A remodeled ivermectin polycaprolactone-based nanoparticles for inhalation as a promising treatment of pulmonary inflammatory diseases
Nagia Sabaa Wafiq Mohammed, Nagia Ahmed El-Megrab, Azza A Hasan, Eman Gomaa
European Journal of Pharmaceutical Sciences, doi:10.1016/j.ejps.2024.106714
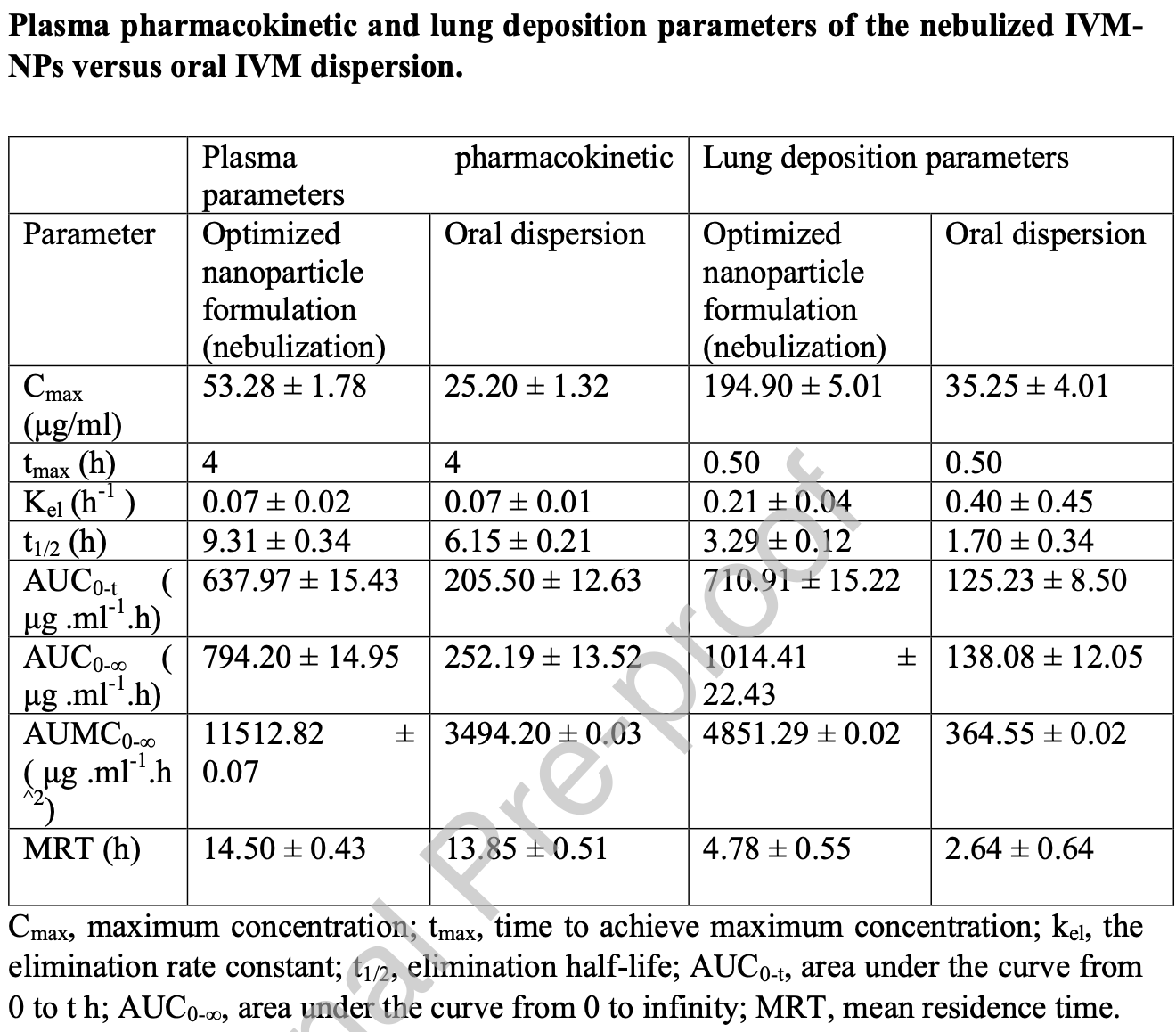
In recent years, ivermectin (IVM), an antiparasitic drug of low water solubility and poor oral bioavailability, has shown a profound effect on inflammatory mediators involved in diseases, such as acute lung injury, lung fibrosis, and COVID-19. In order to maximize drug bioavailability, polymeric nanoparticles can be delivered through nebulizers for pulmonary administration. The aim of this study was to prepare IVMloaded polycaprolactone (PCL) nanoparticles (NPs) by solvent evaporation method. Box-Benkhen design (BBD) was used to optimize entrapment efficiency (Y 1 ), percent drug release after 6 h (Y 2 ), particle size (Y 3 ), and zeta potential (Y 4 ). A study was conducted examining the effects of three independent variables: PCL-IVM ratio (A), polyvinyl alcohol (PVA) concentration (B), and sonication time (C). The optimized formula was also compared to the oral IVM dispersion for lung deposition, in-vivo behavior, and pharmacokinetic parameters. The optimized IVM-PCL-NPs formulation was spherical in shape with entrapment efficiency (% EE) of 93.99 ± 0.96 %, about 62.71 ± 0.53 % released after 6 hours, particle size of 100.07 ± 0.73 nm and zeta potential of -3.30 ± 0.23 mV. Comparing the optimized formulation to IVM-dispersion, the optimized formulation demonstrated greater bioavailability with greater area under the curve AUC 0-t of 710.91 ± 15.22 μg .ml -1 .h for lung and 637.97 ± 15.43 μg .ml -1 .h for plasma. Based on the results, the optimized NPs accumulated better in lung tissues, exhibiting a twofold longer residence time (MRT 4.78 ± 0.55 h) than the IVM-dispersion (MRT 2.64 ± 0.64 h). The optimized nanoparticle formulation also achieved higher c max (194.90 ± 5.01 μg/ml), and lower k el (0.21 ± 0.04 h -1 ) in lungs. Additionally, the level of inflammatory mediators was markedly reduced. To conclude, inhalable IVM-PCL-NPs formulation was suitable for the pulmonary delivery and may be one of the most promising approaches to increase IVM bioavailability for the successful treatment of a variety of lung diseases.
Conflict of interest The authors report no conflicts of interest.
Author contribution
Sabaa
References
Abbas, Labbez, Nordholm, Ahlberg, Size-Dependent Surface Charging of Nanoparticles, The Journal of Physical Chemistry C,
doi:10.1021/jp709667uAhmed, Ibrahim, Samy, Kaseem, Nutan et al., Biodegradable injectable in situ implants and microparticles for sustained release of montelukast: in vitro release, pharmacokinetics, and stability, AAPS PharmSciTech,
doi:10.1208/s12249-014-0101-3Al-Mazidi, Alotaibi, Nedjadi, Chaudhary, Alzoghaibi et al., Blocking of cytokines signalling attenuates evoked and spontaneous neuropathic pain behaviours in the paclitaxel rat model of chemotherapy-induced neuropathy, European Journal of Pain,
doi:10.1002/ejp.1169Allam, Hamdallah, Abdallah, Chitosan-coated diacerein nanosuspensions as a platform for enhancing bioavailability and lowering side effects: preparation, characterization, and ex vivo/in vivo evaluation, International journal of nanomedicine,
doi:10.2147/IJN.S139706Alshehri, Imam, Rizwanullah, Fakhri, Rizvi et al., Effect of chitosan coating on PLGA nanoparticles for oral delivery of thymoquinone: In vitro, ex vivo, and cancer cell line assessments, Coatings,
doi:10.3390/coatings11010006Ayoub, Elantouny, El-Nahas, Ghazy, Injectable PLGA Adefovir microspheres; the way for long term therapy of chronic hepatitis-B, European journal of pharmaceutical sciences,
doi:10.1016/j.ejps.2018.03.016Ayoub, Jasti, Elantouny, Elnahas, Ghazy, Comparative study of PLGA in-situ implant and nanoparticle formulations of entecavir; in-vitro and in-vivo evaluation, Journal of Drug Delivery Science and Technology,
doi:10.1016/j.jddst.2020.101585Badran, Alanazi, Ibrahim, Alshora, Taha et al., Optimization of Bromocriptine-Mesylate-Loaded Polycaprolactone Nanoparticles Coated with Chitosan for Nose-to-Brain Delivery: In Vitro and In Vivo Studies, Polymers,
doi:10.3390/polym15193890Behera, Barik, Joshi, Shah, Formulation and evaluation of Rifampicin loaded poly-ε-caprolactone nano-particles using 3 2 factorial design, International Journal of Pharmaceutial Sciences And Research
Bhattacharya, Genotoxicity and in vitro investigation of Gefitinib-loaded polycaprolactone fabricated nanoparticles for anticancer activity against NCI-H460 cell lines, Journal of Experimental Nanoscience,
doi:10.1080/17458080.2022.2060501Bilati, Allémann, Doelker, Sonication Parameters for the Preparation of Biodegradable Nanocapsulesof Controlled Size by the Double Emulsion Method, Pharmaceutical Development and Technology,
doi:10.1081/PDT-120017517Blum, Saltzman, High loading efficiency and tunable release of plasmid DNA encapsulated in submicron particles fabricated from PLGA conjugated with poly-L-lysine, Journal of controlled release,
doi:10.1016/j.jconrel.2008.04.002Chaccour, Abizanda, Irigoyen-Barrio, Casellas, Aldaz et al., Nebulized ivermectin for COVID-19 and other respiratory diseases, a proof of concept, doseranging study in rats, Scientific reports,
doi:10.1038/s41598-020-74084-yCui, Cun, Tao, Yang, Shi et al., Preparation and characterization of melittin-loaded poly (DL-lactic acid) or poly (DL-lactic-co-glycolic acid) microspheres made by the double emulsion method, Journal of Controlled Release,
doi:10.1016/j.jconrel.2005.07.001De Castro Jr, Gregianin, Burger, Continuous high-dose ivermectin appears to be safe in patients with acute myelogenous leukemia and could inform clinical repurposing for COVID-19 infection, Leukemia & Lymphoma,
doi:10.1080/10428194.2020.1786559Dua, Rapalli, Shukla, Singhvi, Shastri et al., Multi-drug resistant Mycobacterium tuberculosis & oxidative stress complexity: Emerging need for novel drug delivery approaches, Biomedicine & Pharmacotherapy,
doi:10.1016/j.biopha.2018.08.101Elmowafy, Alhakamy, Shalaby, Alshehri, Ali et al., Hybrid polylactic acid/Eudragit L100 nanoparticles: A promising system for enhancement of bioavailability and pharmacodynamic efficacy of luteolin, Journal of Drug Delivery Science and Technology,
doi:10.1016/j.jddst.2021.102727Esmaeili, Atyabi, Dinarvand, Preparation and characterization of estradiol-loaded PLGA nanoparticles using homogenization-solvent diffusion method, DARU Journal of Pharmaceutical Sciences
Fude, Dongmei, Anjin, Mingshi, Kai et al., Preparation and characterization of melittin-loaded poly (dl-lactic acid) or poly (dl-lactic-co-glycolic acid) microspheres made by the double emulsion method, Journal of Controlled Release,
doi:10.1016/j.jconrel.2005.07.001Fulzele, Chatterjee, Shaik, Jackson, Singh, Inhalation delivery and anti-tumor activity of celecoxib in human orthotopic non-small cell lung cancer xenograft model, Pharmaceutical research,
doi:10.1007/s11095-006-9074-6Garg, Singh, Bhatia, Raza, Singh et al., Systematic development of transethosomal gel system of piroxicam: formulation optimization, in vitro evaluation, and ex vivo assessment, AAPS pharmscitech,
doi:10.1208/s12249-016-0489-zGoel, Baboota, Sahni, Ali, Exploring targeted pulmonary delivery for treatment of lung cancer, International journal of pharmaceutical investigation
Guo, Dou, Li, Zhang, Bhutto et al., Ivermectionloaded solid lipid nanoparticles: preparation, characterisation, stability and transdermal behaviour, Artificial Cells, Nanomedicine, and Biotechnology,
doi:10.1080/21691401.2017.1307207Guzzo, Furtek, Porras, Chen, Tipping et al., Safety, tolerability, and pharmacokinetics of escalating high doses of ivermectin in healthy adult subjects, The Journal of Clinical Pharmacology,
doi:10.1177/009127002237994Habib, Sayed, Elsayed, Enhanced transdermal delivery of ondansetron using nanovesicular systems: fabrication, characterization, optimization and ex-vivo permeation study-Box-Cox transformation practical example, European journal of pharmaceutical sciences,
doi:10.1016/j.ejps.2018.01.044Haggag, Matchett, Dakir, Buchanan, Osman et al., Nanoencapsulation of a novel anti-Ran-GTPase peptide for blockade of regulator of chromosome condensation 1 (RCC1) function in MDA-MB-231 breast cancer cells, International journal of pharmaceutics,
doi:10.1016/j.ijpharm.2017.02.006Hernández-Giottonini, Rodríguez-Córdova, Gutiérrez-Valenzuela, Peñuñuri-Miranda, Zavala-Rivera et al., PLGA nanoparticle preparations by emulsification and nanoprecipitation techniques: Effects of formulation parameters, Rsc Advances,
doi:10.1039/C9RA10857BHitzman, Elmquist, Wattenberg, Wiedmann, Development of a respirable, sustained release microcarrier for 5fluorouracil I: In vitro assessment of liposomes, microspheres, and lipid coated nanoparticles, Journal of pharmaceutical sciences,
doi:10.1002/jps.20591Ibraheem, Iqbal, Agusti, Fessi, Elaissari, Effects of process parameters on the colloidal properties of polycaprolactone microparticles prepared by double emulsion like process, Colloids and Surfaces A: Physicochemical and Engineering Aspects,
doi:10.1016/j.colsurfa.2014.01.012Ibrahim, Ayoub, El-Bassossy, El-Nahas, Gomaa, Investigation of Alogliptin-Loaded In Situ Gel Implants by 23 Factorial Design with Glycemic Assessment in Rats, Pharmaceutics,
doi:10.3390/pharmaceutics14091867Ichite, Chougule, Jackson, Fulzele, Safe et al., Enhancement of docetaxel anticancer activity by a novel diindolylmethane compound in human non-small cell lung cancer, Clinical Cancer Research,
doi:10.1158/1078-0432.CCR-08-1558Jahromi, Ghazali, Ashrafi, Azadi, A comparison of models for the analysis of the kinetics of drug release from PLGA-based nanoparticles, Heliyon,
doi:10.1016/j.heliyon.2020.e03451Jaques, Kim, Measurement of total lung deposition of inhaled ultrafine particles in healthy men and women, Inhalation toxicology,
doi:10.1080/08958370050085156Ji, Cen, Lin, Hu, Fang et al., Study on the subacute inhalation toxicity of ivermectin TC in rats, Chinese Journal of Comparative Medicine
Jiang, Klein, Niederacher, Du, Marx et al., C/T polymorphism of the intercellular adhesion molecule-1 gene (exon 6, codon 469). A risk factor for coronary heart disease and myocardial infarction, International journal of cardiology,
doi:10.1016/S0167-5273(02)00138-9Keum, Noh, Baek, Lim, Hwang et al., Practical preparation procedures for docetaxelloaded nanoparticles using polylactic acid-co-glycolic acid, International journal of nanomedicine,
doi:10.2147/IJN.S24547Lalan, Tandel, Lalani, Patel, Misra, Inhalation drug therapy: Emerging trends in nasal and pulmonary drug delivery, Novel Drug Delivery Technologies: Innovative Strategies for Drug Repositioning,
doi:10.1007/978-981-13-3642-3Laskin, Malaviya, Laskin, Role of Macrophages in Acute Lung Injury and Chronic Fibrosis Induced by Pulmonary Toxicants, Toxicological Sciences,
doi:10.1093/toxsci/kfy309Lauweryns, Baert, Alveolar clearance and the role of the pulmonary lymphatics, American Review of Respiratory Disease
Li, Zhan, Anti-parasite drug ivermectin can suppress ovarian cancer by regulating lncRNA-EIF4A3-mRNA axes, EPMA Journal,
doi:10.1007/s13167-020-00209-yLu, Xiong, Sun, Yu, Hu et al., Sustained release ivermectin-loaded solid lipid dispersion for subcutaneous delivery: in vitro and in vivo evaluation, Drug delivery,
doi:10.1080/10717544.2017.1284945Ma, Xu, Wu, Li, Zhong et al., Ivermectin contributes to attenuating the severity of acute lung injury in mice, Biomedicine & Pharmacotherapy,
doi:10.1016/j.biopha.2022.113706Madelung, Østergaard, Bertelsen, Jørgensen, Jacobsen et al., Impact of sodium dodecyl sulphate on the dissolution of poorly soluble drug into biorelevant medium from drug-surfactant discs, International Journal of Pharmaceutics,
doi:10.1016/j.ijpharm.2014.02.043Mansour, Rhee, Wu, Nanomedicine in pulmonary delivery, International journal of nanomedicine,
doi:10.2147/ijn.s4937Mansour, Shamma, Ahmed, Sabry, Esmat et al., Safety of inhaled ivermectin as a repurposed direct drug for treatment of COVID-19: A preclinical tolerance study, International Immunopharmacology,
doi:10.1016/j.intimp.2021.108004Martin, Jans, Antivirals that target the host IMPα/β1-virus interface, Biochemical Society Transactions,
doi:10.1042/BST20200568Mehta, Bothiraja, Kadam, Pawar, Potential of dry powder inhalers for tuberculosis therapy: facts, fidelity and future, Artificial cells, nanomedicine, and biotechnology,
doi:10.1080/21691401.2018.1513938Nnamani, Hansen, Windbergs, Lehr, Development of artemether-loaded nanostructured lipid carrier (NLC) formulation for topical application, International Journal of Pharmaceutics,
doi:10.1016/j.ijpharm.2014.10.004Padhi, Mirza, Verma, Khuroo, Panda et al., Revisiting the nanoformulation design approach for effective delivery of topotecan in its stable form: an appraisal of its in vitro Behavior and tumor amelioration potential, Drug Delivery,
doi:10.3109/10717544.2015.1105323Patel, Raval, Manvar, Airao, Bhatt et al., Lung cancer targeting efficiency of Silibinin loaded Poly Caprolactone/Pluronic F68 Inhalable nanoparticles: In vitro and In vivo study, Plos one,
doi:10.1371/journal.pone.0267257Patlolla, Chougule, Patel, Jackson, Tata et al., Formulation, characterization and pulmonary deposition of nebulized celecoxib encapsulated nanostructured lipid carriers, Journal of Controlled Release,
doi:10.1016/j.jconrel.2010.02.006Rch, Alveolar surface forces and lung architecture, Comparative Biochemistry and Physiology--Part B: Biochemistry and Molecular Biology,
doi:10.1016/S1095-6433(01)00315-4Ruiz, Orozco, Hoyos, Giraldo, Study of sonication parameters on PLA nanoparticles preparation by simple emulsionevaporation solvent technique, European Polymer Journal,
doi:10.1016/j.eurpolymj.2022.111307Rőszer, Understanding the Mysterious M2 Macrophage through Activation Markers and Effector Mechanisms, Mediators of Inflammation,
doi:10.1155/2015/816460Sahoo, Panyam, Prabha, Labhasetwar, Residual polyvinyl alcohol associated with poly (D, L-lactide-co-glycolide) nanoparticles affects their physical properties and cellular uptake, Journal of controlled release,
doi:10.1016/S0168-3659(02)00127-XSaptarshi, Duschl, Lopata, Interaction of nanoparticles with proteins: relation to bio-reactivity of the nanoparticle, Journal of nanobiotechnology
Sarkar, Bhattacharya, Application of statistical design to evaluate critical process parameters and optimize formulation technique of polymeric nanoparticles, Royal Society open science,
doi:10.1098/rsos.190896Schürch, Gehr, Im, Hof, Geiser et al., Etoposide-loaded PLGA and PCL nanoparticles I: preparation and effect of formulation variables, Respiration Physiology,
doi:10.1016/0034-5687Tavares, De Araújo, Fraceto, Ivermectin-loaded polymeric nanoparticles: Screening the effects of polymers, methods, and the usefulness of mathematical models, Journal of Nanoscience and Nanotechnology,
doi:10.1166/jnn.2017.13111Thakur, Chellappan, Dua, Mehta, Satija et al., Patented therapeutic drug delivery strategies for targeting pulmonary diseases, Expert opinion on therapeutic patents,
doi:10.1080/13543776.2020.1741547Triplett, Rathman, Optimization of β-carotene loaded solid lipid nanoparticles preparation using a high shear homogenization technique, Journal of nanoparticle research,
doi:10.1007/s11051-008-9402-3Venkateswarlu, Manjunath, Preparation, characterization and in vitro release kinetics of clozapine solid lipid nanoparticles, Journal of Controlled Release,
doi:10.1016/j.jconrel.2004.01.005Vishwa, Moin, Gowda, Rizvi, Hegazy et al., Pulmonary targeting of inhalable moxifloxacin microspheres for effective management of tuberculosis, Pharmaceutics,
doi:10.3390/pharmaceutics13010079Xu, Khan, Burgess, A quality by design (QbD) case study on liposomes containing hydrophilic API: I. Formulation, processing design and risk assessment, International Journal of Pharmaceutics,
doi:10.1016/j.ijpharm.2011.07.012Yang, Chung, Bai, Chan, Effect of preparation conditions on morphology and release profiles of biodegradable polymeric microspheres containing protein fabricated by double-emulsion method, Chemical Engineering Science,
doi:10.1016/S0009-2509(99)00503-5DOI record:
{
"DOI": "10.1016/j.ejps.2024.106714",
"ISSN": [
"0928-0987"
],
"URL": "http://dx.doi.org/10.1016/j.ejps.2024.106714",
"alternative-id": [
"S0928098724000253"
],
"article-number": "106714",
"assertion": [
{
"label": "This article is maintained by",
"name": "publisher",
"value": "Elsevier"
},
{
"label": "Article Title",
"name": "articletitle",
"value": "A remodeled ivermectin polycaprolactone-based nanoparticles for inhalation as a promising treatment of pulmonary inflammatory diseases"
},
{
"label": "Journal Title",
"name": "journaltitle",
"value": "European Journal of Pharmaceutical Sciences"
},
{
"label": "CrossRef DOI link to publisher maintained version",
"name": "articlelink",
"value": "https://doi.org/10.1016/j.ejps.2024.106714"
},
{
"label": "Content Type",
"name": "content_type",
"value": "article"
},
{
"label": "Copyright",
"name": "copyright",
"value": "© 2024 The Author(s). Published by Elsevier B.V."
}
],
"author": [
{
"affiliation": [],
"family": "Mohammed",
"given": "Sabaa Wafiq",
"sequence": "first"
},
{
"affiliation": [],
"family": "El-Megrab",
"given": "Nagia Ahmed",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hasan",
"given": "Azza A.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gomaa",
"given": "Eman",
"sequence": "additional"
}
],
"container-title": "European Journal of Pharmaceutical Sciences",
"container-title-short": "European Journal of Pharmaceutical Sciences",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"elsevier.com",
"sciencedirect.com"
]
},
"created": {
"date-parts": [
[
2024,
1,
30
]
],
"date-time": "2024-01-30T17:10:55Z",
"timestamp": 1706634655000
},
"deposited": {
"date-parts": [
[
2024,
1,
30
]
],
"date-time": "2024-01-30T17:11:34Z",
"timestamp": 1706634694000
},
"indexed": {
"date-parts": [
[
2024,
1,
31
]
],
"date-time": "2024-01-31T00:40:36Z",
"timestamp": 1706661636776
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2024,
1
]
]
},
"language": "en",
"license": [
{
"URL": "https://www.elsevier.com/tdm/userlicense/1.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
1,
1
]
],
"date-time": "2024-01-01T00:00:00Z",
"timestamp": 1704067200000
}
},
{
"URL": "http://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 28,
"start": {
"date-parts": [
[
2024,
1,
29
]
],
"date-time": "2024-01-29T00:00:00Z",
"timestamp": 1706486400000
}
}
],
"link": [
{
"URL": "https://api.elsevier.com/content/article/PII:S0928098724000253?httpAccept=text/xml",
"content-type": "text/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://api.elsevier.com/content/article/PII:S0928098724000253?httpAccept=text/plain",
"content-type": "text/plain",
"content-version": "vor",
"intended-application": "text-mining"
}
],
"member": "78",
"original-title": [],
"page": "106714",
"prefix": "10.1016",
"published": {
"date-parts": [
[
2024,
1
]
]
},
"published-print": {
"date-parts": [
[
2024,
1
]
]
},
"publisher": "Elsevier BV",
"reference": [
{
"DOI": "10.1021/jp709667u",
"article-title": "Size-Dependent Surface Charging of Nanoparticles",
"author": "Abbas",
"doi-asserted-by": "crossref",
"first-page": "5715",
"journal-title": "The Journal of Physical Chemistry C",
"key": "10.1016/j.ejps.2024.106714_bib0001",
"volume": "112",
"year": "2008"
},
{
"DOI": "10.1208/s12249-014-0101-3",
"article-title": "Biodegradable injectable in situ implants and microparticles for sustained release of montelukast: in vitro release, pharmacokinetics, and stability",
"author": "Ahmed",
"doi-asserted-by": "crossref",
"first-page": "772",
"journal-title": "AAPS PharmSciTech",
"key": "10.1016/j.ejps.2024.106714_bib0002",
"volume": "15",
"year": "2014"
},
{
"DOI": "10.2147/IJN.S139706",
"article-title": "Chitosan-coated diacerein nanosuspensions as a platform for enhancing bioavailability and lowering side effects: preparation, characterization, and ex vivo/in vivo evaluation",
"author": "Allam",
"doi-asserted-by": "crossref",
"first-page": "4733",
"journal-title": "International journal of nanomedicine",
"key": "10.1016/j.ejps.2024.106714_bib0003",
"year": "2017"
},
{
"DOI": "10.1002/ejp.1169",
"article-title": "Blocking of cytokines signalling attenuates evoked and spontaneous neuropathic pain behaviours in the paclitaxel rat model of chemotherapy-induced neuropathy",
"author": "Al-Mazidi",
"doi-asserted-by": "crossref",
"first-page": "810",
"journal-title": "European Journal of Pain",
"key": "10.1016/j.ejps.2024.106714_bib0004",
"volume": "22",
"year": "2018"
},
{
"DOI": "10.1080/10717544.2021.1995078",
"article-title": "Formulation and evaluation of butenafine loaded PLGA-nanoparticulate laden chitosan nano gel",
"author": "Alshehri",
"doi-asserted-by": "crossref",
"first-page": "2348",
"journal-title": "Drug Delivery",
"key": "10.1016/j.ejps.2024.106714_bib0005",
"volume": "28",
"year": "2021"
},
{
"article-title": "Effect of chitosan coating on PLGA nanoparticles for oral delivery of thymoquinone: In vitro, ex vivo, and cancer cell line assessments",
"author": "Alshehri",
"issue": "6",
"journal-title": "Coatings",
"key": "10.1016/j.ejps.2024.106714_bib0006",
"volume": "11",
"year": "2020"
},
{
"author": "Aminu",
"first-page": "37",
"key": "10.1016/j.ejps.2024.106714_bib0007",
"series-title": "Polycaprolactone-based nanoparticles for advanced therapeutic applications",
"year": "2023"
},
{
"DOI": "10.1016/j.ejps.2018.03.016",
"doi-asserted-by": "crossref",
"key": "10.1016/j.ejps.2024.106714_bib0008",
"unstructured": "Ayoub, M. M., N. G. Elantouny, H. M. El-Nahas and F. E.-D. S. Ghazy, 2018. Injectable PLGA Adefovir microspheres; the way for long term therapy of chronic hepatitis-B. European journal of pharmaceutical sciences. 118, 24-31. https://doi.org/10.1016/j.ejps.2018.03.016"
},
{
"DOI": "10.1016/j.jddst.2020.101585",
"article-title": "Comparative study of PLGA in-situ implant and nanoparticle formulations of entecavir; in-vitro and in-vivo evaluation",
"author": "Ayoub",
"doi-asserted-by": "crossref",
"journal-title": "Journal of Drug Delivery Science and Technology",
"key": "10.1016/j.ejps.2024.106714_bib0009",
"volume": "56",
"year": "2020"
},
{
"article-title": "Alveolar surface forces and lung architecture",
"author": "Bachofen",
"journal-title": "Comparative Biochemistry and Physiology–Part B: Biochemistry and Molecular Biology",
"key": "10.1016/j.ejps.2024.106714_bib0010",
"volume": "S8",
"year": "2000"
},
{
"DOI": "10.3390/polym15193890",
"article-title": "Optimization of Bromocriptine-Mesylate-Loaded Polycaprolactone Nanoparticles Coated with Chitosan for Nose-to-Brain Delivery: In Vitro and In Vivo Studies",
"author": "Badran",
"doi-asserted-by": "crossref",
"first-page": "3890",
"journal-title": "Polymers",
"key": "10.1016/j.ejps.2024.106714_bib0011",
"volume": "15",
"year": "2023"
},
{
"article-title": "Formulation and evaluation of Rifampicin loaded poly-ε-caprolactone nano-particles using 32 factorial design",
"author": "Behera",
"first-page": "340",
"journal-title": "International Journal of Pharmaceutial Sciences And Research",
"key": "10.1016/j.ejps.2024.106714_bib0012",
"year": "2012"
},
{
"DOI": "10.1080/17458080.2022.2060501",
"article-title": "Genotoxicity and in vitro investigation of Gefitinib-loaded polycaprolactone fabricated nanoparticles for anticancer activity against NCI-H460 cell lines",
"author": "Bhattacharya",
"doi-asserted-by": "crossref",
"first-page": "214",
"journal-title": "Journal of Experimental Nanoscience",
"key": "10.1016/j.ejps.2024.106714_bib0013",
"volume": "17",
"year": "2022"
},
{
"DOI": "10.1081/PDT-120017517",
"doi-asserted-by": "crossref",
"key": "10.1016/j.ejps.2024.106714_bib0014",
"unstructured": "Bilati, U., E. Allémann and E. Doelker, 2003. Sonication Parameters for the Preparation of Biodegradable Nanocapsulesof Controlled Size by the Double Emulsion Method. Pharmaceutical Development and Technology. 8, 1-9. https://doi.org/10.1081/PDT-120017517"
},
{
"DOI": "10.1016/j.jconrel.2008.04.002",
"article-title": "High loading efficiency and tunable release of plasmid DNA encapsulated in submicron particles fabricated from PLGA conjugated with poly-L-lysine",
"author": "Blum",
"doi-asserted-by": "crossref",
"first-page": "66",
"journal-title": "Journal of controlled release",
"key": "10.1016/j.ejps.2024.106714_bib0015",
"volume": "129",
"year": "2008"
},
{
"article-title": "A comparison of models for the analysis of the kinetics of drug release from PLGA-based nanoparticles",
"author": "Jahromi",
"journal-title": "Heliyon",
"key": "10.1016/j.ejps.2024.106714_bib0016",
"volume": "6",
"year": "2020"
},
{
"DOI": "10.1016/j.antiviral.2020.104787",
"article-title": "The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro",
"author": "Caly",
"doi-asserted-by": "crossref",
"journal-title": "Antiviral Research",
"key": "10.1016/j.ejps.2024.106714_bib0017",
"volume": "178",
"year": "2020"
},
{
"DOI": "10.1038/s41598-020-74084-y",
"article-title": "Nebulized ivermectin for COVID-19 and other respiratory diseases, a proof of concept, dose-ranging study in rats",
"author": "Chaccour",
"doi-asserted-by": "crossref",
"first-page": "17073",
"journal-title": "Scientific reports",
"key": "10.1016/j.ejps.2024.106714_bib0018",
"volume": "10",
"year": "2020"
},
{
"DOI": "10.1016/S2095-3119(19)62613-4",
"article-title": "Preparation and characterization of atrazine-loaded biodegradable PLGA nanospheres",
"author": "Chen",
"doi-asserted-by": "crossref",
"first-page": "1035",
"journal-title": "Journal of Integrative Agriculture",
"key": "10.1016/j.ejps.2024.106714_bib0019",
"volume": "18",
"year": "2019"
},
{
"DOI": "10.1016/j.jconrel.2005.07.001",
"article-title": "Preparation and characterization of melittin-loaded poly (DL-lactic acid) or poly (DL-lactic-co-glycolic acid) microspheres made by the double emulsion method",
"author": "Cui",
"doi-asserted-by": "crossref",
"first-page": "310",
"journal-title": "Journal of Controlled Release",
"key": "10.1016/j.ejps.2024.106714_bib0020",
"volume": "107",
"year": "2005"
},
{
"DOI": "10.1080/10428194.2020.1786559",
"article-title": "Continuous high-dose ivermectin appears to be safe in patients with acute myelogenous leukemia and could inform clinical repurposing for COVID-19 infection",
"author": "de Castro",
"doi-asserted-by": "crossref",
"first-page": "2536",
"journal-title": "Leukemia & Lymphoma",
"key": "10.1016/j.ejps.2024.106714_bib0021",
"volume": "61",
"year": "2020"
},
{
"DOI": "10.1016/j.biopha.2018.08.101",
"article-title": "Multi-drug resistant Mycobacterium tuberculosis & oxidative stress complexity: Emerging need for novel drug delivery approaches",
"author": "Dua",
"doi-asserted-by": "crossref",
"first-page": "1218",
"journal-title": "Biomedicine & Pharmacotherapy",
"key": "10.1016/j.ejps.2024.106714_bib0022",
"volume": "107",
"year": "2018"
},
{
"DOI": "10.1016/j.jddst.2021.102727",
"article-title": "Hybrid polylactic acid/Eudragit L100 nanoparticles: A promising system for enhancement of bioavailability and pharmacodynamic efficacy of luteolin",
"author": "Elmowafy",
"doi-asserted-by": "crossref",
"journal-title": "Journal of Drug Delivery Science and Technology",
"key": "10.1016/j.ejps.2024.106714_bib0023",
"volume": "65",
"year": "2021"
},
{
"article-title": "Preparation and characterization of estradiol-loaded PLGA nanoparticles using homogenization-solvent diffusion method",
"author": "Esmaeili",
"first-page": "196",
"journal-title": "DARU Journal of Pharmaceutical Sciences",
"key": "10.1016/j.ejps.2024.106714_bib0024",
"year": "2008"
},
{
"DOI": "10.1016/j.jconrel.2005.07.001",
"article-title": "Preparation and characterization of melittin-loaded poly (dl-lactic acid) or poly (dl-lactic-co-glycolic acid) microspheres made by the double emulsion method",
"author": "Fude",
"doi-asserted-by": "crossref",
"first-page": "310",
"journal-title": "Journal of Controlled Release",
"key": "10.1016/j.ejps.2024.106714_bib0025",
"volume": "107",
"year": "2005"
},
{
"DOI": "10.1007/s11095-006-9074-6",
"article-title": "Inhalation delivery and anti-tumor activity of celecoxib in human orthotopic non-small cell lung cancer xenograft model",
"author": "Fulzele",
"doi-asserted-by": "crossref",
"first-page": "2094",
"journal-title": "Pharmaceutical research",
"key": "10.1016/j.ejps.2024.106714_bib0026",
"volume": "23",
"year": "2006"
},
{
"DOI": "10.1208/s12249-016-0489-z",
"article-title": "Systematic development of transethosomal gel system of piroxicam: formulation optimization, in vitro evaluation, and ex vivo assessment",
"author": "Garg",
"doi-asserted-by": "crossref",
"first-page": "58",
"journal-title": "AAPS pharmscitech",
"key": "10.1016/j.ejps.2024.106714_bib0027",
"volume": "18",
"year": "2017"
},
{
"DOI": "10.1177/0018578718758972",
"article-title": "A Case of Ivermectin-Induced Warfarin Toxicity: First Published Report",
"author": "Gilbert",
"doi-asserted-by": "crossref",
"first-page": "393",
"journal-title": "Hospital Pharmacy",
"key": "10.1016/j.ejps.2024.106714_bib0028",
"volume": "53",
"year": "2018"
},
{
"article-title": "Exploring targeted pulmonary delivery for treatment of lung cancer",
"author": "Goel",
"issue": "8",
"journal-title": "International journal of pharmaceutical investigation",
"key": "10.1016/j.ejps.2024.106714_bib0029",
"volume": "3",
"year": "2013"
},
{
"article-title": "Ivermection-loaded solid lipid nanoparticles: preparation, characterisation, stability and transdermal behaviour",
"author": "Guo",
"first-page": "255",
"journal-title": "Nanomedicine, and Biotechnology",
"key": "10.1016/j.ejps.2024.106714_bib0030",
"volume": "46",
"year": "2018"
},
{
"DOI": "10.1177/009127002237994",
"article-title": "Safety, tolerability, and pharmacokinetics of escalating high doses of ivermectin in healthy adult subjects",
"author": "Guzzo",
"doi-asserted-by": "crossref",
"first-page": "1122",
"journal-title": "The Journal of Clinical Pharmacology",
"key": "10.1016/j.ejps.2024.106714_bib0031",
"volume": "42",
"year": "2002"
},
{
"DOI": "10.1016/j.ejps.2018.01.044",
"article-title": "Enhanced transdermal delivery of ondansetron using nanovesicular systems: fabrication, characterization, optimization and ex-vivo permeation study-Box-Cox transformation practical example",
"author": "Habib",
"doi-asserted-by": "crossref",
"first-page": "352",
"journal-title": "European journal of pharmaceutical sciences",
"key": "10.1016/j.ejps.2024.106714_bib0032",
"volume": "115",
"year": "2018"
},
{
"DOI": "10.1016/j.ijpharm.2017.02.006",
"article-title": "Nano-encapsulation of a novel anti-Ran-GTPase peptide for blockade of regulator of chromosome condensation 1 (RCC1) function in MDA-MB-231 breast cancer cells",
"author": "Haggag",
"doi-asserted-by": "crossref",
"first-page": "40",
"journal-title": "International journal of pharmaceutics",
"key": "10.1016/j.ejps.2024.106714_bib0033",
"volume": "521",
"year": "2017"
},
{
"DOI": "10.1039/C9RA10857B",
"article-title": "PLGA nanoparticle preparations by emulsification and nanoprecipitation techniques: Effects of formulation parameters",
"author": "Hernández-Giottonini",
"doi-asserted-by": "crossref",
"first-page": "4218",
"journal-title": "Rsc Advances",
"key": "10.1016/j.ejps.2024.106714_bib0034",
"volume": "10",
"year": "2020"
},
{
"DOI": "10.1002/jps.20591",
"article-title": "Development of a respirable, sustained release microcarrier for 5-fluorouracil I: In vitro assessment of liposomes, microspheres, and lipid coated nanoparticles",
"author": "Hitzman",
"doi-asserted-by": "crossref",
"first-page": "1114",
"journal-title": "Journal of pharmaceutical sciences",
"key": "10.1016/j.ejps.2024.106714_bib0035",
"volume": "95",
"year": "2006"
},
{
"DOI": "10.1016/j.colsurfa.2014.01.012",
"article-title": "Effects of process parameters on the colloidal properties of polycaprolactone microparticles prepared by double emulsion like process",
"author": "Ibraheem",
"doi-asserted-by": "crossref",
"first-page": "79",
"journal-title": "Colloids and Surfaces A: Physicochemical and Engineering Aspects",
"key": "10.1016/j.ejps.2024.106714_bib0036",
"volume": "445",
"year": "2014"
},
{
"DOI": "10.3390/pharmaceutics14091867",
"article-title": "Investigation of Alogliptin-Loaded In Situ Gel Implants by 23 Factorial Design with Glycemic Assessment in Rats",
"author": "Ibrahim",
"doi-asserted-by": "crossref",
"first-page": "1867",
"journal-title": "Pharmaceutics",
"key": "10.1016/j.ejps.2024.106714_bib0037",
"volume": "14",
"year": "2022"
},
{
"DOI": "10.1158/1078-0432.CCR-08-1558",
"article-title": "Enhancement of docetaxel anticancer activity by a novel diindolylmethane compound in human non–small cell lung cancer",
"author": "Ichite",
"doi-asserted-by": "crossref",
"first-page": "543",
"journal-title": "Clinical Cancer Research",
"key": "10.1016/j.ejps.2024.106714_bib0038",
"volume": "15",
"year": "2009"
},
{
"DOI": "10.1080/08958370050085156",
"article-title": "Measurement of total lung deposition of inhaled ultrafine particles in healthy men and women",
"author": "Jaques",
"doi-asserted-by": "crossref",
"first-page": "715",
"journal-title": "Inhalation toxicology",
"key": "10.1016/j.ejps.2024.106714_bib0039",
"volume": "12",
"year": "2000"
},
{
"article-title": "Study on the subacute inhalation toxicity of ivermectin TC in rats",
"author": "Ji",
"first-page": "70",
"journal-title": "Chinese Journal of Comparative Medicine",
"key": "10.1016/j.ejps.2024.106714_bib0040",
"year": "2016"
},
{
"DOI": "10.1016/S0167-5273(02)00138-9",
"article-title": "C/T polymorphism of the intercellular adhesion molecule-1 gene (exon 6, codon 469). A risk factor for coronary heart disease and myocardial infarction",
"author": "Jiang",
"doi-asserted-by": "crossref",
"first-page": "171",
"journal-title": "International journal of cardiology",
"key": "10.1016/j.ejps.2024.106714_bib0041",
"volume": "84",
"year": "2002"
},
{
"article-title": "Practical preparation procedures for docetaxel-loaded nanoparticles using polylactic acid-co-glycolic acid",
"author": "Keum",
"first-page": "2225",
"journal-title": "International journal of nanomedicine",
"key": "10.1016/j.ejps.2024.106714_bib0042",
"year": "2011"
},
{
"DOI": "10.1016/j.pt.2017.02.004",
"article-title": "Ivermectin–old drug, new tricks?",
"author": "Laing",
"doi-asserted-by": "crossref",
"first-page": "463",
"journal-title": "Trends in parasitology",
"key": "10.1016/j.ejps.2024.106714_bib0043",
"volume": "33",
"year": "2017"
},
{
"DOI": "10.1007/978-981-13-3642-3_9",
"doi-asserted-by": "crossref",
"key": "10.1016/j.ejps.2024.106714_bib0044",
"unstructured": "Lalan, M., H. Tandel, R. Lalani, V. Patel and A. Misra, 2019. Inhalation drug therapy: Emerging trends in nasal and pulmonary drug delivery. Novel Drug Delivery Technologies: Innovative Strategies for Drug Re-positioning. 291-333. https://doi.org/10.1007/978-981-13-3642-3"
},
{
"DOI": "10.1093/toxsci/kfy309",
"article-title": "Role of Macrophages in Acute Lung Injury and Chronic Fibrosis Induced by Pulmonary Toxicants",
"author": "Laskin",
"doi-asserted-by": "crossref",
"first-page": "287",
"journal-title": "Toxicological Sciences",
"key": "10.1016/j.ejps.2024.106714_bib0045",
"volume": "168",
"year": "2019"
},
{
"article-title": "Alveolar clearance and the role of the pulmonary lymphatics",
"author": "Lauweryns",
"first-page": "625",
"journal-title": "American Review of Respiratory Disease",
"key": "10.1016/j.ejps.2024.106714_bib0046",
"volume": "115",
"year": "1977"
},
{
"DOI": "10.1007/s13167-020-00209-y",
"article-title": "Anti-parasite drug ivermectin can suppress ovarian cancer by regulating lncRNA-EIF4A3-mRNA axes",
"author": "Li",
"doi-asserted-by": "crossref",
"first-page": "289",
"journal-title": "EPMA Journal",
"key": "10.1016/j.ejps.2024.106714_bib0047",
"volume": "11",
"year": "2020"
},
{
"DOI": "10.1080/10717544.2017.1284945",
"article-title": "Sustained release ivermectin-loaded solid lipid dispersion for subcutaneous delivery: in vitro and in vivo evaluation",
"author": "Lu",
"doi-asserted-by": "crossref",
"first-page": "622",
"journal-title": "Drug delivery",
"key": "10.1016/j.ejps.2024.106714_bib0048",
"volume": "24",
"year": "2017"
},
{
"DOI": "10.1016/j.biopha.2022.113706",
"article-title": "Ivermectin contributes to attenuating the severity of acute lung injury in mice",
"author": "Ma",
"doi-asserted-by": "crossref",
"journal-title": "Biomedicine & Pharmacotherapy",
"key": "10.1016/j.ejps.2024.106714_bib0049",
"volume": "155",
"year": "2022"
},
{
"DOI": "10.1016/j.ijpharm.2014.02.043",
"article-title": "Impact of sodium dodecyl sulphate on the dissolution of poorly soluble drug into biorelevant medium from drug-surfactant discs",
"author": "Madelung",
"doi-asserted-by": "crossref",
"first-page": "1",
"journal-title": "International Journal of Pharmaceutics",
"key": "10.1016/j.ejps.2024.106714_bib0050",
"volume": "467",
"year": "2014"
},
{
"DOI": "10.2147/IJN.S4937",
"article-title": "Nanomedicine in pulmonary delivery",
"author": "Mansour",
"doi-asserted-by": "crossref",
"first-page": "299",
"journal-title": "International journal of nanomedicine",
"key": "10.1016/j.ejps.2024.106714_bib0051",
"year": "2009"
},
{
"DOI": "10.1016/j.intimp.2021.108004",
"article-title": "Safety of inhaled ivermectin as a repurposed direct drug for treatment of COVID-19: A preclinical tolerance study",
"author": "Mansour",
"doi-asserted-by": "crossref",
"journal-title": "International Immunopharmacology",
"key": "10.1016/j.ejps.2024.106714_bib0052",
"volume": "99",
"year": "2021"
},
{
"DOI": "10.1042/BST20200568",
"article-title": "Antivirals that target the host IMPα/β1-virus interface",
"author": "Martin",
"doi-asserted-by": "crossref",
"first-page": "281",
"journal-title": "Biochemical Society Transactions",
"key": "10.1016/j.ejps.2024.106714_bib0053",
"volume": "49",
"year": "2021"
},
{
"DOI": "10.1080/21691401.2018.1513938",
"article-title": "Potential of dry powder inhalers for tuberculosis therapy: facts, fidelity and future",
"author": "Mehta",
"doi-asserted-by": "crossref",
"first-page": "S791",
"journal-title": "Artificial cells, nanomedicine, and biotechnology",
"key": "10.1016/j.ejps.2024.106714_bib0054",
"volume": "46",
"year": "2018"
},
{
"DOI": "10.1016/j.ijpharm.2014.10.004",
"article-title": "Development of artemether-loaded nanostructured lipid carrier (NLC) formulation for topical application",
"author": "Nnamani",
"doi-asserted-by": "crossref",
"first-page": "208",
"journal-title": "International Journal of Pharmaceutics",
"key": "10.1016/j.ejps.2024.106714_bib0055",
"volume": "477",
"year": "2014"
},
{
"DOI": "10.3109/10717544.2015.1105323",
"article-title": "Revisiting the nanoformulation design approach for effective delivery of topotecan in its stable form: an appraisal of its in vitro Behavior and tumor amelioration potential",
"author": "Padhi",
"doi-asserted-by": "crossref",
"first-page": "2827",
"journal-title": "Drug Delivery",
"key": "10.1016/j.ejps.2024.106714_bib0056",
"volume": "23",
"year": "2016"
},
{
"DOI": "10.1371/journal.pone.0267257",
"article-title": "Lung cancer targeting efficiency of Silibinin loaded Poly Caprolactone/Pluronic F68 Inhalable nanoparticles: In vitro and In vivo study",
"author": "Patel",
"doi-asserted-by": "crossref",
"journal-title": "Plos one",
"key": "10.1016/j.ejps.2024.106714_bib0057",
"volume": "17",
"year": "2022"
},
{
"DOI": "10.1016/j.jconrel.2010.02.006",
"article-title": "Formulation, characterization and pulmonary deposition of nebulized celecoxib encapsulated nanostructured lipid carriers",
"author": "Patlolla",
"doi-asserted-by": "crossref",
"first-page": "233",
"journal-title": "Journal of Controlled Release",
"key": "10.1016/j.ejps.2024.106714_bib0058",
"volume": "144",
"year": "2010"
},
{
"DOI": "10.1155/2015/816460",
"article-title": "2015. Understanding the Mysterious M2 Macrophage through Activation Markers and Effector Mechanisms",
"author": "Rőszer",
"doi-asserted-by": "crossref",
"journal-title": "Mediators of Inflammation",
"key": "10.1016/j.ejps.2024.106714_bib0059",
"year": "2015"
},
{
"DOI": "10.1016/j.eurpolymj.2022.111307",
"article-title": "Study of sonication parameters on PLA nanoparticles preparation by simple emulsion-evaporation solvent technique",
"author": "Ruiz",
"doi-asserted-by": "crossref",
"journal-title": "European Polymer Journal",
"key": "10.1016/j.ejps.2024.106714_bib0060",
"volume": "173",
"year": "2022"
},
{
"DOI": "10.1016/S0168-3659(02)00127-X",
"article-title": "Residual polyvinyl alcohol associated with poly (D, L-lactide-co-glycolide) nanoparticles affects their physical properties and cellular uptake",
"author": "Sahoo",
"doi-asserted-by": "crossref",
"first-page": "105",
"journal-title": "Journal of controlled release",
"key": "10.1016/j.ejps.2024.106714_bib0061",
"volume": "82",
"year": "2002"
},
{
"DOI": "10.1186/1477-3155-11-26",
"article-title": "Interaction of nanoparticles with proteins: relation to bio-reactivity of the nanoparticle",
"author": "Saptarshi",
"doi-asserted-by": "crossref",
"first-page": "1",
"journal-title": "Journal of nanobiotechnology",
"key": "10.1016/j.ejps.2024.106714_bib0062",
"volume": "11",
"year": "2013"
},
{
"DOI": "10.1098/rsos.190896",
"article-title": "Application of statistical design to evaluate critical process parameters and optimize formulation technique of polymeric nanoparticles",
"author": "Sarkar",
"doi-asserted-by": "crossref",
"journal-title": "Royal Society open science",
"key": "10.1016/j.ejps.2024.106714_bib0063",
"volume": "6",
"year": "2019"
},
{
"DOI": "10.1016/0034-5687(90)90003-H",
"article-title": "Surfactant displaces particles toward the epithelium in airways and alveoli",
"author": "Schürch",
"doi-asserted-by": "crossref",
"first-page": "17",
"journal-title": "Respiration Physiology",
"key": "10.1016/j.ejps.2024.106714_bib0064",
"volume": "80",
"year": "1990"
},
{
"DOI": "10.1080/10717540802174662",
"article-title": "Etoposide-loaded PLGA and PCL nanoparticles I: preparation and effect of formulation variables",
"author": "Snehalatha",
"doi-asserted-by": "crossref",
"first-page": "267",
"journal-title": "Drug Delivery",
"key": "10.1016/j.ejps.2024.106714_bib0065",
"volume": "15",
"year": "2008"
},
{
"DOI": "10.1166/jnn.2017.13111",
"article-title": "Ivermectin-loaded polymeric nanoparticles: Screening the effects of polymers, methods, and the usefulness of mathematical models",
"author": "Tavares",
"doi-asserted-by": "crossref",
"first-page": "4218",
"journal-title": "Journal of Nanoscience and Nanotechnology",
"key": "10.1016/j.ejps.2024.106714_bib0066",
"volume": "17",
"year": "2017"
},
{
"DOI": "10.1080/13543776.2020.1741547",
"doi-asserted-by": "crossref",
"key": "10.1016/j.ejps.2024.106714_bib0067",
"unstructured": "Thakur, A. K., D. K. Chellappan, K. Dua, M. Mehta, S. Satija and I. Singh, 2020. Patented therapeutic drug delivery strategies for targeting pulmonary diseases. Expert opinion on therapeutic patents. 30, 375-387. https://doi.org/10.1080/13543776.2020.1741547"
},
{
"DOI": "10.1007/s11051-008-9402-3",
"article-title": "Optimization of β-carotene loaded solid lipid nanoparticles preparation using a high shear homogenization technique",
"author": "Triplett",
"doi-asserted-by": "crossref",
"first-page": "601",
"journal-title": "Journal of nanoparticle research",
"key": "10.1016/j.ejps.2024.106714_bib0068",
"volume": "11",
"year": "2009"
},
{
"DOI": "10.1016/j.jconrel.2004.01.005",
"article-title": "Preparation, characterization and in vitro release kinetics of clozapine solid lipid nanoparticles",
"author": "Venkateswarlu",
"doi-asserted-by": "crossref",
"first-page": "627",
"journal-title": "Journal of Controlled Release",
"key": "10.1016/j.ejps.2024.106714_bib0069",
"volume": "95",
"year": "2004"
},
{
"article-title": "Pulmonary targeting of inhalable moxifloxacin microspheres for effective management of tuberculosis",
"author": "Vishwa",
"issue": "79",
"journal-title": "Pharmaceutics",
"key": "10.1016/j.ejps.2024.106714_bib0070",
"volume": "13",
"year": "2021"
},
{
"DOI": "10.1016/j.ijpharm.2011.07.012",
"article-title": "A quality by design (QbD) case study on liposomes containing hydrophilic API: I. Formulation, processing design and risk assessment",
"author": "Xu",
"doi-asserted-by": "crossref",
"first-page": "52",
"journal-title": "International Journal of Pharmaceutics",
"key": "10.1016/j.ejps.2024.106714_bib0071",
"volume": "419",
"year": "2011"
},
{
"DOI": "10.1016/j.cca.2010.07.006",
"article-title": "MCP-1: chemoattractant with a role beyond immunity: a review",
"author": "Yadav",
"doi-asserted-by": "crossref",
"first-page": "1570",
"journal-title": "Clinica chimica acta",
"key": "10.1016/j.ejps.2024.106714_bib0072",
"volume": "411",
"year": "2010"
},
{
"DOI": "10.1016/S0009-2509(99)00503-5",
"article-title": "Effect of preparation conditions on morphology and release profiles of biodegradable polymeric microspheres containing protein fabricated by double-emulsion method",
"author": "Yang",
"doi-asserted-by": "crossref",
"first-page": "2223",
"journal-title": "Chemical Engineering Science",
"key": "10.1016/j.ejps.2024.106714_bib0073",
"volume": "55",
"year": "2000"
},
{
"DOI": "10.1016/j.biopha.2022.113888",
"article-title": "Enhancement of the functionality of attenuating acute lung injury by a microemulsion formulation with volatile oil of Angelicae Sinensis Radix and Ligusticum Chuanxiong Rhizoma encapsulated",
"author": "Zhang",
"doi-asserted-by": "crossref",
"journal-title": "Biomedicine & Pharmacotherapy",
"key": "10.1016/j.ejps.2024.106714_bib0074",
"volume": "156",
"year": "2022"
},
{
"DOI": "10.1016/j.cmpb.2010.01.007",
"doi-asserted-by": "crossref",
"key": "10.1016/j.ejps.2024.106714_bib0075",
"unstructured": "Zhang, Y., M. Huo, J. Zhou and S. Xie, 2010. PKSolver: An add-in program for pharmacokinetic and pharmacodynamic data analysis in Microsoft Excel. Computer methods and programs in biomedicine. 99, 306-314. https://doi.org/10.1016/j.cmpb.2010.01.007"
}
],
"reference-count": 75,
"references-count": 75,
"relation": {},
"resource": {
"primary": {
"URL": "https://linkinghub.elsevier.com/retrieve/pii/S0928098724000253"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Pharmaceutical Science"
],
"subtitle": [],
"title": "A remodeled ivermectin polycaprolactone-based nanoparticles for inhalation as a promising treatment of pulmonary inflammatory diseases",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1016/elsevier_cm_policy"
}