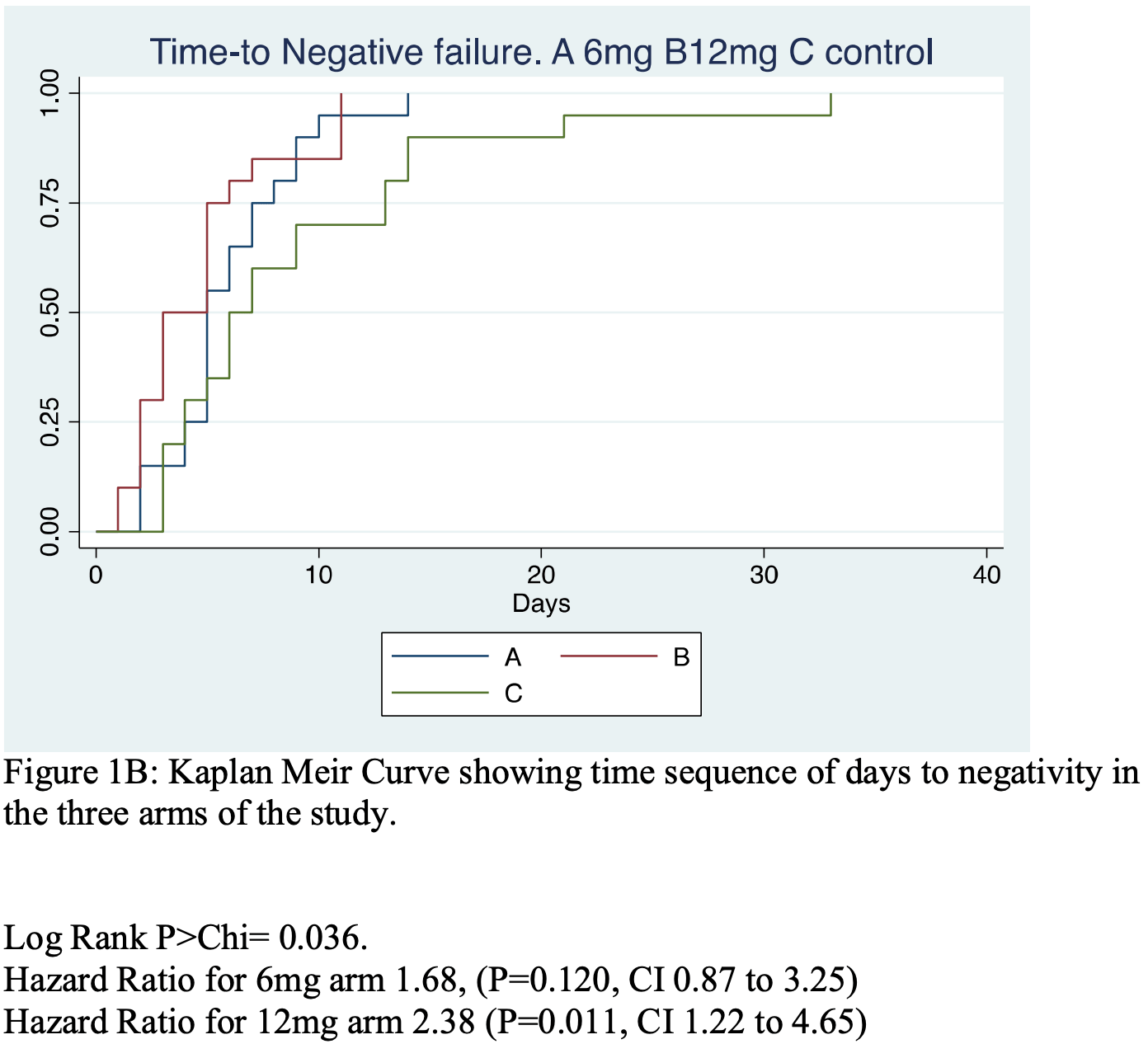
Ivermectin shows clinical benefits in mild to moderate COVID19: a randomized controlled double-blind, dose-response study in Lagos
Prof O E Babalola, C O Bode, A A Ajayi, F M Alakaloko, I E Akase, E Otrofanowei, O B Salu, W L Adeyemo, A O Ademuyiwa, S Omilabu
QJM: An International Journal of Medicine, doi:10.1093/qjmed/hcab035
Introduction: In vitro studies have shown the efficacy of Ivermectin (IV) to inhibit the SARS-CoV-2 viral replication, but questions remained as to in-vivo applications. We set out to explore the efficacy and safety of Ivermectin in persons infected with COVID19. Methods: We conducted a translational proof of concept randomized, double blind placebo controlled, dose response and parallel group study of IV efficacy in RT-polymerase chain reaction proven COVID 19 positive patients. Sixty-two patients were randomized to three treatment groups. (A) IV 6 mg regime, (B) IV 12 mg regime (given Q84 h for 2 weeks) (C, control) Lopinavir/Ritonavir. All groups plus standard of Care. Results: The Days to COVID negativity (DTN) was significantly and dose dependently reduced by IV (P ¼ 0.0066). The DTN for Control were, ¼ 9.1þ/-5.2, for A 6.0 þ/-2.9 and for B 4.6 þ/-3.2. Two way repeated measures ANOVA of ranked COVID 19 þ/scores at 0, 84, 168 and252h showed a significant IV treatment effect (P ¼ 0.035) and time effect (P < 0.0001). IV also tended to increase SPO2% compared to controls, P ¼ 0.073, 95% CI-0.39 to 2.59 and increased platelet count compared to C (P ¼ 0.037) 95%CI 5.55-162.55 Â 10 3 /ml. The platelet count increase was inversely correlated to DTN (r ¼ -0.52, P ¼ 0.005). No SAE was reported.
Conflict of interest. None declared.
References
Abiose, Jones, Murdoch, Cassels-Brown, Babalola et al., Reduction in incidence of optic nerve disease with annual ivermectin to control onchocerciasis, Lancet
Ahmed, Karim, Ross, Hossain, Clemens et al., A five-day course of ivermectin for the treatment of COVID-19 may reduce the duration of illness, Int J Infect Dis
Anderson, Rouphael, Widge, Jackson, Roberts et al., Safety and immunogenicity of SARS-CoV-2 mRNA-1273 vaccine in older adults, N Engl J Med
Arshad, Pertinez, Box, Tatham, Rajoli et al., Prioritisation of anti-SARS-Cov-2 drug repurposing opportunities based on plasma and target site concentrations derived from their established human pharmacokinetics, Clin Pharmacol Ther
Babalola, None
Caly, Druce, Catton, Jans, Wagstaff, The FDAapproved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro, Antiviral Res
Carfı, Bernabei, Landi, For the Gemelli against COVID-19 post-acute care study group). Persistent symptoms in patients after acute COVID-19, JAMA
Cucinotta, Vanelli, WHO Declares COVID-19 a pandemic, Acta Bio Medica Atenei Parm
Dinicolantonio, Barroso, Mccarty, Ivermectin may be a clinically useful anti-inflammatory agent for late-stage COVID-19, Open Heart
Dunn, Laboratory Diagnostics and Testing Guidance for COVID-19: Laboratory Studies, Specimen Selection, Collection, and Transport, Nucleic Acid Detection
Elfein, Number of Coronavirus (COVID-19) Cases, Recoveries and Deaths Worldwide as of February 8
Elgazzar, Hany, Youssef, Hafez, Moussa, Efficacy and safety of Ivermectin for treatment and prophylaxis of COVID-19 pandemic
Heidary, Gharebaghi, Ivermectin: a systematic review from antiviral effects to COVID-19 complementary regimen, J Antibiot
Huang, Wang, Li, Ren, Zhao et al., Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China, Lancet
Jackson, Weiss, Schwarzenberg, Nelson, Sutter et al., Global Economic Effects of COVID-19
Jans, Wagstaff, The broad-spectrum host-directed agent ivermectin as an antiviral for SARS-CoV-2?, Biochem Biophys Res Commun
Khullar, Bond, Schpero, COVID-19 and the financial health of US hospitals, JAMA
Liao, Zhou, Luo, Xu, Wang et al., Haematological characteristics and risk factors in the classification and prognosis evaluation of COVID-19: a retrospective cohort study, Lancet Haematol
Luo, Li, Jiang, Wang, Ye, Signaling to control cytokine release syndrome in COVID-19, Trends Pharmacol Sci
Mojtabavi, Saghazadeh, Rezaei, Interleukin-6 and severe COVID-19: a systematic review and meta-analysis, Eur Cytokine Netw
Moore, June, Cytokine release syndrome in severe COVID-19, Science
Muñoz, Ballester, Antonijoan, Gich, Rodrı ´guez et al., Safety and pharmacokinetic profile of fixed-dose ivermectin with an innovative 18mg Tablet in healthy adult volunteers, PLoS Negl Trop Dis
Nandini, Sundararaj, Akihide, Interpreting diagnostic tests for SARS-CoV-2 Au, JAMA
Parvez, Karim, Hasan, Jaman, Karim et al., Prediction of potential inhibitors for RNA-dependent RNA polymerase of SARS-CoV-2 using comprehensive drug repurposing and molecular docking approach
Polack, Thomas, Kitchin, Absalon, Gurtman et al., Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine, N Engl J Med
Rajter, Sherman, Fatteh, Vogel, Sacks et al., Use of Ivermectin is associated with lower mortality in hospitalized patients with coronavirus disease 2019: the ICON Study, Chest
Schmith, Zhou, Lohmer, The approved dose of Ivermectin alone is not the ideal dose for the treatment of COVID-19, Clin Pharmacol Ther
Sjoding, Dickson, Iwashyna, Gay, Valley, Racial bias in pulse oximetry measurement, N Engl J Med
Yang, Atkinson, Wang, Lee, Bogoyevitch et al., The broad spectrum antiviral ivermectin targets the host nuclear transport importin a/b1 heterodimer, Antiviral Res
Zhang, Song, Ci, Ju, Li, Ivermectin inhibits LPS-induced production of inflammatory cytokines and improves LPS-induced survival in mice, Inflamm Res
DOI record:
{
"DOI": "10.1093/qjmed/hcab035",
"ISSN": [
"1460-2725",
"1460-2393"
],
"URL": "http://dx.doi.org/10.1093/qjmed/hcab035",
"abstract": "<jats:title>Summary</jats:title>\n <jats:sec>\n <jats:title>Introduction</jats:title>\n <jats:p>In vitro studies have shown the efficacy of Ivermectin (IV) to inhibit the SARS—CoV-2 viral replication, but questions remained as to in-vivo applications. We set out to explore the efficacy and safety of Ivermectin in persons infected with COVID19.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Methods</jats:title>\n <jats:p>We conducted a translational proof of concept randomized, double blind placebo controlled, dose response and parallel group study of IV efficacy in RT—polymerase chain reaction proven COVID 19 positive patients. Sixty-two patients were randomized to three treatment groups. (A) IV 6 mg regime, (B) IV 12 mg regime (given Q84 h for 2 weeks) (C, control) Lopinavir/Ritonavir. All groups plus standard of Care.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Results</jats:title>\n <jats:p>The Days to COVID negativity (DTN) was significantly and dose dependently reduced by IV (P = 0.0066). The DTN for Control were, = 9.1+/–5.2, for A 6.0 +/– 2.9 and for B 4.6 +/–3.2. Two way repeated measures ANOVA of ranked COVID 19 +/– scores at 0, 84, 168 and252h showed a significant IV treatment effect (P = 0.035) and time effect (P &lt; 0.0001). IV also tended to increase SPO2% compared to controls, P = 0.073, 95% CI—0.39 to 2.59 and increased platelet count compared to C (P = 0.037) 95%CI 5.55—162.55 × 103/ml. The platelet count increase was inversely correlated to DTN (r = –0.52, P = 0.005). No SAE was reported.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Conclusions</jats:title>\n <jats:p>12mg IV regime given twice a week may have superior efficacy over 6mg IV given twice a week, and certainly over the non IV arm of the study. IV should be considered for use in clinical management of SARS-COV2, and may find applications in prophylaxis in high risk areas.</jats:p>\n </jats:sec>",
"author": [
{
"affiliation": [
{
"name": "From the Department of Ophthalmology, Bingham University, Karu/Jos, Nassarawa/Plateau state, Nigeria"
}
],
"family": "Babalola",
"given": "O E",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Department of Surgery, College of Medicine and Lagos University Teaching Hospital, Lagos, Nigeria"
}
],
"family": "Bode",
"given": "C O",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Division of Hypertension and Clinical pharmacology, Keck Department of Medicine, Baylor College of Medicine Houston Texas, TX 77030, USA"
}
],
"family": "Ajayi",
"given": "A A",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Surgery, Lagos University Teaching Hospital, Lagos, Nigeria"
}
],
"family": "Alakaloko",
"given": "F M",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medicine, Lagos University Teaching Hospital, Lagos, Nigeria"
}
],
"family": "Akase",
"given": "I E",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medicine, Faculty of Clinical Sciences, College of Medicine/Lagos University Teaching Hospital, Lagos, Nigeria"
}
],
"family": "Otrofanowei",
"given": "E",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Central Research Laboratory/Department of Medical Microbiology and Parasitology, Centre for Human and Zoonotic Virology, College of Medicine, University of Lagos, Lagos, Nigeria"
}
],
"family": "Salu",
"given": "O B",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Oral and Maxillofacial Surgery, College of Medicine, University of Lagos, Lagos, Nigeria"
}
],
"family": "Adeyemo",
"given": "W L",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Surgery, College of Medicine and Lagos University Teaching Hospital, Lagos, Nigeria"
}
],
"family": "Ademuyiwa",
"given": "A O",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Central Research Laboratory/Department of Medical Microbiology and Parasitology, Centre for Human and Zoonotic Virology, College of Medicine, University of Lagos, Lagos, Nigeria"
}
],
"family": "Omilabu",
"given": "S",
"sequence": "additional"
}
],
"container-title": "QJM: An International Journal of Medicine",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2021,
2,
15
]
],
"date-time": "2021-02-15T13:19:18Z",
"timestamp": 1613395158000
},
"deposited": {
"date-parts": [
[
2022,
1,
7
]
],
"date-time": "2022-01-07T15:27:44Z",
"timestamp": 1641569264000
},
"funder": [
{
"name": "The Central Bank of Nigeria Health Sector Research and Development Intervention Scheme"
}
],
"indexed": {
"date-parts": [
[
2024,
4,
8
]
],
"date-time": "2024-04-08T17:18:07Z",
"timestamp": 1712596687572
},
"is-referenced-by-count": 32,
"issue": "11",
"issued": {
"date-parts": [
[
2021,
2,
18
]
]
},
"journal-issue": {
"issue": "11",
"published-online": {
"date-parts": [
[
2021,
2,
18
]
]
},
"published-print": {
"date-parts": [
[
2022,
1,
5
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://academic.oup.com/journals/pages/open_access/funder_policies/chorus/standard_publication_model",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
2,
18
]
],
"date-time": "2021-02-18T00:00:00Z",
"timestamp": 1613606400000
}
}
],
"link": [
{
"URL": "http://academic.oup.com/qjmed/advance-article-pdf/doi/10.1093/qjmed/hcab035/37968229/hcab035.pdf",
"content-type": "application/pdf",
"content-version": "am",
"intended-application": "syndication"
},
{
"URL": "https://academic.oup.com/qjmed/article-pdf/114/11/780/42079773/hcab035.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "syndication"
},
{
"URL": "https://academic.oup.com/qjmed/article-pdf/114/11/780/42079773/hcab035.pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "286",
"original-title": [],
"page": "780-788",
"prefix": "10.1093",
"published": {
"date-parts": [
[
2021,
2,
18
]
]
},
"published-online": {
"date-parts": [
[
2021,
2,
18
]
]
},
"published-other": {
"date-parts": [
[
2021,
11,
1
]
]
},
"published-print": {
"date-parts": [
[
2022,
1,
5
]
]
},
"publisher": "Oxford University Press (OUP)",
"reference": [
{
"DOI": "10.1016/S0140-6736(20)30183-5",
"article-title": "Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China",
"author": "Huang",
"doi-asserted-by": "crossref",
"first-page": "497",
"journal-title": "Lancet",
"key": "2022010715264238000_hcab035-B1",
"volume": "395",
"year": "2020"
},
{
"article-title": "WHO Declares COVID-19 a pandemic",
"author": "Cucinotta",
"first-page": "157",
"journal-title": "Acta Bio Medica Atenei Parm",
"key": "2022010715264238000_hcab035-B2",
"volume": "91",
"year": "2020"
},
{
"author": "Elfein",
"key": "2022010715264238000_hcab035-B3",
"year": "2021"
},
{
"DOI": "10.1001/jama.2020.12603",
"article-title": "Persistent symptoms in patients after acute COVID-19",
"author": "Carfì",
"doi-asserted-by": "crossref",
"first-page": "603",
"journal-title": "JAMA",
"key": "2022010715264238000_hcab035-B4",
"volume": "324",
"year": "2020"
},
{
"DOI": "10.1001/jama.2020.6269",
"article-title": "COVID-19 and the financial health of US hospitals",
"author": "Khullar",
"doi-asserted-by": "crossref",
"first-page": "2127",
"journal-title": "JAMA",
"key": "2022010715264238000_hcab035-B5",
"volume": "323",
"year": "2020"
},
{
"author": "Jackson",
"key": "2022010715264238000_hcab035-B6"
},
{
"DOI": "10.1056/NEJMoa2034577",
"article-title": "Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine",
"author": "Polack",
"doi-asserted-by": "crossref",
"first-page": "2603",
"journal-title": "N Engl J Med",
"key": "2022010715264238000_hcab035-B7",
"volume": "383",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2028436",
"article-title": "Safety and immunogenicity of SARS-CoV-2 mRNA-1273 vaccine in older adults",
"author": "Anderson",
"doi-asserted-by": "crossref",
"first-page": "2427",
"journal-title": "N Engl J Med",
"key": "2022010715264238000_hcab035-B8",
"volume": "383",
"year": "2020"
},
{
"DOI": "10.1038/s41429-020-0336-z",
"article-title": "Ivermectin: a systematic review from antiviral effects to COVID-19 complementary regimen",
"author": "Heidary",
"doi-asserted-by": "crossref",
"first-page": "593",
"journal-title": "J Antibiot (Tokyo)",
"key": "2022010715264238000_hcab035-B9",
"volume": "73",
"year": "2020"
},
{
"author": "Jans",
"key": "2022010715264238000_hcab035-B10",
"year": "2020"
},
{
"DOI": "10.1016/0140-6736(93)90002-X",
"article-title": "Reduction in incidence of optic nerve disease with annual ivermectin to control onchocerciasis",
"author": "Abiose",
"doi-asserted-by": "crossref",
"first-page": "130",
"journal-title": "Lancet",
"key": "2022010715264238000_hcab035-B11",
"volume": "341",
"year": "1993"
},
{
"DOI": "10.1016/j.antiviral.2020.104787",
"article-title": "The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro",
"author": "Caly",
"doi-asserted-by": "crossref",
"first-page": "104787",
"journal-title": "Antiviral Res",
"key": "2022010715264238000_hcab035-B12",
"volume": "178",
"year": "2020"
},
{
"author": "Parvez",
"key": "2022010715264238000_hcab035-B13",
"year": "2020"
},
{
"DOI": "10.1016/j.antiviral.2020.104760",
"article-title": "The broad spectrum antiviral ivermectin targets the host nuclear transport importin α/β1 heterodimer",
"author": "Yang",
"doi-asserted-by": "crossref",
"first-page": "104760",
"journal-title": "Antiviral Res",
"key": "2022010715264238000_hcab035-B14",
"volume": "177",
"year": "2020"
},
{
"DOI": "10.1002/cpt.1889",
"article-title": "The approved dose of Ivermectin alone is not the ideal dose for the treatment of COVID-19",
"author": "Schmith",
"doi-asserted-by": "crossref",
"first-page": "762",
"journal-title": "Clin Pharmacol Ther",
"key": "2022010715264238000_hcab035-B15",
"volume": "108",
"year": "2020"
},
{
"DOI": "10.1002/cpt.1909",
"article-title": "Prioritisation of anti-SARS-Cov-2 drug repurposing opportunities based on plasma and target site concentrations derived from their established human pharmacokinetics",
"author": "Arshad",
"doi-asserted-by": "crossref",
"journal-title": "Clin Pharmacol Ther",
"key": "2022010715264238000_hcab035-B16",
"year": "2020"
},
{
"DOI": "10.1371/journal.pntd.0006020",
"article-title": "Safety and pharmacokinetic profile of fixed-dose ivermectin with an innovative 18mg Tablet in healthy adult volunteers",
"author": "Muñoz",
"doi-asserted-by": "crossref",
"first-page": "e0006020",
"journal-title": "PLoS Negl Trop Dis",
"key": "2022010715264238000_hcab035-B17",
"volume": "12",
"year": "2018"
},
{
"DOI": "10.1007/s00011-008-8007-8",
"article-title": "Ivermectin inhibits LPS-induced production of inflammatory cytokines and improves LPS-induced survival in mice",
"author": "Zhang",
"doi-asserted-by": "crossref",
"first-page": "524",
"journal-title": "Inflamm Res",
"key": "2022010715264238000_hcab035-B18",
"volume": "57",
"year": "2008"
},
{
"article-title": "Interpreting diagnostic tests for SARS-CoV-2 Au",
"author": "Nandini",
"journal-title": "JAMA",
"key": "2022010715264238000_hcab035-B19",
"year": "2020"
},
{
"author": "Dunn",
"key": "2022010715264238000_hcab035-B20"
},
{
"article-title": "A five-day course of ivermectin for the treatment of COVID-19 may reduce the duration of illness",
"author": "Ahmed",
"journal-title": "Int J Infect Dis",
"key": "2022010715264238000_hcab035-B21",
"year": "2020"
},
{
"author": "Elgazzar",
"key": "2022010715264238000_hcab035-B22",
"year": "2020"
},
{
"DOI": "10.1056/NEJMc2029240",
"article-title": "Racial bias in pulse oximetry measurement",
"author": "Sjoding",
"doi-asserted-by": "crossref",
"first-page": "2477",
"journal-title": "N Engl J Med",
"key": "2022010715264238000_hcab035-B23",
"volume": "383",
"year": "2020"
},
{
"DOI": "10.1016/S2352-3026(20)30217-9",
"article-title": "Haematological characteristics and risk factors in the classification and prognosis evaluation of COVID-19: a retrospective cohort study",
"author": "Liao",
"doi-asserted-by": "crossref",
"first-page": "e671",
"journal-title": "Lancet Haematol",
"key": "2022010715264238000_hcab035-B24",
"volume": "7",
"year": "2020"
},
{
"DOI": "10.1016/j.chest.2020.10.009",
"article-title": "Use of Ivermectin is associated with lower mortality in hospitalized patients with coronavirus disease 2019: the ICON Study",
"author": "Rajter",
"doi-asserted-by": "crossref",
"first-page": "85",
"journal-title": "Chest",
"key": "2022010715264238000_hcab035-B25",
"volume": "159",
"year": "2020"
},
{
"DOI": "10.1126/science.abb8925",
"article-title": "Cytokine release syndrome in severe COVID-19",
"author": "Moore",
"doi-asserted-by": "crossref",
"first-page": "473",
"journal-title": "Science",
"key": "2022010715264238000_hcab035-B26",
"volume": "368",
"year": "2020"
},
{
"DOI": "10.1136/openhrt-2020-001350",
"article-title": "Ivermectin may be a clinically useful anti-inflammatory agent for late-stage COVID-19",
"author": "DiNicolantonio",
"doi-asserted-by": "crossref",
"first-page": "e001350",
"journal-title": "Open Heart",
"key": "2022010715264238000_hcab035-B27",
"volume": "7",
"year": "2020"
},
{
"DOI": "10.3389/fphar.2020.01169",
"article-title": "Cellular and molecular pathways of COVID-19 and potential points of therapeutic intervention",
"author": "Hussman",
"doi-asserted-by": "crossref",
"journal-title": "Front Pharmacol",
"key": "2022010715264238000_hcab035-B28",
"volume": "11",
"year": "2020"
},
{
"DOI": "10.1684/ecn.2020.0448",
"article-title": "Interleukin-6 and severe COVID-19: a systematic review and meta-analysis",
"author": "Mojtabavi",
"doi-asserted-by": "crossref",
"first-page": "44",
"journal-title": "Eur Cytokine Netw",
"key": "2022010715264238000_hcab035-B29",
"volume": "31",
"year": "2020"
},
{
"DOI": "10.1016/j.tips.2020.06.007",
"article-title": "Signaling to control cytokine release syndrome in COVID-19",
"author": "Luo",
"doi-asserted-by": "crossref",
"first-page": "531",
"journal-title": "Trends Pharmacol Sci",
"key": "2022010715264238000_hcab035-B30",
"volume": "41",
"year": "2020"
}
],
"reference-count": 30,
"references-count": 30,
"relation": {},
"resource": {
"primary": {
"URL": "https://academic.oup.com/qjmed/article/114/11/780/6143037"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"General Medicine"
],
"subtitle": [],
"title": "Ivermectin shows clinical benefits in mild to moderate COVID19: a randomized controlled double-blind, dose-response study in Lagos",
"type": "journal-article",
"volume": "114"
}