Use of Ivermectin as a Potential Chemoprophylaxis for COVID-19 in Egypt: A Randomised Clinical Trial
Waheed M Shoumann, Dr Abdelmonem Awad Hegazy, Ramadan M Nafae, Moustafa I Ragab, Saad R Samra, Dalia Anas Ibrahim, Tarek H Al-Mahrouky, Ashraf E Sileem
JOURNAL OF CLINICAL AND DIAGNOSTIC RESEARCH, doi:10.7860/jcdr/2021/46795.14529
Introduction: The rate of secondary attacks of SARS-COV-2 is high among household close contacts. Social distancing, isolation and infection control measures are important for preventing exposure to infection, but insufficient.
Aim: The study aimed to evaluate possible role of oral ivermectin as a chemoprophylaxis in asymptomatic family close contacts with COVID-19 patients.
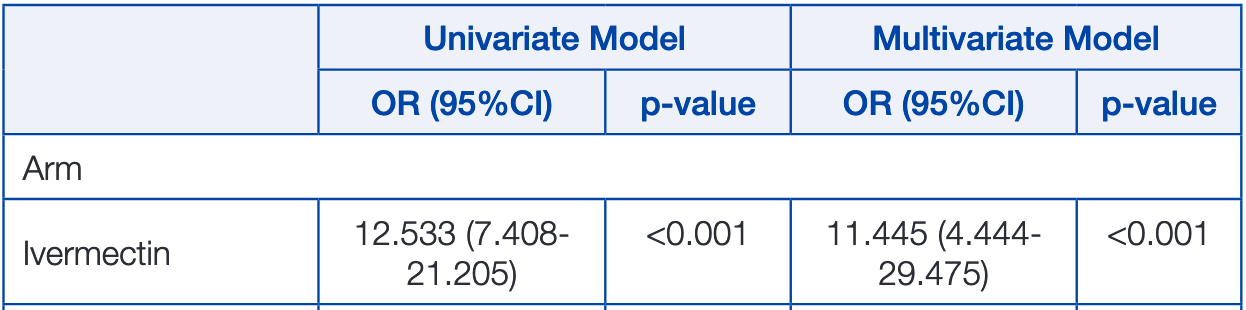
Materials and Methods: A prospective interventional randomised open label-controlled study was conducted (registered at clinicaltrials.gov; NCT04422561) during June and July 2020. Two arms were designed according to use of ivermectin. In ivermectin arm, contacts received ivermectin according to Body Weight (BW) on day of the diagnosis of their index case. The non-intervention group received no treatment. Both groups were followed-up for two weeks for development of symptoms suggestive of COVID-19. Results: Ivermectin group included 203 contacts (to 52 index cases) aged 39.75±14.94 years; 52.2% were males. Nonintervention group included 101 contacts (to a total of 24 index cases) aged 37.69±16.96 years, 49.5% were males. Fifteen contacts (7.4%) developed COVID-19 in the ivermectin arm compared to 59 (58.4%) in the non-intervention arm (p<0.001). The protection rate for ivermectin was more prominent in contacts aged less than 60-year-old (6.2% infected compared to 58.7% if no treatment). Ivermectin in the protection against SARS-CoV-2 infection had an OR of 12.533 and 11.445 (compared to nontreatment) in both univariate and multivariate models, respectively. Side effects of ivermectin were reported in 5.4%; they were mild.
Conclusion: Ivermectin is suggested to be a promising, effective and safe chemoprophylactic drug in management of COVID-19.
References
Am, Liébana, Martínez, None
Azeem, Ashraf, Rasheed, Anjum, Hameed, Nuclear import and export inhibitors alter capsid protein distribution in mammalian cells and reduce Venezuelan Equine Encephalitis Virus replication,
doi:10.1016/j.antiviral.2013.10.004Guzzo, Furtek, Porras, Chen, Tipping et al., None
Hegazy, Hegazy, COVID-19
Heidary, Gharebaghi, Ivermectin: A systematic review from antiviral effects
Ketkar, Yang, Wormser, Wang, Lack of efficacy of ivermectin for
Liu, Yan, Wan, Xiang, Le et al., from the pharmacokinetic point of view: Antiviral levels are not likely attainable with known dosing regimens, Biotechnology & Biotechnological Equipment,
doi:10.1080/13102818.2020.1775118Mastrangelo, Pezzullo, Burghgraeve, Kaptein, Pastorino et al., None
Mitjà, Clotet, Use of antiviral drugs to reduce COVID-19 transmission, Lancet
Momekov, Momekova, Ivermectin as a potential COVID-19 treatment
Morris-Jones, Oral ivermectin for infants and children under 15 kg appears to
Petersen, Koopmans, Go, Hamer, Petrosillo et al., None
Shah, Das, Jain, Misra, Negi, A systematic review of the prophylactic
Wagstaff, None, Sci Rep
Yang, Zheng, Gou, Pu, Chen et al., Prevalence of comorbidities
DOI record:
{
"DOI": "10.7860/jcdr/2021/46795.14529",
"ISSN": [
"2249-782X"
],
"URL": "http://dx.doi.org/10.7860/JCDR/2021/46795.14529",
"abstract": "<jats:p>Introduction: The rate of secondary attacks of SARS-COV-2 is high among household close contacts. Social distancing, isolation and infection control measures are important for preventing exposure to infection, but insufficient. Aim: The study aimed to evaluate possible role of oral ivermectin as a chemoprophylaxis in asymptomatic family close contacts with COVID-19 patients. Materials and Methods: A prospective interventional randomised open label-controlled study was conducted (registered at clinicaltrials.gov; NCT04422561) during June and July 2020. Two arms were designed according to use of ivermectin. In ivermectin arm, contacts received ivermectin according to Body Weight (BW) on day of the diagnosis of their index case. The non-intervention group received no treatment. Both groups were followed-up for two weeks for development of symptoms suggestive of COVID-19. Results: Ivermectin group included 203 contacts (to 52 index cases) aged 39.75±14.94 years; 52.2% were males. Non-intervention group included 101 contacts (to a total of 24 index cases) aged 37.69±16.96 years, 49.5% were males. Fifteen contacts (7.4%) developed COVID-19 in the ivermectin arm compared to 59 (58.4%) in the non-intervention arm (p<0.001). The protection rate for ivermectin was more prominent in contacts aged less than 60-year-old (6.2% infected compared to 58.7% if no treatment). Ivermectin in the protection against SARS-CoV-2 infection had an OR of 12.533 and 11.445 (compared to nontreatment) in both univariate and multivariate models, respectively. Side effects of ivermectin were reported in 5.4%; they were mild. Conclusion: Ivermectin is suggested to be a promising, effective and safe chemoprophylactic drug in management of COVID-19.</jats:p>",
"author": [
{
"affiliation": [],
"family": "Shoumann",
"given": "Waheed M",
"sequence": "first"
},
{
"affiliation": [],
"family": "Hegazy",
"given": "Abdelmonem Awad",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Nafae",
"given": "Ramadan M",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ragab",
"given": "Moustafa I",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Samra",
"given": "Saad R",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ibrahim",
"given": "Dalia Anas",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Al-Mahrouky",
"given": "Tarek H",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sileem",
"given": "Ashraf E",
"sequence": "additional"
}
],
"container-title": "JOURNAL OF CLINICAL AND DIAGNOSTIC RESEARCH",
"container-title-short": "JCDR",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2021,
4,
6
]
],
"date-time": "2021-04-06T07:23:49Z",
"timestamp": 1617693829000
},
"deposited": {
"date-parts": [
[
2021,
4,
6
]
],
"date-time": "2021-04-06T07:23:49Z",
"timestamp": 1617693829000
},
"indexed": {
"date-parts": [
[
2024,
3,
28
]
],
"date-time": "2024-03-28T16:35:46Z",
"timestamp": 1711643746441
},
"is-referenced-by-count": 15,
"issued": {
"date-parts": [
[
2021
]
]
},
"link": [
{
"URL": "https://jcdr.net/article_fulltext.asp?issn=0973-709x&year=2021&volume=15&issue=2&page=OC27&issn=0973-709x&id=14529",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "4601",
"original-title": [],
"prefix": "10.7860",
"published": {
"date-parts": [
[
2021
]
]
},
"published-online": {
"date-parts": [
[
2021
]
]
},
"publisher": "JCDR Research and Publications",
"reference-count": 0,
"references-count": 0,
"relation": {},
"resource": {
"primary": {
"URL": "https://jcdr.net/article_fulltext.asp?issn=0973-709x&year=2021&volume=15&issue=2&page=OC27&issn=0973-709x&id=14529"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Use of Ivermectin as a Potential Chemoprophylaxis for COVID-19 in Egypt: A Randomised Clinical Trial",
"type": "journal-article"
}