The effect of early treatment with ivermectin on viral load, symptoms and humoral response in patients with non-severe COVID-19: A pilot, double-blind, placebo-controlled, randomized clinical trial
Carlos Chaccour, Aina Casellas, Andrés Blanco-Di Matteo, Iñigo Pineda, Alejandro Fernandez-Montero, Paula Ruiz-Castillo, Mary-Ann Richardson, Mariano Rodríguez-Mateos, Carlota Jordán-Iborra, Joe Brew, Francisco Carmona-Torre, Miriam Giráldez, Ester Laso, Juan C Gabaldón-Figueira, Carlota Dobaño, Gemma Moncunill, José R Yuste, Jose L Del Pozo, N Regina Rabinovich, Verena Schöning, Felix Hammann, Gabriel Reina, Belen Sadaba, Mirian Fernández-Alonso
EClinicalMedicine, doi:10.1016/j.eclinm.2020.100720
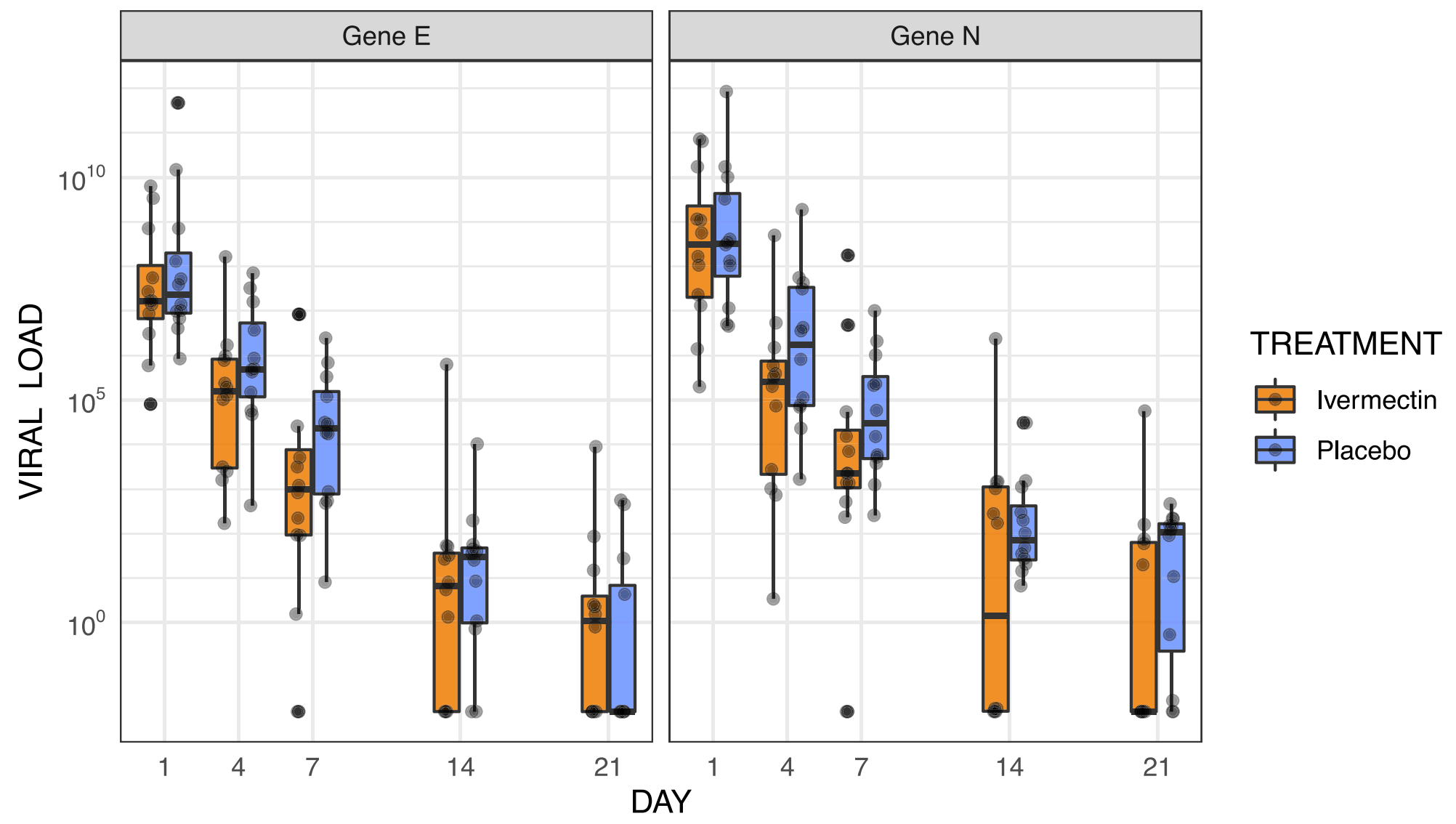
Background: Ivermectin inhibits the replication of SARS-CoV-2 in vitro at concentrations not readily achievable with currently approved doses. There is limited evidence to support its clinical use in COVID-19 patients. We conducted a Pilot, randomized, double-blind, placebo-controlled trial to evaluate the efficacy of a single dose of ivermectin reduce the transmission of SARS-CoV-2 when administered early after disease onset. Methods: Consecutive patients with non-severe COVID-19 and no risk factors for complicated disease attending the emergency room of the Clínica Universidad de Navarra between July 31, 2020 and September 11, 2020 were enrolled. All enrollments occurred within 72 h of onset of fever or cough. Patients were randomized 1:1 to receive ivermectin, 400 mcg/kg, single dose (n = 12) or placebo (n = 12). The primary outcome measure was the proportion of patients with detectable SARS-CoV-2 RNA by PCR from nasopharyngeal swab at day 7 post-treatment. The primary outcome was supported by determination of the viral load and infectivity of each sample. The differences between ivermectin and placebo were calculated using Fisher's exact test and presented as a relative risk ratio. This study is registered at ClinicalTrials.gov: NCT04390022. Findings: All patients recruited completed the trial (median age, 26 [IQR 19À36 in the ivermectin and 21À44 in the controls] years; 12 [50%] women; 100% had symptoms at recruitment, 70% reported headache, 62% reported fever, 50% reported general malaise and 25% reported cough). At day 7, there was no difference in the proportion of PCR positive patients (RR 0¢92, 95% CI: 0¢77À1¢09, p = 1¢0). The ivermectin group had non-statistically significant lower viral loads at day 4 (p = 0¢24 for gene E; p = 0¢18 for gene N) and day 7 (p = 0¢16 for gene E; p = 0¢18 for gene N) post treatment as well as lower IgG titers at day 21 post treatment (p = 0¢24). Patients in the ivermectin group recovered earlier from hyposmia/anosmia (76 vs 158 patient-days; p < 0.001). Interpretation: Among patients with non-severe COVID-19 and no risk factors for severe disease receiving a single 400 mcg/kg dose of ivermectin within 72 h of fever or cough onset there was no difference in the
treatment in the ivermectin group with differences increasing from 3-fold lower at day 4 (p = 0¢24 for gene E; p = 0¢18 for gene N) to around 18-fold lower at day 7 (p = 0¢16 for gene E; p = 0¢18 for gene N) (Fig. 2 and Table S1 ). A similar tendency remained for the viral load at days 14 and 21, with values from patients in the ivermectin group consistently lower for at least one of the genes, the difference was not statistically significant at any single point (Fig. 2 and Table S1 ). The values of cycle thresholds had a very similar behavior (Figure S1 ). Summary statistics for viral kinetics are provided in Table S2 . a Hours before dosing b The slightly higher median systolic blood pressure in the placebo group at baseline was not seen in subsequent study visits and was judged as non-clinically significant, see table S3 for the evolution of all vital signs throughout the study, *Reported or measured fever. IQR: interquartile range
Author contributions Carlos Chaccour and Aina Casellas had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Conceptualization
Supplementary materials Supplementary material associated with this article can be found in the online version at doi:10.1016/j.eclinm.2020.100720.
References
Ahmed, Karim, Ross, A five day course of ivermectin for the treatment of COVID-19 may reduce the duration of illness, Int J Infect Dis
Arshad, Pertinez, Box, Prioritization of anti-SARS-Cov-2 drug repurposing opportunities based on plasma and target site concentrations derived from their established human pharmacokinetics, Clin Pharmacol Ther
Barrows, Campos, Powell, A screen of FDA-approved drugs for inhibitors of Zika virus infection, Cell Host Microbe
Benefield, Skrip, Clement, Althouse, Chang et al., SARS-CoV-2 viral load peaks prior to symptom onset: a systematic review and individualpooled analysis of coronavirus viral load from 66 studies, medRxiv
Bray, Rayner, Noel, Jans, Wagstaff, Ivermectin and COVID-19: a report in antiviral research, widespread interest, an FDA warning, two letters to the editor and the authors' responses, Antiviral Res
Caly, Druce, Catton, Jans, The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro, Antiviral Res
Cevik, Tate, Lloyd, Maraolo, Schafers et al., SARS-CoV-2, SARS-CoV, and MERS-CoV viral load dynamics, duration of viral shedding, and infectiousness: a systematic review and meta-analysis, Lancet Microbe,
doi:10.1016/S2666-5247(20)30172-5Chaccour, Abizanda, Irigoyen-Barrio, Nebulized ivermectin for COVID-19 and other respiratory diseases, a proof of concept, dose-ranging study in rats, Sci Rep
Chaccour, Brew, Garcia-Basteiro, Ivermectin and COVID-19: how a flawed database shaped the pandemic response of several Latin-American countries
Chaccour, Ruiz-Castillo, Richardson, The SARS-CoV-2 Ivermectin Navarra-ISGlobal Trial (SAINT) to evaluate the potential of ivermectin to reduce COVID-19 transmission in low risk, non-severe COVID-19 patients in the first 48 h after symptoms onset: a structured summary of a study protocol for a randomized control pilot trial, Trials
Changeux J-P, Amoura, Rey, Miyara, A nicotinic hypothesis for Covid-19 with preventive and therapeutic implications, C R Biol
Dahdouh, Perona, Romero-Gomez, Mingorance, Garcia-Rodriguez, Ct values from SARS-CoV-2 diagnostic PCR assays should not be used as direct estimates of viral load, J Infect
De M Enonville, Rosignoli, Soares, Topical treatment of rosacea with ivermectin inhibits gene expression of cathelicidin innate immune mediators, LL-37 and KLK5, in reconstructed and ex vivo skin models, Dermatol Ther (Heidelb)
De Melo, Lazarini, Larrous, Anti-COVID-19 efficacy of ivermectin in the golden hamster, bioRxiv
European_Medicines_Agency, EMA decision of 18
Fontanet, Cauchemez, COVID-19 herd immunity: where are we?, Nat Rev Immunol
Gombart, The vitamin D-antimicrobial peptide pathway and its role in protection against infection, Fut Microbiol
Gomez-Ambrosi, Silva, Galofre, Body mass index classification misses subjects with increased cardiometabolic risk factors related to elevated adiposity, Int J Obes (Lond)
Han, Singh, Robinson-Bostom, Vezeridis, Weinstock et al., MeTC7, a novel vitamin D receptor (VDR) antagonist, induces cytotoxicity in metastatic melanoma cell lines and inhibits importin-mediated VDR nuclear transport and signaling, J Am Acad Dermatol
He, Lau, Wu, Temporal dynamics in viral shedding and transmissibility of COVID-19, Nat Med
Hoffmann, Mosbauer, Hofmann-Winkler, Chloroquine does not inhibit infection of human lung cells with SARS-CoV-2, Nature
Kahlenberg, Kaplan, Little peptide, big effects: the role of LL-37 in inflammation and autoimmune disease, J Immunol
Krolewiecki, Lifschitz, Moragas, Antiviral effect of high-dose ivermectin in adults with COVID-19: a pilot randomised, controlled, open label,
doi:10.2139/ssrn.3714649Lauer, Grantz, Bi, The incubation period of coronavirus disease 2019 (COVID-19) from publicly reported confirmed cases: estimation and application, Ann Intern Med
Liu, Yan, Wan, Viral dynamics in mild and severe cases of COVID-19, Lancet Infect Dis
Marklund, Leach, Axelsson, Serum-IgG responses to SARS-CoV-2 after mild and severe COVID-19 infection and analysis of IgG non-responders, PLoS One
Mastrangelo, Pezzullo, Burghgraeve, Ivermectin is a potent inhibitor of flavivirus replication specifically targeting NS3 helicase activity: new prospects for an old drug, J Antimicrob Chemother
Miyauchi, Michigami, Sakaguchi, Importin 4 is responsible for ligandindependent nuclear translocation of vitamin D receptor, J Biol Chem
Rajter, Sherman, Fatteh, Vogel, Sacks et al., Use of ivermectin is associated with lower mortality in hospitalized patients with coronavirus disease 2019: the ICON study, Chest
Rhee, Kanjilal, Baker, Klompas, Duration of SARS-CoV-2 infectivity: when is it safe to discontinue isolation?, Clin Infect Dis,
doi:10.1093/cid/ciaa1249/5896916Rubin, Difficult to determine herd immunity threshold for COVID-19, JAMA
R€ Oltgen, Powell, Wirz, Defining the features and duration of antibody responses to SARS-CoV-2 infection associated with disease severity and outcome, Sci Immunol
Schaller, Gonser, Belge, Dual anti-inflammatory and anti-parasitic action of topical ivermectin 1% in papulopustular rosacea, J Eur Acad Dermatol Venereol
Torabi, Mohammadbagheri, Dilmaghani, Proinflammatory cytokines in the olfactory mucosa result in COVID-19 induced anosmia, ACS Chem Neurosci
Wagstaff, Sivakumaran, Heaton, Harrich, Jans, Ivermectin is a specific inhibitor of importin a/b-mediated nuclear import able to inhibit replication of HIV-1 and dengue virus, Biochem J
Wagstaff, Sivakumaran, Heaton, Harrich, Jans, Ivermectin is a specific inhibitor of importin alpha/beta-mediated nuclear import able to inhibit replication of HIV-1 and dengue virus, Biochem J
Who, COVID-19 Weekly epidemiological update -15
DOI record:
{
"DOI": "10.1016/j.eclinm.2020.100720",
"ISSN": [
"2589-5370"
],
"URL": "http://dx.doi.org/10.1016/j.eclinm.2020.100720",
"alternative-id": [
"S2589537020304648"
],
"article-number": "100720",
"author": [
{
"ORCID": "http://orcid.org/0000-0001-9812-050X",
"affiliation": [],
"authenticated-orcid": false,
"family": "Chaccour",
"given": "Carlos",
"sequence": "first"
},
{
"affiliation": [],
"family": "Casellas",
"given": "Aina",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-0674-2841",
"affiliation": [],
"authenticated-orcid": false,
"family": "Blanco-Di Matteo",
"given": "Andrés",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Pineda",
"given": "Iñigo",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Fernandez-Montero",
"given": "Alejandro",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ruiz-Castillo",
"given": "Paula",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Richardson",
"given": "Mary-Ann",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rodríguez-Mateos",
"given": "Mariano",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Jordán-Iborra",
"given": "Carlota",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Brew",
"given": "Joe",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Carmona-Torre",
"given": "Francisco",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Giráldez",
"given": "Miriam",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Laso",
"given": "Ester",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gabaldón-Figueira",
"given": "Juan C.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Dobaño",
"given": "Carlota",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Moncunill",
"given": "Gemma",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Yuste",
"given": "José R.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Del Pozo",
"given": "Jose L.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rabinovich",
"given": "N.Regina",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Schöning",
"given": "Verena",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hammann",
"given": "Felix",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Reina",
"given": "Gabriel",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sadaba",
"given": "Belen",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Fernández-Alonso",
"given": "Mirian",
"sequence": "additional"
}
],
"container-title": "EClinicalMedicine",
"container-title-short": "EClinicalMedicine",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2021,
1,
19
]
],
"date-time": "2021-01-19T10:01:45Z",
"timestamp": 1611050505000
},
"deposited": {
"date-parts": [
[
2021,
2,
26
]
],
"date-time": "2021-02-26T11:22:48Z",
"timestamp": 1614338568000
},
"funder": [
{
"DOI": "10.13039/100017168",
"doi-asserted-by": "publisher",
"name": "Barcelona Institute of Science and Technology"
}
],
"indexed": {
"date-parts": [
[
2024,
4,
8
]
],
"date-time": "2024-04-08T17:21:13Z",
"timestamp": 1712596873136
},
"is-referenced-by-count": 135,
"issued": {
"date-parts": [
[
2021,
2
]
]
},
"language": "en",
"license": [
{
"URL": "https://www.elsevier.com/tdm/userlicense/1.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
2,
1
]
],
"date-time": "2021-02-01T00:00:00Z",
"timestamp": 1612137600000
}
},
{
"URL": "http://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2020,
12,
30
]
],
"date-time": "2020-12-30T00:00:00Z",
"timestamp": 1609286400000
}
}
],
"link": [
{
"URL": "https://api.elsevier.com/content/article/PII:S2589537020304648?httpAccept=text/xml",
"content-type": "text/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://api.elsevier.com/content/article/PII:S2589537020304648?httpAccept=text/plain",
"content-type": "text/plain",
"content-version": "vor",
"intended-application": "text-mining"
}
],
"member": "78",
"original-title": [],
"page": "100720",
"prefix": "10.1016",
"published": {
"date-parts": [
[
2021,
2
]
]
},
"published-print": {
"date-parts": [
[
2021,
2
]
]
},
"publisher": "Elsevier BV",
"reference": [
{
"key": "10.1016/j.eclinm.2020.100720_bib0001",
"unstructured": "WHO. COVID-19 Weekly epidemiological update - 15 December 2020. Available at https://www.who.int/publications/m/item/weekly-epidemiological-update—15-december-2020 (Accessed December 21, 2020)."
},
{
"article-title": "Difficult to determine herd immunity threshold for COVID-19",
"author": "Rubin",
"first-page": "732",
"issue": "8",
"journal-title": "JAMA",
"key": "10.1016/j.eclinm.2020.100720_bib0002",
"volume": "324",
"year": "2020"
},
{
"DOI": "10.1038/s41577-020-00451-5",
"article-title": "COVID-19 herd immunity: where are we?",
"author": "Fontanet",
"doi-asserted-by": "crossref",
"first-page": "583",
"issue": "10",
"journal-title": "Nat Rev Immunol",
"key": "10.1016/j.eclinm.2020.100720_bib0003",
"volume": "20",
"year": "2020"
},
{
"key": "10.1016/j.eclinm.2020.100720_bib0004",
"unstructured": "WHO. Newsroom - coronavirus disease (COVID-19): vaccines. Available at https://www.who.int/news-room/q-a-detail/coronavirus-disease-(covid-19)-vaccines (accessed December 21, 2020)."
},
{
"DOI": "10.1042/BJ20120150",
"article-title": "Ivermectin is a specific inhibitor of importin alpha/beta-mediated nuclear import able to inhibit replication of HIV-1 and dengue virus",
"author": "Wagstaff",
"doi-asserted-by": "crossref",
"first-page": "851",
"issue": "3",
"journal-title": "Biochem J",
"key": "10.1016/j.eclinm.2020.100720_bib0005",
"volume": "443",
"year": "2012"
},
{
"DOI": "10.1016/j.chom.2016.07.004",
"article-title": "A screen of FDA-approved drugs for inhibitors of Zika virus infection",
"author": "Barrows",
"doi-asserted-by": "crossref",
"first-page": "259",
"issue": "2",
"journal-title": "Cell Host Microbe",
"key": "10.1016/j.eclinm.2020.100720_bib0006",
"volume": "20",
"year": "2016"
},
{
"DOI": "10.1093/jac/dks147",
"article-title": "Ivermectin is a potent inhibitor of flavivirus replication specifically targeting NS3 helicase activity: new prospects for an old drug",
"author": "Mastrangelo",
"doi-asserted-by": "crossref",
"first-page": "1884",
"issue": "8",
"journal-title": "J Antimicrob Chemother",
"key": "10.1016/j.eclinm.2020.100720_bib0007",
"volume": "67",
"year": "2012"
},
{
"DOI": "10.1016/j.antiviral.2020.104787",
"article-title": "The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro",
"author": "Caly",
"doi-asserted-by": "crossref",
"journal-title": "Antiviral Res",
"key": "10.1016/j.eclinm.2020.100720_bib0008",
"volume": "178",
"year": "2020"
},
{
"key": "10.1016/j.eclinm.2020.100720_bib0009",
"unstructured": "Chaccour C, Brew J, Garcia-Basteiro A. Ivermectin and COVID-19: how a flawed database shaped the pandemic response of several Latin-American countries. Available at: https://www.isglobal.org/en/healthisglobal/-/custom-blog-portlet/ivermectin-and-covid-19-how-a-flawed-database-shaped-the-covid-19-response-of-several-latin-american-countries/2877257/0 (Accessed Nov 2020)."
},
{
"key": "10.1016/j.eclinm.2020.100720_bib0010",
"unstructured": "Clinical trial of ivermectin plus doxycycline for the treatment of confirmed COVID-19 infection. NCT04523831. Available at: https://clinicaltrials.gov/ct2/show/results/NCT04523831 (accessed Nov 2020)."
},
{
"article-title": "A five day course of ivermectin for the treatment of COVID-19 may reduce the duration of illness",
"author": "Ahmed",
"journal-title": "Int J Infect Dis",
"key": "10.1016/j.eclinm.2020.100720_bib0011",
"year": "2020"
},
{
"DOI": "10.2139/ssrn.3714649",
"doi-asserted-by": "crossref",
"key": "10.1016/j.eclinm.2020.100720_bib0012",
"unstructured": "Krolewiecki A, Lifschitz A, Moragas M, et al. Antiviral effect of high-dose ivermectin in adults with COVID-19: a pilot randomised, controlled, open label, multicentre trial. http://dx.doi.org/10.2139/ssrn.3714649 (accessed Nov 2020)."
},
{
"article-title": "Use of ivermectin is associated with lower mortality in hospitalized patients with coronavirus disease 2019: the ICON study",
"author": "Rajter",
"journal-title": "Chest",
"key": "10.1016/j.eclinm.2020.100720_bib0013",
"year": "2020"
},
{
"DOI": "10.1186/s13063-020-04421-z",
"author": "Chaccour",
"doi-asserted-by": "crossref",
"first-page": "498",
"issue": "1",
"journal-title": "Trials",
"key": "10.1016/j.eclinm.2020.100720_bib0014",
"volume": "21",
"year": "2020"
},
{
"key": "10.1016/j.eclinm.2020.100720_bib0015",
"unstructured": "European_Medicines_Agency. EMA decision of 18 December 2012 on the granting of a product-specific waiver for ivermectin. http://www.ema.europa.eu/docs/en_GB/document_library/PIP_decision/WC500138600.pdf (accessed july 2015)."
},
{
"DOI": "10.1093/cid/ciaa1249",
"article-title": "Duration of SARS-CoV-2 infectivity: when is it safe to discontinue isolation?",
"author": "Rhee",
"doi-asserted-by": "crossref",
"journal-title": "Clin Infect Dis",
"key": "10.1016/j.eclinm.2020.100720_bib0016",
"year": "2020"
},
{
"article-title": "SARS-CoV-2 viral load peaks prior to symptom onset: a systematic review and individual-pooled analysis of coronavirus viral load from 66 studies",
"author": "Benefield",
"journal-title": "medRxiv",
"key": "10.1016/j.eclinm.2020.100720_bib0017",
"year": "2020"
},
{
"article-title": "Temporal dynamics in viral shedding and transmissibility of COVID-19",
"author": "He",
"journal-title": "Nat Med",
"key": "10.1016/j.eclinm.2020.100720_bib0018",
"year": "2020"
},
{
"DOI": "10.7326/M20-0504",
"article-title": "The incubation period of coronavirus disease 2019 (COVID-19) from publicly reported confirmed cases: estimation and application",
"author": "Lauer",
"doi-asserted-by": "crossref",
"first-page": "577",
"issue": "9",
"journal-title": "Ann Intern Med",
"key": "10.1016/j.eclinm.2020.100720_bib0019",
"volume": "172",
"year": "2020"
},
{
"DOI": "10.1038/ijo.2011.100",
"article-title": "Body mass index classification misses subjects with increased cardiometabolic risk factors related to elevated adiposity",
"author": "Gomez-Ambrosi",
"doi-asserted-by": "crossref",
"first-page": "286",
"issue": "2",
"journal-title": "Int J Obes (Lond)",
"key": "10.1016/j.eclinm.2020.100720_bib0020",
"volume": "36",
"year": "2012"
},
{
"DOI": "10.1016/j.antiviral.2020.104805",
"article-title": "Ivermectin and COVID-19: a report in antiviral research, widespread interest, an FDA warning, two letters to the editor and the authors' responses",
"author": "Bray",
"doi-asserted-by": "crossref",
"journal-title": "Antiviral Res",
"key": "10.1016/j.eclinm.2020.100720_bib0021",
"year": "2020"
},
{
"DOI": "10.1002/cpt.1909",
"article-title": "Prioritization of anti-SARS-Cov-2 drug repurposing opportunities based on plasma and target site concentrations derived from their established human pharmacokinetics",
"author": "Arshad",
"doi-asserted-by": "crossref",
"first-page": "775",
"issue": "4",
"journal-title": "Clin Pharmacol Ther",
"key": "10.1016/j.eclinm.2020.100720_bib0022",
"volume": "108",
"year": "2020"
},
{
"DOI": "10.1038/s41586-020-2575-3",
"article-title": "Chloroquine does not inhibit infection of human lung cells with SARS-CoV-2",
"author": "Hoffmann",
"doi-asserted-by": "crossref",
"first-page": "588",
"issue": "7826",
"journal-title": "Nature",
"key": "10.1016/j.eclinm.2020.100720_bib0023",
"volume": "585",
"year": "2020"
},
{
"article-title": "Anti-COVID-19 efficacy of ivermectin in the golden hamster",
"author": "de Melo",
"journal-title": "bioRxiv",
"key": "10.1016/j.eclinm.2020.100720_bib0024",
"year": "2020"
},
{
"DOI": "10.1371/journal.pone.0241104",
"article-title": "Serum-IgG responses to SARS-CoV-2 after mild and severe COVID-19 infection and analysis of IgG non-responders",
"author": "Marklund",
"doi-asserted-by": "crossref",
"issue": "10",
"journal-title": "PLoS One",
"key": "10.1016/j.eclinm.2020.100720_bib0025",
"volume": "15",
"year": "2020"
},
{
"DOI": "10.1126/sciimmunol.abe0240",
"article-title": "Defining the features and duration of antibody responses to SARS-CoV-2 infection associated with disease severity and outcome",
"author": "Röltgen",
"doi-asserted-by": "crossref",
"first-page": "eabe0240",
"issue": "54",
"journal-title": "Sci Immunol",
"key": "10.1016/j.eclinm.2020.100720_bib0026",
"volume": "5",
"year": "2020"
},
{
"article-title": "A nicotinic hypothesis for Covid-19 with preventive and therapeutic implications",
"author": "Changeux",
"first-page": "33",
"issue": "1",
"journal-title": "C R Biol",
"key": "10.1016/j.eclinm.2020.100720_bib0027",
"volume": "343",
"year": "2020"
},
{
"DOI": "10.1021/acschemneuro.0c00249",
"article-title": "Proinflammatory cytokines in the olfactory mucosa result in COVID-19 induced anosmia",
"author": "Torabi",
"doi-asserted-by": "crossref",
"first-page": "1909",
"issue": "13",
"journal-title": "ACS Chem Neurosci",
"key": "10.1016/j.eclinm.2020.100720_bib0028",
"volume": "11",
"year": "2020"
},
{
"DOI": "10.1111/jdv.14437",
"article-title": "Dual anti-inflammatory and anti-parasitic action of topical ivermectin 1% in papulopustular rosacea",
"author": "Schaller",
"doi-asserted-by": "crossref",
"first-page": "1907",
"issue": "11",
"journal-title": "J Eur Acad Dermatol Venereol",
"key": "10.1016/j.eclinm.2020.100720_bib0029",
"volume": "31",
"year": "2017"
},
{
"DOI": "10.1007/s13555-017-0176-3",
"article-title": "Topical treatment of rosacea with ivermectin inhibits gene expression of cathelicidin innate immune mediators, LL-37 and KLK5, in reconstructed and ex vivo skin models",
"author": "Thibaut de Ménonville",
"doi-asserted-by": "crossref",
"first-page": "213",
"issue": "2",
"journal-title": "Dermatol Ther (Heidelb)",
"key": "10.1016/j.eclinm.2020.100720_bib0030",
"volume": "7",
"year": "2017"
},
{
"DOI": "10.4049/jimmunol.1302005",
"article-title": "Little peptide, big effects: the role of LL-37 in inflammation and autoimmune disease",
"author": "Kahlenberg",
"doi-asserted-by": "crossref",
"first-page": "4895",
"issue": "10",
"journal-title": "J Immunol",
"key": "10.1016/j.eclinm.2020.100720_bib0031",
"volume": "191",
"year": "2013"
},
{
"DOI": "10.1016/j.jaad.2015.02.705",
"article-title": "MeTC7, a novel vitamin D receptor (VDR) antagonist, induces cytotoxicity in metastatic melanoma cell lines and inhibits importin-mediated VDR nuclear transport and signaling",
"author": "Han",
"doi-asserted-by": "crossref",
"first-page": "AB172",
"issue": "5",
"journal-title": "J Am Acad Dermatol",
"key": "10.1016/j.eclinm.2020.100720_bib0032",
"volume": "72",
"year": "2015"
},
{
"DOI": "10.1042/BJ20120150",
"article-title": "Ivermectin is a specific inhibitor of importin α/β-mediated nuclear import able to inhibit replication of HIV-1 and dengue virus",
"author": "Wagstaff",
"doi-asserted-by": "crossref",
"first-page": "851",
"issue": "3",
"journal-title": "Biochem J",
"key": "10.1016/j.eclinm.2020.100720_bib0033",
"volume": "443",
"year": "2012"
},
{
"DOI": "10.1074/jbc.M509347200",
"article-title": "Importin 4 is responsible for ligand-independent nuclear translocation of vitamin D receptor",
"author": "Miyauchi",
"doi-asserted-by": "crossref",
"first-page": "40901",
"issue": "49",
"journal-title": "J Biol Chem",
"key": "10.1016/j.eclinm.2020.100720_bib0034",
"volume": "280",
"year": "2005"
},
{
"DOI": "10.2217/fmb.09.87",
"article-title": "The vitamin D-antimicrobial peptide pathway and its role in protection against infection",
"author": "Gombart",
"doi-asserted-by": "crossref",
"first-page": "1151",
"issue": "9",
"journal-title": "Fut Microbiol",
"key": "10.1016/j.eclinm.2020.100720_bib0035",
"volume": "4",
"year": "2009"
},
{
"DOI": "10.1038/s41598-020-74084-y",
"article-title": "Nebulized ivermectin for COVID-19 and other respiratory diseases, a proof of concept, dose-ranging study in rats",
"author": "Chaccour",
"doi-asserted-by": "crossref",
"first-page": "17073",
"issue": "1",
"journal-title": "Sci Rep",
"key": "10.1016/j.eclinm.2020.100720_bib0036",
"volume": "10",
"year": "2020"
},
{
"DOI": "10.1016/S1473-3099(20)30232-2",
"article-title": "Viral dynamics in mild and severe cases of COVID-19",
"author": "Liu",
"doi-asserted-by": "crossref",
"first-page": "656",
"issue": "6",
"journal-title": "Lancet Infect Dis",
"key": "10.1016/j.eclinm.2020.100720_bib0037",
"volume": "20",
"year": "2020"
},
{
"article-title": "SARS-CoV-2, SARS-CoV, and MERS-CoV viral load dynamics, duration of viral shedding, and infectiousness: a systematic review and meta-analysis",
"author": "Cevik",
"journal-title": "Lancet Microbe",
"key": "10.1016/j.eclinm.2020.100720_bib0038",
"year": "2020"
},
{
"DOI": "10.1016/j.jinf.2020.10.017",
"article-title": "Ct values from SARS-CoV-2 diagnostic PCR assays should not be used as direct estimates of viral load",
"author": "Dahdouh",
"doi-asserted-by": "crossref",
"journal-title": "J Infect",
"key": "10.1016/j.eclinm.2020.100720_bib0039",
"year": "2020"
}
],
"reference-count": 39,
"references-count": 39,
"relation": {
"has-preprint": [
{
"asserted-by": "object",
"id": "10.21203/rs.3.rs-116547/v1",
"id-type": "doi"
}
]
},
"resource": {
"primary": {
"URL": "https://linkinghub.elsevier.com/retrieve/pii/S2589537020304648"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"General Medicine"
],
"subtitle": [],
"title": "The effect of early treatment with ivermectin on viral load, symptoms and humoral response in patients with non-severe COVID-19: A pilot, double-blind, placebo-controlled, randomized clinical trial",
"type": "journal-article",
"volume": "32"
}