Preparation and Characterization of Inhalable Ivermectin Powders as a Potential COVID-19 Therapy
MS Ahmed H Albariqi, PhD Wei-Ren Ke, PhD Dipesh Khanal, MD Stefanie Kalfas, PhD Patricia Tang, PhD Warwick J Britton, PhD John Drago, PhD, DSc Hak-Kim Chan
Journal of Aerosol Medicine and Pulmonary Drug Delivery, doi:10.1089/jamp.2021.0059
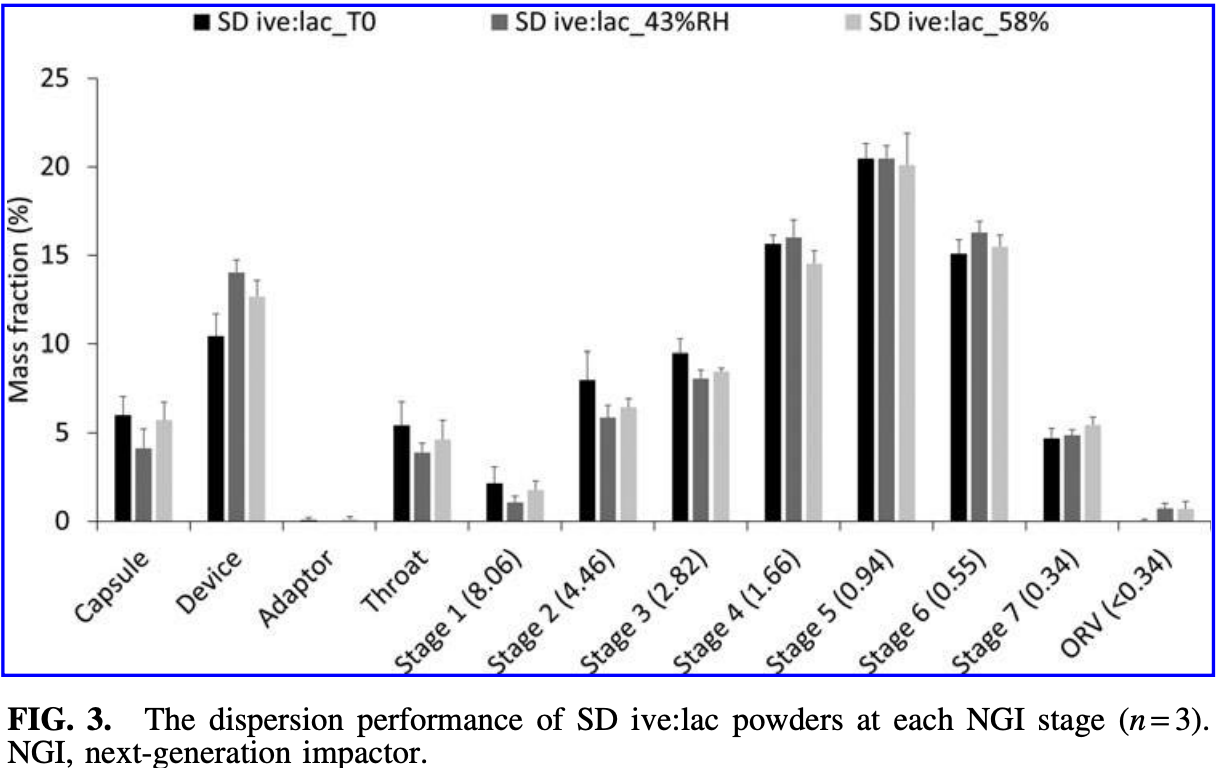
Background: Ivermectin has received worldwide attention as a potential COVID-19 treatment after showing antiviral activity against SARS-CoV-2 in vitro. However, the pharmacokinetic limitations associated with oral administration have been postulated as limiting factors to its bioavailability and efficacy. These limitations can be overcome by targeted delivery to the lungs. In this study, inhalable dry powders of ivermectin and lactose crystals were prepared and characterized for the potential treatment of COVID-19. Methods: Ivermectin was co-spray dried with lactose monohydrate crystals and conditioned by storage at two different relative humidity points (43% and 58% RH) for a week. The in vitro dispersion performance of the stored powders was examined using a medium-high resistance Osmohaler connecting to a next-generation impactor at 60 L/min flow rate. The solid-state characteristics including particle size distribution and morphology, crystallinity, and moisture sorption profiles of raw and spray-dried ivermectin samples were assessed by laser diffraction, scanning electron microscopy, Raman spectroscopy, X-ray powder diffraction, thermogravimetric analysis, differential scanning calorimetry, and dynamic vapor sorption. Results: All the freshly spray-dried formulation (T0) and the conditioned samples could achieve the anticipated therapeutic dose with fine particle dose of 300 lg, FPF recovered of 70%, and FPF emitted of 83%. In addition, the formulations showed a similar volume median diameter of 4.3 lm and span of 1.9. The spray-dried formulations were stable even after conditioning and exposing to different RH points as ivermectin remained amorphous with predominantly crystalline lactose. Conclusion: An inhalable and stable dry powder of ivermectin and lactose crystals was successfully formulated. This powder inhaler ivermectin candidate therapy appears to be able to deliver doses that could be safe and effective to treat the SARS-COV-2 infection. Further development of this therapy is warranted.
Funding Information Jack & Robert Smorgon Families Foundation provided financial support as a gift to the University of Sydney for this study.
References
Albariqi, Chang, Tai, Ke, Chow et al., Inhalable hydroxychloroquine powders for potential treatment of COVID-19, J Aerosol Med Pulm Drug Deliv
Bhugra, Role of thermodynamic, molecular, and kinetic factors in crystallization from the amorphous state, J Pharm Sci
Broadhead, Rouan, Rhodes, The spray drying of pharmaceuticals, Drug Dev Indus Pharm
Caly, Druce, Catton, Jans, Wagstaff, The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro, Antiviral Res
Chaccour, Abizanda, Irigoyen-Barrio, Casellas, Aldaz et al., Nebulized ivermectin for COVID-19 and other respiratory diseases, a proof of concept, dose-ranging study in rats, Sci Rep
Chen, Huang, Shift of aerosol penetration in respirable cyclone samplers, Am Ind Hyg Assoc J
Cornberg, Buti, Eberhardt, Grossi, EASL position paper on the use of COVID-19 vaccines in patients with chronic liver diseases, hepatobiliary cancer and liver transplant recipients, J Hepatol
Dixit, Yadav, Singh, Ivermectin: Potential role as repurposed drug for COVID-19, Malays J Med Sci
Errecalde, Lifschitz, Vecchioli, Ceballos, Errecalde et al., Safety and pharmacokinetic assessments of a novel ivermectin nasal spray formulation in a Pig Model, J Pharm Sci
Frohlich, Mercuri, Wu, Measurements of deposition, lung surface area and lung fluid for simulation of inhaled compounds, Front Pharmacol
Geller, Comparing clinical features of the nebulizer, metered-dose inhaler, and dry powder inhaler, Respir Care
Gombas, Szabo, ´ve ´sz, Regdon, Quantitative determination of crystallinity of a-lactose monohydrate by DSC, J Therm Anal Calorim
Gonzalez Canga, Prieto, Diez Liebana, Martinez, Vega et al., The pharmacokinetics and metabolism of ivermectin in domestic animal species, Vet J
Heidary, Gharebaghi, Ivermectin: A systematic review from antiviral effects to COVID-19 complementary regimen, J Antibiot
Ji, Cen, Lin, Hu, Fang et al., Study on the subacute inhalation toxicity of ivermectin 567 in TC rats, Chin J Comp Med
Kachrimanis, Fucke, Noisternig, Siebenhaar, Griesser, Effects of moisture and residual solvent on the phase stability of orthorhombic paracetamol, Pharm Res
Karim, De Oliveira, New SARS-CoV-2 variants-Clinical, public health, and vaccine implications, N Engl J Med
Ke, Chang, Kwok, Chen, Hk, Spray drying lactose from organic solvent suspensions for aerosol delivery to the lungs, Int J Pharm
Ke, Kwok, Khanal, Chang, Hk, Co-spray dried hydrophobic drug formulations with crystalline lactose for inhalation aerosol delivery, Int J Pharm
Keehner, Horton, Pfeffer, Longhurst, Schooley et al., SARS-CoV-2 infection after vaccination in Health Care Workers in California, N Engl J Med
Kow, Merchant, Mustafa, Hasan, The association between the use of ivermectin and mortality in patients with COVID-19: A meta-analysis, Pharmacol Rep
Kumar, Ellen, Bouma, Troost, Van De Pol et al., Moxidectin and ivermectin inhibit Sars-Cov-2 replication in Vero E6 cells but not in human primary airway epithelium cells, Antimicrob Agents Chemother
Lespine, Alvinerie, Sutra, Pors, Chartier, Influence of the route of administration on efficacy and tissue distribution of ivermectin in goat, Vet Parasitol
Lifschitz, Virkel, Sallovitz, Sutra, Galtier et al., Comparative distribution of ivermectin and doramectin to parasite location tissues in cattle, Vet Parasitol
Lin, Chen, Huang, Kuo, Gui, Effect of aerosol loading on separation performance of PM2. 5 cyclone separators, Aerosol Air Qual Res
Lu, Xiong, Sun, Yu, Hu et al., Sustained release ivermectin-loaded solid lipid dispersion for subcutaneous delivery: In vitro and in vivo evaluation, Drug Deliv
Mansour, Shamma, Ahmed, Sabry, Esmat et al., Safety of inhaled ivermectin as a repurposed direct drug for treatment of COVID-19: A preclinical tolerance study, Int Immunopharmacol
Mitchell, Berlinski, Canisius, Cipolla, Dolovich et al., Urgent Appeal from International Society for Aerosols in Medicine (ISAM) during COVID-19: Clinical decision makers and governmental agencies should consider the inhaled route of administration: A statement from the ISAM Regulatory and Standardization Issues Networking Group, J Aerosol Med Pulm Drug Deliv
Munoz-Fontela, Dowling, Funnell, Gsell, Riveros-Balta et al., Animal models for COVID-19, Nature
Newman, Hk, In vitro/in vivo comparisons in pulmonary drug delivery, J Aerosol Med Pulm Drug Deliv
Pilcer, Amighi, Formulation strategy and use of excipients in pulmonary drug delivery, Int J Pharm
Rahimpour, Kouhsoltani, Hamishehkar, Alternative carriers in dry powder inhaler formulations, Drug Discov Today
Rolim, Santos, Chaves, Gonc ¸alves, Freitas-Neto et al., Preformulation study of ivermectin raw material, J Therm Anal Calorim
Sallam, COVID-19 Vaccine Hesitancy Worldwide: A concise systematic review of vaccine acceptance rates, Vaccines
Sibum, Hagedoorn, De Boer, Frijlink, Grasmeijer, Challenges for pulmonary delivery of high powder doses, Int J Pharm
Singh, Van Den Mooter, Spray drying formulation of amorphous solid dispersions, Adv Drug Deliv Rev
Strohl, Thomas, Jean, Schlenker, Koletsky et al., Ventilation and metabolism among rat strains, J Appl Physiol
Tepper, Kuehl, Cracknell, Nikula, Pei et al., Symposium summary: ''Breathe In, Breathe Out, Its Easy: What You Need to Know About Developing Inhaled Drugs', Int J Toxicol
Vankeirsbilck, Vercauteren, Baeyens, Van Der Weken, Verpoort et al., Applications of Raman spectroscopy in pharmaceutical analysis, TrAC Trends Anal Chem
Walters, Lung lining liquid-the hidden depths. The 5th Nils W. Svenningsen memorial lecture, Biol Neonate
Wouters, Shadlen, Salcher-Konrad, Pollard, Larson et al., Challenges in ensuring global access to COVID-19 vaccines: Production, affordability, allocation, and deployment, Lancet
Yamada, Poverty, wealth, and access to pandemic influenza vaccines, N Engl J Med
DOI record:
{
"DOI": "10.1089/jamp.2021.0059",
"ISSN": [
"1941-2711",
"1941-2703"
],
"URL": "http://dx.doi.org/10.1089/jamp.2021.0059",
"alternative-id": [
"10.1089/jamp.2021.0059"
],
"author": [
{
"ORCID": "http://orcid.org/0000-0002-1937-6775",
"affiliation": [
{
"name": "Advanced Drug Delivery Group, Sydney Pharmacy School, Faculty of Medicine and Health, The University of Sydney, Sydney, Australia."
},
{
"name": "The Department of Pharmaceutics, Faculty of Pharmacy, Jazan University, Jazan, Saudi Arabia."
}
],
"authenticated-orcid": false,
"family": "Albariqi",
"given": "Ahmed H.",
"sequence": "first"
},
{
"ORCID": "http://orcid.org/0000-0002-1303-6497",
"affiliation": [
{
"name": "Advanced Drug Delivery Group, Sydney Pharmacy School, Faculty of Medicine and Health, The University of Sydney, Sydney, Australia."
},
{
"name": "School of Pharmacy, Collage of Medicine, National Taiwan University, Taipei, Taiwan."
}
],
"authenticated-orcid": false,
"family": "Ke",
"given": "Wei-Ren",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Advanced Drug Delivery Group, Sydney Pharmacy School, Faculty of Medicine and Health, The University of Sydney, Sydney, Australia."
}
],
"family": "Khanal",
"given": "Dipesh",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Florey Institute of Neuroscience and Mental Health, Melbourne, Australia."
}
],
"family": "Kalfas",
"given": "Stefanie",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Advanced Drug Delivery Group, Sydney Pharmacy School, Faculty of Medicine and Health, The University of Sydney, Sydney, Australia."
}
],
"family": "Tang",
"given": "Patricia",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Centenary Institute, The University of Sydney, Sydney, Australia."
},
{
"name": "Department of Clinical Immunology, Royal Prince Alfred Hospital, Camperdown, Australia."
}
],
"family": "Britton",
"given": "Warwick J",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Florey Institute of Neuroscience and Mental Health, Melbourne, Australia."
},
{
"name": "Department of Medicine, St Vincent's Hospital, University of Melbourne, Melbourne, Australia."
}
],
"family": "Drago",
"given": "John",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-7054-3137",
"affiliation": [
{
"name": "Advanced Drug Delivery Group, Sydney Pharmacy School, Faculty of Medicine and Health, The University of Sydney, Sydney, Australia."
}
],
"authenticated-orcid": false,
"family": "Chan",
"given": "Hak-Kim",
"sequence": "additional"
}
],
"container-title": [
"Journal of Aerosol Medicine and Pulmonary Drug Delivery"
],
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2022,
3,
11
]
],
"date-time": "2022-03-11T19:04:26Z",
"timestamp": 1647025466000
},
"deposited": {
"date-parts": [
[
2022,
3,
11
]
],
"date-time": "2022-03-11T19:04:30Z",
"timestamp": 1647025470000
},
"indexed": {
"date-parts": [
[
2022,
4,
4
]
],
"date-time": "2022-04-04T05:43:55Z",
"timestamp": 1649051035432
},
"is-referenced-by-count": 0,
"issn-type": [
{
"type": "print",
"value": "1941-2711"
},
{
"type": "electronic",
"value": "1941-2703"
}
],
"issued": {
"date-parts": [
[
2022,
3,
11
]
]
},
"language": "en",
"license": [
{
"URL": "https://www.liebertpub.com/nv/resources-tools/text-and-data-mining-policy/121/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
3,
11
]
],
"date-time": "2022-03-11T00:00:00Z",
"timestamp": 1646956800000
}
}
],
"link": [
{
"URL": "https://www.liebertpub.com/doi/full-xml/10.1089/jamp.2021.0059",
"content-type": "application/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://www.liebertpub.com/doi/pdf/10.1089/jamp.2021.0059",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "278",
"original-title": [],
"prefix": "10.1089",
"published": {
"date-parts": [
[
2022,
3,
11
]
]
},
"published-online": {
"date-parts": [
[
2022,
3,
11
]
]
},
"publisher": "Mary Ann Liebert Inc",
"reference": [
{
"DOI": "10.1056/NEJMc2100362",
"doi-asserted-by": "publisher",
"key": "B2"
},
{
"DOI": "10.1056/NEJMp0906972",
"doi-asserted-by": "publisher",
"key": "B3"
},
{
"DOI": "10.1016/S0140-6736(21)00306-8",
"doi-asserted-by": "publisher",
"key": "B4"
},
{
"DOI": "10.3390/vaccines9020160",
"doi-asserted-by": "publisher",
"key": "B5"
},
{
"DOI": "10.1056/NEJMc2101927",
"doi-asserted-by": "publisher",
"key": "B6"
},
{
"DOI": "10.1016/j.jhep.2021.01.032",
"doi-asserted-by": "publisher",
"key": "B7"
},
{
"DOI": "10.1016/j.antiviral.2020.104787",
"doi-asserted-by": "publisher",
"key": "B8"
},
{
"DOI": "10.1128/AAC.01543-21",
"doi-asserted-by": "publisher",
"key": "B9"
},
{
"author": "Dixit A",
"first-page": "154",
"journal-title": "Malays J Med Sci",
"key": "B11",
"volume": "27",
"year": "2020"
},
{
"DOI": "10.1038/s41429-020-0336-z",
"doi-asserted-by": "publisher",
"key": "B12"
},
{
"DOI": "10.1007/s43440-021-00245-z",
"doi-asserted-by": "publisher",
"key": "B13"
},
{
"DOI": "10.1038/s41598-020-74084-y",
"doi-asserted-by": "publisher",
"key": "B14"
},
{
"DOI": "10.1016/j.tvjl.2007.07.011",
"doi-asserted-by": "publisher",
"key": "B15"
},
{
"DOI": "10.1016/j.intimp.2021.108004",
"doi-asserted-by": "publisher",
"key": "B16"
},
{
"author": "Ji L",
"first-page": "70",
"journal-title": "Chin J Comp Med",
"key": "B17",
"volume": "26",
"year": "2016"
},
{
"DOI": "10.1152/jappl.1997.82.1.317",
"doi-asserted-by": "publisher",
"key": "B18"
},
{
"DOI": "10.1177/1091581815624080",
"doi-asserted-by": "publisher",
"key": "B19"
},
{
"DOI": "10.1159/000056764",
"doi-asserted-by": "publisher",
"key": "B20"
},
{
"DOI": "10.3389/fphar.2016.00181",
"doi-asserted-by": "publisher",
"key": "B21"
},
{
"DOI": "10.1016/j.ijpharm.2018.07.008",
"doi-asserted-by": "publisher",
"key": "B22"
},
{
"author": "Geller DE",
"first-page": "1313",
"journal-title": "Respir Care",
"key": "B23",
"volume": "50",
"year": "2005"
},
{
"DOI": "10.1016/j.ijpharm.2010.03.017",
"doi-asserted-by": "publisher",
"key": "B24"
},
{
"DOI": "10.1016/j.drudis.2013.11.013",
"doi-asserted-by": "publisher",
"key": "B25"
},
{
"DOI": "10.3109/03639049209046327",
"doi-asserted-by": "publisher",
"key": "B26"
},
{
"DOI": "10.1089/jamp.2020.1622",
"doi-asserted-by": "publisher",
"key": "B27"
},
{
"DOI": "10.1089/jamp.2020.1648",
"doi-asserted-by": "publisher",
"key": "B28"
},
{
"DOI": "10.1023/A:1016039819247",
"doi-asserted-by": "publisher",
"key": "B33"
},
{
"DOI": "10.1007/s10973-014-3691-9",
"doi-asserted-by": "publisher",
"key": "B34"
},
{
"DOI": "10.1080/10717544.2017.1284945",
"doi-asserted-by": "publisher",
"key": "B35"
},
{
"DOI": "10.1007/s11095-007-9529-4",
"doi-asserted-by": "publisher",
"key": "B36"
},
{
"DOI": "10.1016/j.addr.2015.12.010",
"doi-asserted-by": "publisher",
"key": "B37"
},
{
"DOI": "10.1016/j.ijpharm.2020.119984",
"doi-asserted-by": "publisher",
"key": "B38"
},
{
"DOI": "10.1089/jamp.2007.0643",
"doi-asserted-by": "publisher",
"key": "B39"
},
{
"DOI": "10.1080/00028899908984494",
"doi-asserted-by": "publisher",
"key": "B40"
},
{
"DOI": "10.4209/aaqr.2017.11.0458",
"doi-asserted-by": "publisher",
"key": "B41"
},
{
"DOI": "10.1016/S0165-9936(02)01208-6",
"doi-asserted-by": "publisher",
"key": "B42"
},
{
"DOI": "10.1002/jps.21138",
"doi-asserted-by": "publisher",
"key": "B43"
},
{
"DOI": "10.1016/j.ijpharm.2021.120608",
"doi-asserted-by": "publisher",
"key": "B44"
},
{
"DOI": "10.1016/j.xphs.2021.01.017",
"doi-asserted-by": "publisher",
"key": "B45"
},
{
"DOI": "10.1016/S0304-4017(99)00175-2",
"doi-asserted-by": "publisher",
"key": "B46"
},
{
"DOI": "10.1016/j.vetpar.2004.11.028",
"doi-asserted-by": "publisher",
"key": "B47"
},
{
"DOI": "10.1038/s41586-020-2787-6",
"doi-asserted-by": "publisher",
"key": "B48"
}
],
"reference-count": 42,
"references-count": 42,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.liebertpub.com/doi/10.1089/jamp.2021.0059"
}
},
"score": 1,
"short-container-title": [
"Journal of Aerosol Medicine and Pulmonary Drug Delivery"
],
"short-title": [],
"source": "Crossref",
"subject": [
"Pharmacology (medical)",
"Pharmaceutical Science",
"Pulmonary and Respiratory Medicine"
],
"subtitle": [],
"title": [
"Preparation and Characterization of Inhalable Ivermectin Powders as a Potential COVID-19 Therapy"
],
"type": "journal-article"
}