izutsu, Tsumoto et al., Next generation drying technologies for pharmaceutical applications, J. Pharm. Sci
Wang, Zhang, Deng, Wang, Complexation of hydrophobic drugs with hydroxypropyl-β-cyclodextrin by lyophilization using a tertiary butyl alcohol system, J. Incl. Phenom. Macrocycl. Chem
Who, Proposal to waive in vivo bioequivalence requirements for the who model list of essential medicines immediate release, solid oral dosage forms
Who, WHO advises that ivermectin only be used to treat COVID-19 within clinical trials
Yadav, Saini, Arora, MCP-1: chemoattractant with a role beyond immunity: a review, Clin. Chim. Acta
Yamanel, Kaldirim, Oztas, Coskun, Poyrazoglu et al., Ozone therapy and hyperbaric oxygen treatment in lung injury in septic rats, Int. J. Med. Sci
Zhang, Dong, Sun, Wang, Cai et al., Tumor-associated inflammatory microenvironment in non-small cell lung cancer: correlation with FGFR1 and TLR4 expression via PI3K/Akt pathway, J Cancer
Zhang, Zhu, Lin, Han, Jiang et al., Hydroxypropyl-betacyclodextrin functionalized calcium carbonate microparticles as a potential carrier for enhancing oral delivery of water-insoluble drugs, Int. J. Nanomed
DOI record:
{
"DOI": "10.1016/j.intimp.2021.108004",
"ISSN": [
"1567-5769"
],
"URL": "http://dx.doi.org/10.1016/j.intimp.2021.108004",
"alternative-id": [
"S1567576921006408"
],
"article-number": "108004",
"assertion": [
{
"label": "This article is maintained by",
"name": "publisher",
"value": "Elsevier"
},
{
"label": "Article Title",
"name": "articletitle",
"value": "Safety of inhaled ivermectin as a repurposed direct drug for treatment of COVID-19: A preclinical tolerance study"
},
{
"label": "Journal Title",
"name": "journaltitle",
"value": "International Immunopharmacology"
},
{
"label": "CrossRef DOI link to publisher maintained version",
"name": "articlelink",
"value": "https://doi.org/10.1016/j.intimp.2021.108004"
},
{
"label": "Content Type",
"name": "content_type",
"value": "article"
},
{
"label": "Copyright",
"name": "copyright",
"value": "© 2021 Elsevier B.V. All rights reserved."
}
],
"author": [
{
"affiliation": [],
"family": "M. Mansour",
"given": "Suzan",
"sequence": "first"
},
{
"affiliation": [],
"family": "N. Shamma",
"given": "Rehab",
"sequence": "additional"
},
{
"affiliation": [],
"family": "A. Ahmed",
"given": "Kawkab",
"sequence": "additional"
},
{
"affiliation": [],
"family": "A. Sabry",
"given": "Nirmeen",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Esmat",
"given": "Gamal",
"sequence": "additional"
},
{
"affiliation": [],
"family": "A. Mahmoud",
"given": "Azza",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Maged",
"given": "Amr",
"sequence": "additional"
}
],
"container-title": "International Immunopharmacology",
"container-title-short": "International Immunopharmacology",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"elsevier.com",
"sciencedirect.com"
]
},
"created": {
"date-parts": [
[
2021,
7,
23
]
],
"date-time": "2021-07-23T12:23:55Z",
"timestamp": 1627043035000
},
"deposited": {
"date-parts": [
[
2023,
4,
15
]
],
"date-time": "2023-04-15T09:30:09Z",
"timestamp": 1681551009000
},
"indexed": {
"date-parts": [
[
2024,
4,
15
]
],
"date-time": "2024-04-15T23:16:47Z",
"timestamp": 1713223007948
},
"is-referenced-by-count": 10,
"issued": {
"date-parts": [
[
2021,
10
]
]
},
"language": "en",
"license": [
{
"URL": "https://www.elsevier.com/tdm/userlicense/1.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
10,
1
]
],
"date-time": "2021-10-01T00:00:00Z",
"timestamp": 1633046400000
}
},
{
"URL": "https://doi.org/10.15223/policy-017",
"content-version": "stm-asf",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
10,
1
]
],
"date-time": "2021-10-01T00:00:00Z",
"timestamp": 1633046400000
}
},
{
"URL": "https://doi.org/10.15223/policy-037",
"content-version": "stm-asf",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
10,
1
]
],
"date-time": "2021-10-01T00:00:00Z",
"timestamp": 1633046400000
}
},
{
"URL": "https://doi.org/10.15223/policy-012",
"content-version": "stm-asf",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
10,
1
]
],
"date-time": "2021-10-01T00:00:00Z",
"timestamp": 1633046400000
}
},
{
"URL": "https://doi.org/10.15223/policy-029",
"content-version": "stm-asf",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
10,
1
]
],
"date-time": "2021-10-01T00:00:00Z",
"timestamp": 1633046400000
}
},
{
"URL": "https://doi.org/10.15223/policy-004",
"content-version": "stm-asf",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
10,
1
]
],
"date-time": "2021-10-01T00:00:00Z",
"timestamp": 1633046400000
}
}
],
"link": [
{
"URL": "https://api.elsevier.com/content/article/PII:S1567576921006408?httpAccept=text/xml",
"content-type": "text/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://api.elsevier.com/content/article/PII:S1567576921006408?httpAccept=text/plain",
"content-type": "text/plain",
"content-version": "vor",
"intended-application": "text-mining"
}
],
"member": "78",
"original-title": [],
"page": "108004",
"prefix": "10.1016",
"published": {
"date-parts": [
[
2021,
10
]
]
},
"published-print": {
"date-parts": [
[
2021,
10
]
]
},
"publisher": "Elsevier BV",
"reference": [
{
"key": "10.1016/j.intimp.2021.108004_b0005",
"unstructured": "Centers for Disease Control and Prevention. Coronavirus (COVID-19): Get the Facts About Coronavirus. 2020 [cited 2020 6/9/2020]; Available from: https://www.cdc.gov/coronavirus/2019-ncov/index.html."
},
{
"article-title": "Efficacy of chloroquine and hydroxychloroquine in the treatment of COVID-19",
"author": "Meo",
"first-page": "4539",
"issue": "8",
"journal-title": "Eur. Rev. Med. Pharmacol. Sci.",
"key": "10.1016/j.intimp.2021.108004_b0010",
"volume": "24",
"year": "2020"
},
{
"DOI": "10.1016/j.nmni.2020.100680",
"article-title": "Review on the global epidemiological situation and the efficacy of chloroquine and hydroxychloroquine for the treatment of COVID-19",
"author": "Saqrane",
"doi-asserted-by": "crossref",
"journal-title": "New Microbes New Infect.",
"key": "10.1016/j.intimp.2021.108004_b0015",
"volume": "35",
"year": "2020"
},
{
"DOI": "10.1136/emermed-2020-210221.2",
"article-title": "BET 1: Lopinavir-ritonavir and COVID-19",
"author": "Dolan",
"doi-asserted-by": "crossref",
"first-page": "450",
"issue": "7",
"journal-title": "Emerg. Med. J.",
"key": "10.1016/j.intimp.2021.108004_b0020",
"volume": "37",
"year": "2020"
},
{
"DOI": "10.3390/jcm9072050",
"article-title": "Role of Lopinavir/Ritonavir in the Treatment of Covid-19: A Review of Current Evidence, Guideline Recommendations, and Perspectives",
"author": "Meini",
"doi-asserted-by": "crossref",
"issue": "7",
"journal-title": "J. Clin. Med.",
"key": "10.1016/j.intimp.2021.108004_b0025",
"volume": "9",
"year": "2020"
},
{
"DOI": "10.1016/S2055-6640(20)30016-9",
"article-title": "A review of the safety of favipiravir - a potential treatment in the COVID-19 pandemic?",
"author": "Pilkington",
"doi-asserted-by": "crossref",
"first-page": "45",
"issue": "2",
"journal-title": "J. Virus Erad.",
"key": "10.1016/j.intimp.2021.108004_b0030",
"volume": "6",
"year": "2020"
},
{
"DOI": "10.1186/s13063-020-04616-4",
"author": "Akram",
"doi-asserted-by": "crossref",
"first-page": "702",
"issue": "1",
"journal-title": "Trials.",
"key": "10.1016/j.intimp.2021.108004_b0035",
"volume": "21",
"year": "2020"
},
{
"article-title": "The impact of sofosbuvir/daclatasvir or ribavirin in patients with severe COVID-19",
"author": "Eslami",
"journal-title": "J. Antimicrob. Chemother.",
"key": "10.1016/j.intimp.2021.108004_b0040",
"volume": "19",
"year": "2020"
},
{
"article-title": "Evaluation of the efficacy of sofosbuvir plus daclatasvir in combination with ribavirin for hospitalized COVID-19 patients with moderate disease compared with standard care: a single-centre, randomized controlled trial",
"author": "Abbaspour Kasgari",
"journal-title": "J. Antimicrob. Chemother.",
"key": "10.1016/j.intimp.2021.108004_b0045",
"volume": "19",
"year": "2020"
},
{
"DOI": "10.1186/s12941-020-00368-w",
"article-title": "Ivermectin, a new candidate therapeutic against SARS-CoV-2/COVID-19",
"author": "Sharun",
"doi-asserted-by": "crossref",
"first-page": "23",
"issue": "1",
"journal-title": "Ann Clin Microbiol Antimicrob.",
"key": "10.1016/j.intimp.2021.108004_b0050",
"volume": "19",
"year": "2020"
},
{
"DOI": "10.1038/s41429-020-0336-z",
"article-title": "Ivermectin: a systematic review from antiviral effects to COVID-19 complementary regimen",
"author": "Heidary",
"doi-asserted-by": "crossref",
"first-page": "593",
"issue": "9",
"journal-title": "J. Antibiot. (Tokyo)",
"key": "10.1016/j.intimp.2021.108004_b0055",
"volume": "73",
"year": "2020"
},
{
"DOI": "10.1208/s12248-007-9000-9",
"article-title": "The pharmacokinetics and interactions of ivermectin in humans–a mini-review",
"author": "Gonzalez Canga",
"doi-asserted-by": "crossref",
"first-page": "42",
"issue": "1",
"journal-title": "AAPS J.",
"key": "10.1016/j.intimp.2021.108004_b0060",
"volume": "10",
"year": "2008"
},
{
"DOI": "10.1002/14651858.CD012994",
"article-title": "Ivermectin and permethrin for treating scabies",
"author": "Rosumeck",
"doi-asserted-by": "crossref",
"journal-title": "Cochrane Database Syst. Rev.",
"key": "10.1016/j.intimp.2021.108004_b0065",
"year": "2018"
},
{
"DOI": "10.1038/ja.2017.11",
"article-title": "Ivermectin: enigmatic multifaceted 'wonder' drug continues to surprise and exceed expectations",
"author": "Crump",
"doi-asserted-by": "crossref",
"first-page": "495",
"issue": "5",
"journal-title": "J. Antibiot. (Tokyo)",
"key": "10.1016/j.intimp.2021.108004_b0070",
"volume": "70",
"year": "2017"
},
{
"article-title": "The multitargeted drug ivermectin: from an antiparasitic agent to a repositioned cancer drug",
"author": "Juarez",
"first-page": "317",
"issue": "2",
"journal-title": "Am J Cancer Res.",
"key": "10.1016/j.intimp.2021.108004_b0075",
"volume": "8",
"year": "2018"
},
{
"DOI": "10.1016/j.bmcl.2019.04.045",
"article-title": "Doxycycline, salinomycin, monensin and ivermectin repositioned as cancer drugs",
"author": "Markowska",
"doi-asserted-by": "crossref",
"first-page": "1549",
"issue": "13",
"journal-title": "Bioorg. Med. Chem. Lett.",
"key": "10.1016/j.intimp.2021.108004_b0080",
"volume": "29",
"year": "2019"
},
{
"DOI": "10.1016/j.diagmicrobio.2019.03.012",
"article-title": "Lack of efficacy of ivermectin for prevention of a lethal Zika virus infection in a murine system",
"author": "Ketkar",
"doi-asserted-by": "crossref",
"first-page": "38",
"issue": "1",
"journal-title": "Diagn. Microbiol. Infect. Dis.",
"key": "10.1016/j.intimp.2021.108004_b0085",
"volume": "95",
"year": "2019"
},
{
"DOI": "10.1042/BJ20120150",
"article-title": "Ivermectin is a specific inhibitor of importin alpha/beta-mediated nuclear import able to inhibit replication of HIV-1 and dengue virus",
"author": "Wagstaff",
"doi-asserted-by": "crossref",
"first-page": "851",
"issue": "3",
"journal-title": "Biochem. J.",
"key": "10.1016/j.intimp.2021.108004_b0090",
"volume": "443",
"year": "2012"
},
{
"DOI": "10.1016/j.antiviral.2020.104787",
"article-title": "The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro",
"author": "Caly",
"doi-asserted-by": "crossref",
"journal-title": "Antiviral Res.",
"key": "10.1016/j.intimp.2021.108004_b0095",
"volume": "178",
"year": "2020"
},
{
"article-title": "Binding mechanism and structural insights into the identified protein target of COVID-19 and importin-alpha with in-vitro effective drug ivermectin",
"author": "Sen Gupta",
"first-page": "1",
"journal-title": "J. Biomol. Struct. Dyn.",
"key": "10.1016/j.intimp.2021.108004_b0100",
"volume": "28",
"year": "2020"
},
{
"DOI": "10.1021/acsptsci.0c00140",
"article-title": "Ivermectin, Famotidine, and Doxycycline: A Suggested Combinatorial Therapeutic for the Treatment of COVID-19",
"author": "Sen Gupta",
"doi-asserted-by": "crossref",
"first-page": "1037",
"issue": "5",
"journal-title": "ACS Pharmacol Transl Sci.",
"key": "10.1016/j.intimp.2021.108004_b0105",
"volume": "3",
"year": "2020"
},
{
"DOI": "10.1080/13102818.2020.1775118",
"article-title": "Ivermectin as a potential COVID-19 treatment from the pharmacokinetic point of view: antiviral levels are not likely attainable with known dosing regimens",
"author": "Momekov",
"doi-asserted-by": "crossref",
"first-page": "469",
"issue": "1",
"journal-title": "Biotechnol. Biotechnol. Equip.",
"key": "10.1016/j.intimp.2021.108004_b0110",
"volume": "34",
"year": "2020"
},
{
"DOI": "10.4269/ajtmh.20-0271",
"article-title": "Ivermectin and COVID-19: Keeping Rigor in Times of Urgency",
"author": "Chaccour",
"doi-asserted-by": "crossref",
"first-page": "1156",
"issue": "6",
"journal-title": "Am. J. Trop. Med. Hyg.",
"key": "10.1016/j.intimp.2021.108004_b0115",
"volume": "102",
"year": "2020"
},
{
"DOI": "10.1177/009127002237994",
"article-title": "Safety, tolerability, and pharmacokinetics of escalating high doses of ivermectin in healthy adult subjects",
"author": "Guzzo",
"doi-asserted-by": "crossref",
"first-page": "1122",
"issue": "10",
"journal-title": "J. Clin. Pharmacol.",
"key": "10.1016/j.intimp.2021.108004_b0120",
"volume": "42",
"year": "2002"
},
{
"key": "10.1016/j.intimp.2021.108004_b0125",
"unstructured": "INCHEM. Ivermectin. 2020 [cited 2020 7/9/2020]; Available from: http://www.inchem.org/documents/pims/pharm/ivermect.htm."
},
{
"key": "10.1016/j.intimp.2021.108004_b0130",
"unstructured": "Merck Sharp Dohme. Product information for AusPAR Stromectol Ivermectin. 2013."
},
{
"DOI": "10.1002/cpt.1909",
"article-title": "Prioritization of Anti-SARS-Cov-2 Drug Repurposing Opportunities Based on Plasma and Target Site Concentrations Derived from their Established Human Pharmacokinetics",
"author": "Arshad",
"doi-asserted-by": "crossref",
"first-page": "775",
"issue": "4",
"journal-title": "Clin. Pharmacol. Ther.",
"key": "10.1016/j.intimp.2021.108004_b0135",
"volume": "108",
"year": "2020"
},
{
"DOI": "10.1002/cpt.1889",
"article-title": "The Approved Dose of Ivermectin Alone is not the Ideal Dose for the Treatment of COVID-19",
"author": "Schmith",
"doi-asserted-by": "crossref",
"journal-title": "Clin. Pharmacol. Ther.",
"key": "10.1016/j.intimp.2021.108004_b0140",
"year": "2020"
},
{
"DOI": "10.7150/jca.26277",
"article-title": "Tumor-associated inflammatory microenvironment in non-small cell lung cancer: correlation with FGFR1 and TLR4 expression via PI3K/Akt pathway",
"author": "Zhang",
"doi-asserted-by": "crossref",
"first-page": "1004",
"issue": "4",
"journal-title": "J Cancer.",
"key": "10.1016/j.intimp.2021.108004_b0145",
"volume": "10",
"year": "2019"
},
{
"author": "Javadzadeh",
"first-page": "619",
"key": "10.1016/j.intimp.2021.108004_b0150",
"series-title": "Therapeutic nanostructures for pulmonary drug delivery. Nanostructures for Drug Delivery",
"year": "2017"
},
{
"DOI": "10.1016/j.ejps.2018.06.015",
"article-title": "Design and characterization of emulsified spray dried alginate microparticles as a carrier for the dually acting drug roflumilast",
"author": "Mahmoud",
"doi-asserted-by": "crossref",
"first-page": "64",
"issue": "122",
"journal-title": "Eur. J. Pharm. Sci.",
"key": "10.1016/j.intimp.2021.108004_b0155",
"volume": "15",
"year": "2018"
},
{
"DOI": "10.1016/S0928-0987(01)00208-1",
"article-title": "Physicochemical characterization and in vitro dissolution behavior of nicardipine-cyclodextrins inclusion compounds",
"author": "Fernandes",
"doi-asserted-by": "crossref",
"first-page": "79",
"issue": "1",
"journal-title": "Eur. J. Pharm. Sci.",
"key": "10.1016/j.intimp.2021.108004_b0160",
"volume": "15",
"year": "2002"
},
{
"DOI": "10.1016/S0378-5173(98)00068-4",
"article-title": "Formation of cyclodextrin inclusion complexes with corticosteroids: their characterization and stability",
"author": "Vianna",
"doi-asserted-by": "crossref",
"first-page": "205",
"issue": "1–2",
"journal-title": "Int. J. Pharm.",
"key": "10.1016/j.intimp.2021.108004_b0165",
"volume": "167",
"year": "1998"
},
{
"DOI": "10.1016/0378-5173(95)04214-8",
"article-title": "Characterization and bioavailability of danazol-hydroxypropyl β-cyclodextrin coprecipitates",
"author": "Badawy",
"doi-asserted-by": "crossref",
"first-page": "45",
"issue": "1–2",
"journal-title": "Int. J. Pharm.",
"key": "10.1016/j.intimp.2021.108004_b0170",
"volume": "128",
"year": "1996"
},
{
"DOI": "10.1021/acs.molpharmaceut.6b00167",
"article-title": "Nano Spray Drying Technique as a Novel Approach To Formulate Stable Econazole Nitrate Nanosuspension Formulations for Ocular Use",
"author": "Maged",
"doi-asserted-by": "crossref",
"first-page": "2951",
"issue": "9",
"journal-title": "Mol. Pharm.",
"key": "10.1016/j.intimp.2021.108004_b0175",
"volume": "13",
"year": "2016"
},
{
"DOI": "10.4172/2380-9477.1000121",
"article-title": "Hydroxypropyl-beta-cyclodextrin as cryoprotectant in nanoparticles prepared by nano-spray drying technique",
"author": "Maged",
"doi-asserted-by": "crossref",
"issue": "1",
"journal-title": "J. Pharm. Sci. Emerg. Drugs",
"key": "10.1016/j.intimp.2021.108004_b0180",
"volume": "5",
"year": "2017"
},
{
"DOI": "10.1016/j.addr.2011.08.003",
"article-title": "Protein stabilization by cyclodextrins in the liquid and dried state",
"author": "Serno",
"doi-asserted-by": "crossref",
"first-page": "1086",
"issue": "13",
"journal-title": "Adv. Drug Deliv. Rev.",
"key": "10.1016/j.intimp.2021.108004_b0185",
"volume": "63",
"year": "2011"
},
{
"DOI": "10.1007/s11095-004-7691-5",
"article-title": "Effects of hydrophilic cyclodextrins on aggregation of recombinant human growth hormone",
"author": "Tavornvipas",
"doi-asserted-by": "crossref",
"first-page": "2369",
"issue": "12",
"journal-title": "Pharm. Res.",
"key": "10.1016/j.intimp.2021.108004_b0190",
"volume": "21",
"year": "2004"
},
{
"DOI": "10.1023/A:1015847604654",
"article-title": "Cyclodextrins as nasal absorption promoters of insulin: mechanistic evaluations",
"author": "Shao",
"doi-asserted-by": "crossref",
"first-page": "1157",
"issue": "9",
"journal-title": "Pharm. Res.",
"key": "10.1016/j.intimp.2021.108004_b0195",
"volume": "9",
"year": "1992"
},
{
"DOI": "10.1023/A:1023013523416",
"article-title": "Improvement in the physicochemical properties of ketoconazole through complexation with cyclodextrin derivatives",
"author": "Taneri",
"doi-asserted-by": "crossref",
"first-page": "257",
"issue": "1",
"journal-title": "J Incl Macrocycl Chem.",
"key": "10.1016/j.intimp.2021.108004_b0200",
"volume": "44",
"year": "2002"
},
{
"DOI": "10.1016/0378-5173(91)90331-H",
"article-title": "Studies of cyclodextrin inclusion complexes. IV. The pulmonary absorption of salbutamol from a complex with 2-hydroxypropyl-β-cyclodextrin in rabbits",
"author": "Marques",
"doi-asserted-by": "crossref",
"first-page": "303",
"issue": "2–3",
"journal-title": "Int. J. Pharm.",
"key": "10.1016/j.intimp.2021.108004_b0205",
"volume": "77",
"year": "1991"
},
{
"DOI": "10.1208/s12249-020-01889-5",
"article-title": "Aerosolization Performance, Antitussive Effect and Local Toxicity of Naringenin-Hydroxypropyl-beta-Cyclodextrin Inhalation Solution for Pulmonary Delivery",
"author": "Guan",
"doi-asserted-by": "crossref",
"first-page": "20",
"issue": "1",
"journal-title": "AAPS PharmSciTech.",
"key": "10.1016/j.intimp.2021.108004_b0210",
"volume": "22",
"year": "2021"
},
{
"DOI": "10.1080/03639045.2020.1724131",
"article-title": "Hydroxypropyl beta cyclodextrin: a water-replacement agent or a surfactant upon spray freeze-drying of IgG with enhanced stability and aerosolization",
"author": "Milani",
"doi-asserted-by": "crossref",
"first-page": "403",
"issue": "3",
"journal-title": "Drug Dev. Ind. Pharm.",
"key": "10.1016/j.intimp.2021.108004_b0215",
"volume": "46",
"year": "2020"
},
{
"key": "10.1016/j.intimp.2021.108004_b0220",
"unstructured": "D. Cataldo, B. Evrard, A. Noel, J.-M. Foldart, Inventors; Use of cyclodextrin for treatment and prevention of bronchial inflammatory diseases, 2015."
},
{
"key": "10.1016/j.intimp.2021.108004_b0225",
"unstructured": "A. Martin, Physical pharmacy: physical chemical principles in the pharmaceutical sciences: BI Waverly. Pvt Ltd; 1993."
},
{
"DOI": "10.1002/jps.23998",
"article-title": "Next generation drying technologies for pharmaceutical applications",
"author": "Walters",
"doi-asserted-by": "crossref",
"first-page": "2673",
"issue": "9",
"journal-title": "J. Pharm. Sci.",
"key": "10.1016/j.intimp.2021.108004_b0230",
"volume": "103",
"year": "2014"
},
{
"key": "10.1016/j.intimp.2021.108004_b0235",
"unstructured": "Lubrizol CDMO. Lyophilization of Pharmaceuticals: An Overview. 2019; Available from: https://lubrizolcdmo.com/blog/lyophilization-of-pharmaceuticals-an-overview/."
},
{
"DOI": "10.1208/s12249-008-9042-z",
"article-title": "Physicochemical properties and dissolution studies of dexamethasone acetate-beta-cyclodextrin inclusion complexes produced by different methods",
"author": "Doile",
"doi-asserted-by": "crossref",
"first-page": "314",
"issue": "1",
"journal-title": "AAPS PharmSciTech.",
"key": "10.1016/j.intimp.2021.108004_b0240",
"volume": "9",
"year": "2008"
},
{
"key": "10.1016/j.intimp.2021.108004_b0245",
"unstructured": "WHO. WHO advises that ivermectin only be used to treat COVID-19 within clinical trials. 2021 [cited 2021 July]; Available from: https://www.who.int/news-room/feature-stories/detail/who-advises-that-ivermectin-only-be-used-to-treat-covid-19-within-clinical-trials."
},
{
"DOI": "10.1016/j.ejpb.2009.04.005",
"article-title": "In vitro and in vivo evaluation of nimesulide lyophilized orally disintegrating tablets",
"author": "Shoukri",
"doi-asserted-by": "crossref",
"first-page": "162",
"issue": "1",
"journal-title": "Eur. J. Pharm. Biopharm. : Off. J. Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik eV [Comparative Study Randomized Controlled Trial].",
"key": "10.1016/j.intimp.2021.108004_b0250",
"volume": "73",
"year": "2009"
},
{
"DOI": "10.1016/j.pharmthera.2017.04.003",
"article-title": "Inhaled efficacious dose translation from rodent to human: A retrospective analysis of clinical standards for respiratory diseases",
"author": "Phillips",
"doi-asserted-by": "crossref",
"first-page": "141",
"journal-title": "Pharmacol. Ther.",
"key": "10.1016/j.intimp.2021.108004_b0255",
"volume": "178",
"year": "2017"
},
{
"DOI": "10.1007/s10787-020-00748-w",
"article-title": "MiR-200a inversely correlates with Hedgehog and TGF-beta canonical/non-canonical trajectories to orchestrate the anti-fibrotic effect of Tadalafil in a bleomycin-induced pulmonary fibrosis model",
"author": "Mansour",
"doi-asserted-by": "crossref",
"first-page": "167",
"issue": "1",
"journal-title": "Inflammopharmacology",
"key": "10.1016/j.intimp.2021.108004_b0260",
"volume": "29",
"year": "2021"
},
{
"author": "Suvarna",
"key": "10.1016/j.intimp.2021.108004_b0265",
"series-title": "Bancroft's theory and practice of histological techniques E-Book",
"year": "2018"
},
{
"DOI": "10.7150/ijms.8.48",
"article-title": "Ozone therapy and hyperbaric oxygen treatment in lung injury in septic rats",
"author": "Yamanel",
"doi-asserted-by": "crossref",
"first-page": "48",
"issue": "1",
"journal-title": "Int. J. Med. Sci.",
"key": "10.1016/j.intimp.2021.108004_b0270",
"volume": "8",
"year": "2011"
},
{
"DOI": "10.1007/s43440-020-00195-y",
"article-title": "Ivermectin as a potential drug for treatment of COVID-19: an in-sync review with clinical and computational attributes",
"author": "Kaur",
"doi-asserted-by": "crossref",
"journal-title": "Pharmacol. Rep.",
"key": "10.1016/j.intimp.2021.108004_b0275",
"year": "2021"
},
{
"DOI": "10.1016/j.jconrel.2020.10.009",
"article-title": "Ivermectin: an award-winning drug with expected antiviral activity against COVID-19",
"author": "Formiga",
"doi-asserted-by": "crossref",
"first-page": "758",
"issue": "329",
"journal-title": "J. Control. Release",
"key": "10.1016/j.intimp.2021.108004_b0280",
"volume": "10",
"year": "2021"
},
{
"DOI": "10.1038/s41598-020-74084-y",
"article-title": "Nebulized ivermectin for COVID-19 and other respiratory diseases, a proof of concept, dose-ranging study in rats",
"author": "Chaccour",
"doi-asserted-by": "crossref",
"first-page": "17073",
"issue": "1",
"journal-title": "Sci. Rep.",
"key": "10.1016/j.intimp.2021.108004_b0285",
"volume": "10",
"year": "2020"
},
{
"DOI": "10.1016/j.mehy.2020.110364",
"article-title": "Inhaled route and anti-inflammatory action of ivermectin: Do they hold promise in fighting against COVID-19?",
"author": "Mittal",
"doi-asserted-by": "crossref",
"journal-title": "Med. Hypotheses",
"key": "10.1016/j.intimp.2021.108004_b0290",
"volume": "146",
"year": "2021"
},
{
"key": "10.1016/j.intimp.2021.108004_b0295",
"unstructured": "WHO. Proposal to waive in vivo bioequivalence requirements for the who model list of essential medicines immediate release, solid oral dosage forms. Working document QAS/04109/Rev1. 2005."
},
{
"DOI": "10.1007/s10847-006-9261-4",
"article-title": "Complexation of hydrophobic drugs with hydroxypropyl-β-cyclodextrin by lyophilization using a tertiary butyl alcohol system",
"author": "Wang",
"doi-asserted-by": "crossref",
"first-page": "349",
"issue": "1",
"journal-title": "J. Incl. Phenom. Macrocycl. Chem.",
"key": "10.1016/j.intimp.2021.108004_b0300",
"volume": "57",
"year": "2007"
},
{
"DOI": "10.1517/17425247.2.1.335",
"article-title": "Cyclodextrins in drug delivery",
"author": "Loftsson",
"doi-asserted-by": "crossref",
"first-page": "335",
"issue": "2",
"journal-title": "Expert Opinion on Drug Delivery.",
"key": "10.1016/j.intimp.2021.108004_b0305",
"volume": "2",
"year": "2005"
},
{
"DOI": "10.3390/molecules23051161",
"article-title": "Solubility of Cyclodextrins and Drug/Cyclodextrin Complexes",
"author": "Saokham",
"doi-asserted-by": "crossref",
"issue": "5",
"journal-title": "Molecules",
"key": "10.1016/j.intimp.2021.108004_b0310",
"volume": "23",
"year": "2018"
},
{
"DOI": "10.1016/j.addr.2007.05.012",
"article-title": "Cyclodextrins as pharmaceutical solubilizers",
"author": "Brewster",
"doi-asserted-by": "crossref",
"first-page": "645",
"issue": "7",
"journal-title": "Adv. Drug Deliv. Rev.",
"key": "10.1016/j.intimp.2021.108004_b0315",
"volume": "59",
"year": "2007"
},
{
"DOI": "10.1021/js960075u",
"article-title": "Pharmaceutical applications of cyclodextrins. 2. In vivo drug delivery",
"author": "Rajewski",
"doi-asserted-by": "crossref",
"first-page": "1142",
"issue": "11",
"journal-title": "J. Pharm. Sci.",
"key": "10.1016/j.intimp.2021.108004_b0320",
"volume": "85",
"year": "1996"
},
{
"DOI": "10.1208/s12249-014-0257-x",
"article-title": "Effects of the preparation method on the formation of true nimodipine SBE-β-CD/HP-β-CD inclusion complexes and their dissolution rates enhancement",
"author": "Semcheddine",
"doi-asserted-by": "crossref",
"first-page": "704",
"issue": "3",
"journal-title": "AAPS PharmSciTech.",
"key": "10.1016/j.intimp.2021.108004_b0325",
"volume": "16",
"year": "2015"
},
{
"DOI": "10.1016/j.jconrel.2019.02.019",
"article-title": "Continuous alternative to freeze drying: Manufacturing of cyclodextrin-based reconstitution powder from aqueous solution using scaled-up electrospinning",
"author": "Vass",
"doi-asserted-by": "crossref",
"first-page": "120",
"journal-title": "J. Control. Release",
"key": "10.1016/j.intimp.2021.108004_b0330",
"volume": "298",
"year": "2019"
},
{
"DOI": "10.1016/j.jpba.2003.12.005",
"article-title": "Effects of alpha- and beta-cyclodextrin complexation on the physico-chemical properties and antioxidant activity of some 3-hydroxyflavones",
"author": "Calabro",
"doi-asserted-by": "crossref",
"first-page": "365",
"issue": "2",
"journal-title": "J. Pharm. Biomed. Anal. [Comparative Study].",
"key": "10.1016/j.intimp.2021.108004_b0335",
"volume": "35",
"year": "2004"
},
{
"DOI": "10.1016/j.ijpharm.2005.11.024",
"article-title": "Formulation and biological evaluation of glimepiride-cyclodextrin-polymer systems",
"author": "Ammar",
"doi-asserted-by": "crossref",
"first-page": "129",
"issue": "1–2",
"journal-title": "Int. J. Pharm.",
"key": "10.1016/j.intimp.2021.108004_b0340",
"volume": "309",
"year": "2006"
},
{
"DOI": "10.2147/IJN.S78814",
"article-title": "Hydroxypropyl-beta-cyclodextrin functionalized calcium carbonate microparticles as a potential carrier for enhancing oral delivery of water-insoluble drugs",
"author": "Zhang",
"doi-asserted-by": "crossref",
"first-page": "3291",
"journal-title": "Int. J. Nanomed.",
"key": "10.1016/j.intimp.2021.108004_b0345",
"volume": "10",
"year": "2015"
},
{
"DOI": "10.1021/cm9601188",
"article-title": "Vitrification and crystallization of organic liquids confined to nanoscale pores",
"author": "Jackson",
"doi-asserted-by": "crossref",
"first-page": "2128",
"issue": "8",
"journal-title": "Chem. Mater.",
"key": "10.1016/j.intimp.2021.108004_b0350",
"volume": "8",
"year": "1996"
},
{
"DOI": "10.1136/thoraxjnl-2015-208032",
"article-title": "Alveolar macrophage-derived microvesicles mediate acute lung injury",
"author": "Soni",
"doi-asserted-by": "crossref",
"first-page": "1020",
"issue": "11",
"journal-title": "Thorax",
"key": "10.1016/j.intimp.2021.108004_b0355",
"volume": "71",
"year": "2016"
},
{
"DOI": "10.5507/bp.2017.029",
"article-title": "Inflammatory, anti-inflammatory and regulatory cytokines in relatively healthy lung tissue as an essential part of the local immune system",
"author": "Vitenberga",
"doi-asserted-by": "crossref",
"first-page": "164",
"issue": "2",
"journal-title": "Biomed. Pap. Med. Fac. Univ. Palacky Olomouc Czech Repub.",
"key": "10.1016/j.intimp.2021.108004_b0360",
"volume": "161",
"year": "2017"
},
{
"DOI": "10.1093/toxsci/kfy309",
"article-title": "Role of Macrophages in Acute Lung Injury and Chronic Fibrosis Induced by Pulmonary Toxicants",
"author": "Laskin",
"doi-asserted-by": "crossref",
"first-page": "287",
"issue": "2",
"journal-title": "Toxicol. Sci.",
"key": "10.1016/j.intimp.2021.108004_b0365",
"volume": "168",
"year": "2019"
},
{
"DOI": "10.1155/2015/816460",
"article-title": "Understanding the Mysterious M2 Macrophage through Activation Markers and Effector Mechanisms",
"author": "Roszer",
"doi-asserted-by": "crossref",
"journal-title": "Mediators Inflamm.",
"key": "10.1016/j.intimp.2021.108004_b0370",
"volume": "2015",
"year": "2015"
},
{
"DOI": "10.1084/jem.20020903",
"article-title": "Regulation and function of the interleukin 13 receptor alpha 2 during a T helper cell type 2-dominant immune response",
"author": "Chiaramonte",
"doi-asserted-by": "crossref",
"first-page": "687",
"issue": "6",
"journal-title": "J. Exp. Med.",
"key": "10.1016/j.intimp.2021.108004_b0375",
"volume": "197",
"year": "2003"
},
{
"DOI": "10.4049/jimmunol.171.5.2684",
"article-title": "Therapeutic attenuation of pulmonary fibrosis via targeting of IL-4- and IL-13-responsive cells",
"author": "Jakubzick",
"doi-asserted-by": "crossref",
"first-page": "2684",
"issue": "5",
"journal-title": "J. Immunol.",
"key": "10.1016/j.intimp.2021.108004_b0380",
"volume": "171",
"year": "2003"
},
{
"article-title": "Circulating Procollagen type III N-terminal peptide (P3NP) and Physical Function in Adults from The Long Life Family Study",
"author": "Santanasto",
"journal-title": "J. Gerontol. A Biol. Sci. Med. Sci.",
"key": "10.1016/j.intimp.2021.108004_b0385",
"volume": "14",
"year": "2020"
},
{
"DOI": "10.1164/ajrccm.162.5.2001061",
"article-title": "Fibroproliferation occurs early in the acute respiratory distress syndrome and impacts on outcome",
"author": "Marshall",
"doi-asserted-by": "crossref",
"first-page": "1783",
"issue": "5",
"journal-title": "Am. J. Respir. Crit. Care Med.",
"key": "10.1016/j.intimp.2021.108004_b0390",
"volume": "162",
"year": "2000"
},
{
"DOI": "10.1097/MD.0000000000006617",
"article-title": "Association of serum levels of laminin, type IV collagen, procollagen III N-terminal peptide, and hyaluronic acid with the progression of interstitial lung disease",
"author": "Su",
"doi-asserted-by": "crossref",
"issue": "18",
"journal-title": "Medicine (Baltimore).",
"key": "10.1016/j.intimp.2021.108004_b0395",
"volume": "96",
"year": "2017"
},
{
"DOI": "10.1136/thx.44.2.126",
"article-title": "Hyaluronan and type III procollagen peptide concentrations in bronchoalveolar lavage fluid in idiopathic pulmonary fibrosis",
"author": "Bjermer",
"doi-asserted-by": "crossref",
"first-page": "126",
"issue": "2",
"journal-title": "Thorax",
"key": "10.1016/j.intimp.2021.108004_b0400",
"volume": "44",
"year": "1989"
},
{
"DOI": "10.1164/ajrccm.163.2.2004168",
"article-title": "Deficiencies in lung surfactant proteins A and D are associated with lung infection in very premature neonatal baboons",
"author": "Awasthi",
"doi-asserted-by": "crossref",
"first-page": "389",
"issue": "2",
"journal-title": "Am. J. Respir. Crit. Care Med.",
"key": "10.1016/j.intimp.2021.108004_b0405",
"volume": "163",
"year": "2001"
},
{
"DOI": "10.1034/j.1399-0004.2000.580305.x",
"article-title": "Polymorphisms of human SP-A, SP-B, and SP-D genes: association of SP-B Thr131Ile with ARDS",
"author": "Lin",
"doi-asserted-by": "crossref",
"first-page": "181",
"issue": "3",
"journal-title": "Clin. Genet.",
"key": "10.1016/j.intimp.2021.108004_b0410",
"volume": "58",
"year": "2000"
},
{
"DOI": "10.1165/ajrcmb.25.6.f221",
"article-title": "Surfactant protein A (SP-A)-mediated bacterial clearance: SP-A and cystic fibrosis",
"author": "Korfhagen",
"doi-asserted-by": "crossref",
"first-page": "668",
"issue": "6",
"journal-title": "Am. J. Respir. Cell Mol. Biol.",
"key": "10.1016/j.intimp.2021.108004_b0415",
"volume": "25",
"year": "2001"
},
{
"DOI": "10.1164/ajrccm/147.3.723",
"article-title": "Elevated levels of lung surfactant protein A in sera from patients with idiopathic pulmonary fibrosis and pulmonary alveolar proteinosis",
"author": "Kuroki",
"doi-asserted-by": "crossref",
"first-page": "723",
"issue": "3",
"journal-title": "Am. Rev. Respir. Dis.",
"key": "10.1016/j.intimp.2021.108004_b0420",
"volume": "147",
"year": "1993"
},
{
"DOI": "10.1152/ajpheart.00400.2008",
"article-title": "Leukocyte-endothelial cell interactions are linked to vascular permeability via ICAM-1-mediated signaling",
"author": "Sumagin",
"doi-asserted-by": "crossref",
"first-page": "H969",
"issue": "3",
"journal-title": "Am. J. Physiol. Heart Circ. Physiol.",
"key": "10.1016/j.intimp.2021.108004_b0425",
"volume": "295",
"year": "2008"
},
{
"DOI": "10.1097/BCR.0b013e3181e4c58c",
"article-title": "Decreased pulmonary inflammation after ethanol exposure and burn injury in intercellular adhesion molecule-1 knockout mice",
"author": "Bird",
"doi-asserted-by": "crossref",
"first-page": "652",
"issue": "4",
"journal-title": "J. Burn Care Res.",
"key": "10.1016/j.intimp.2021.108004_b0430",
"volume": "31",
"year": "2010"
},
{
"DOI": "10.1183/09031936.93.05070815",
"article-title": "Intercellular adhesion molecule-1 (ICAM-1) and endothelial leucocyte adhesion molecule-1 (ELAM-1) expression in the bronchial mucosa of normal and asthmatic subjects",
"author": "Montefort",
"doi-asserted-by": "crossref",
"first-page": "815",
"issue": "7",
"journal-title": "Eur. Respir. J.",
"key": "10.1016/j.intimp.2021.108004_b0435",
"volume": "5",
"year": "1992"
},
{
"DOI": "10.1016/S0167-5273(02)00138-9",
"article-title": "C/T polymorphism of the intercellular adhesion molecule-1 gene (exon 6, codon 469). A risk factor for coronary heart disease and myocardial infarction",
"author": "Jiang",
"doi-asserted-by": "crossref",
"first-page": "171",
"issue": "2–3",
"journal-title": "Int. J. Cardiol.",
"key": "10.1016/j.intimp.2021.108004_b0440",
"volume": "84",
"year": "2002"
},
{
"DOI": "10.1093/jnci/94.10.733",
"article-title": "Effects of intercellular adhesion molecule 1 (ICAM-1) null mutation on radiation-induced pulmonary fibrosis and respiratory insufficiency in mice",
"author": "Hallahan",
"doi-asserted-by": "crossref",
"first-page": "733",
"issue": "10",
"journal-title": "J. Natl Cancer Inst.",
"key": "10.1016/j.intimp.2021.108004_b0445",
"volume": "94",
"year": "2002"
},
{
"DOI": "10.1016/j.cca.2010.07.006",
"article-title": "MCP-1: chemoattractant with a role beyond immunity: a review",
"author": "Yadav",
"doi-asserted-by": "crossref",
"first-page": "1570",
"issue": "21–22",
"journal-title": "Clin. Chim. Acta",
"key": "10.1016/j.intimp.2021.108004_b0450",
"volume": "411",
"year": "2010"
},
{
"DOI": "10.1016/j.cyto.2012.06.018",
"article-title": "Monocyte Chemoattractant Protein 1 (MCP-1) in obesity and diabetes",
"author": "Panee",
"doi-asserted-by": "crossref",
"first-page": "1",
"issue": "1",
"journal-title": "Cytokine",
"key": "10.1016/j.intimp.2021.108004_b0455",
"volume": "60",
"year": "2012"
},
{
"DOI": "10.1016/j.humimm.2015.10.005",
"article-title": "TLR stimulation of human neutrophils lead to increased release of MCP-1, MIP-1alpha, IL-1beta, IL-8 and TNF during tuberculosis",
"author": "Hilda",
"doi-asserted-by": "crossref",
"first-page": "63",
"issue": "1",
"journal-title": "Hum. Immunol.",
"key": "10.1016/j.intimp.2021.108004_b0460",
"volume": "77",
"year": "2016"
},
{
"DOI": "10.1002/ejp.1169",
"article-title": "Blocking of cytokines signalling attenuates evoked and spontaneous neuropathic pain behaviours in the paclitaxel rat model of chemotherapy-induced neuropathy",
"author": "Al-Mazidi",
"doi-asserted-by": "crossref",
"first-page": "810",
"issue": "4",
"journal-title": "Eur. J. Pain",
"key": "10.1016/j.intimp.2021.108004_b0465",
"volume": "22",
"year": "2018"
},
{
"DOI": "10.1007/s13555-017-0176-3",
"article-title": "Topical Treatment of Rosacea with Ivermectin Inhibits Gene Expression of Cathelicidin Innate Immune Mediators, LL-37 and KLK5, in Reconstructed and Ex Vivo Skin Models",
"author": "Thibaut de Menonville",
"doi-asserted-by": "crossref",
"first-page": "213",
"issue": "2",
"journal-title": "Dermatol Ther (Heidelb)",
"key": "10.1016/j.intimp.2021.108004_b0470",
"volume": "7",
"year": "2017"
},
{
"DOI": "10.1016/j.micinf.2011.12.002",
"article-title": "Chemokines and cytokines in patients with an occult Onchocerca volvulus infection",
"author": "Lechner",
"doi-asserted-by": "crossref",
"first-page": "438",
"issue": "5",
"journal-title": "Microbes Infect.",
"key": "10.1016/j.intimp.2021.108004_b0475",
"volume": "14",
"year": "2012"
}
],
"reference-count": 95,
"references-count": 95,
"relation": {},
"resource": {
"primary": {
"URL": "https://linkinghub.elsevier.com/retrieve/pii/S1567576921006408"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Safety of inhaled ivermectin as a repurposed direct drug for treatment of COVID-19: A preclinical tolerance study",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1016/elsevier_cm_policy",
"volume": "99"
}