Systematic review and meta-analysis of ivermectin for treatment of COVID-19: evidence beyond the hype
Milena Soriano Marcolino, Karina Cardoso Meira, Nathalia Sernizon Guimarães, Paula Perdigão Motta, Victor Schulthais Chagas, Silvana Márcia Bruschi Kelles, Laura Caetano De Sá, Reginaldo Aparecido Valacio, Patrícia Klarmann Ziegelmann
BMC Infectious Diseases, doi:10.1186/s12879-022-07589-8
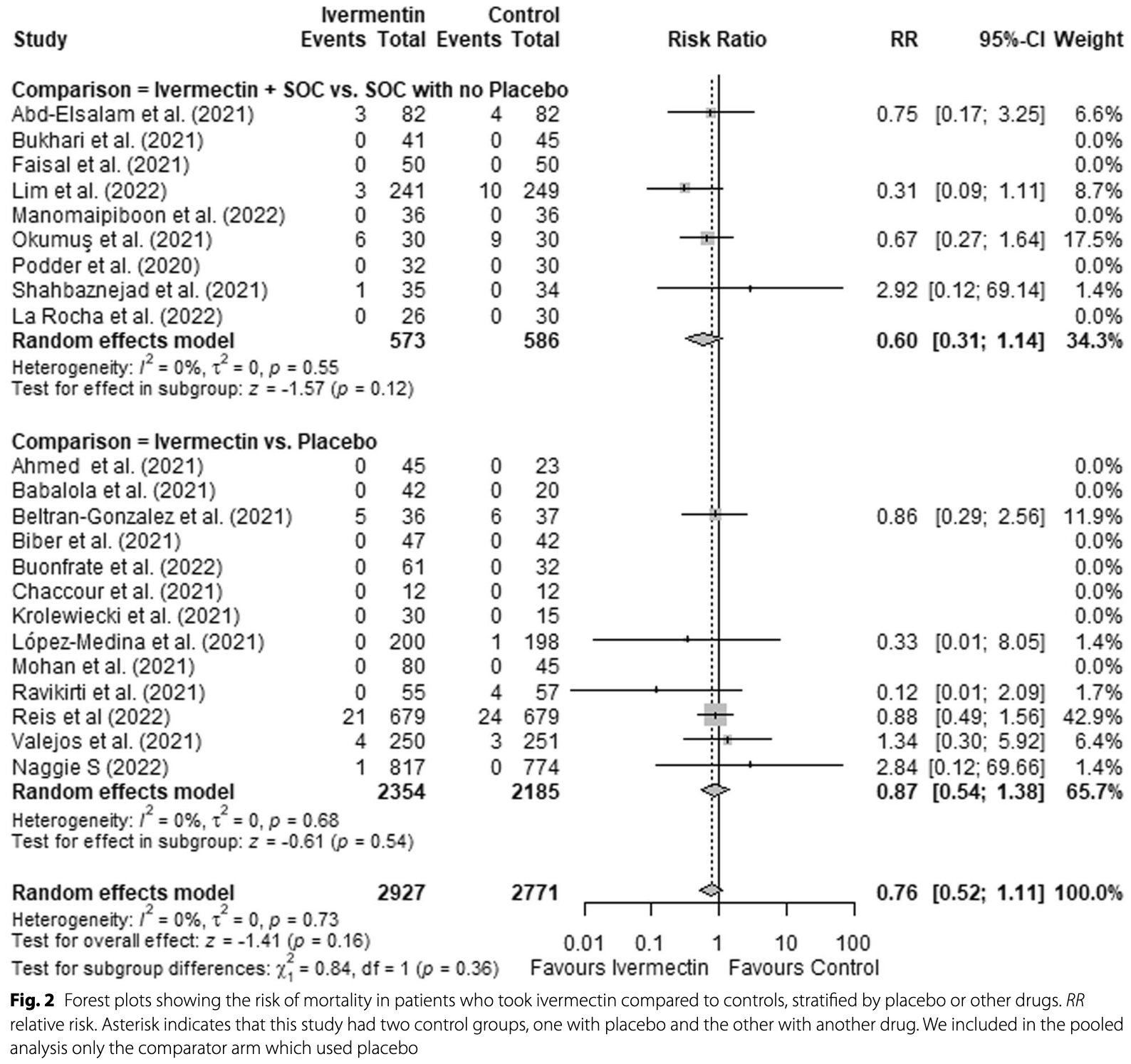
Background: The role of ivermectin in the treatment of COVID-19 is still under debate, yet the drug has been widely used in some parts of the world, as shown by impressive market data. The available body of evidence may have changed over the last months, as studies have been retracted and "standards of care" (SOC) used in control groups have changed with rapidly evolving knowledge on COVID-19. This review aims to summarize and critically appraise the evidence of randomized controlled trials (RCTs) of ivermectin, assessing clinical outcomes in COVID-19 patients. Methods: RCTs evaluating the effects of ivermectin in adult patients with COVID-19 were searched through June 22, 2022, in four databases, L.OVE platform, clinical trial registries and pre-prints platforms. Primary endpoints included allcause mortality and invasive ventilation requirement. Secondary endpoint was the occurrence of adverse events. Risk of bias was evaluated using the Cochrane Risk of Bias 2.0 tool. Meta-analysis included only studies which compared ivermectin to placebo or SOC. Random-effects were used to pool the risk ratios (RRs) of individual trials. The quality of evidence was evaluated using GRADE. The protocol was register in PROSPERO (CRD42021257471). Results: Twenty-five RCTs fulfilled inclusion criteria (n = 6310). Of those, 14 compared ivermectin with placebo, in night ivermectin associated with SOC was compared to SOC and two studies compared ivermectin to an active comparator. Most RCTs had some concerns or high risk of bias, mostly due to lack of concealment of the randomization sequence and allocation, lack of blinding and high number of missing cases. Ivermectin did not show an effect in reducing mortality (RR = 0.76; 95%CI: 0.52-1.11) or mechanical ventilation (RR = 0.74; 95%CI: 0.48-1.16). This effect was consistent when comparing ivermectin vs. placebo, and ivermectin associated with SOC vs. SOC, as well as in sensitivity analysis. Additionally, there was very low quality of evidence regarding adverse effects (RR = 1.07; 95%CI: 0.84-1.35).
Conclusions: The evidence suggests that ivermectin does not reduce mortality risk and the risk of mechanical ventilation requirement. Although we did not observe an increase in the risk of adverse effects, the evidence is very uncertain regarding this endpoint.
Abbreviations
Supplementary Information The online version contains supplementary material available at https:// doi. org/ 10. 1186/ s12879-022-07589-8.
Additional
Author contributions Substantial contributions to the conception or design of the work: MSM and NSG. Substantial contributions to the acquisition, analysis, or interpretation of data for the work: MSM, NSG, KCM, VSC, LCS, SMBK, RAV, PKZ, PPM. Drafted the manuscript: MSM, KCM, VSC, NSG. Revised the manuscript critically for important intellectual content: MSM, NSG, KCM, VSC, LCS, SMBK, RAV, PKZ, PPM. Final approval of the version to be published: MSM, NSG, KCM, VSC, LCS, SMBK, RAV, PKZ, PPM. Agreement to be accountable for the author's own contributions and to ensure that questions related to the accuracy or integrity of any part of the work, even ones in which the author was not personally involved, are appropriately investigated, resolved, and the resolution documented in the literature: MSM, NSG, KCM, VSC, LCS, SMBK, RAV, PKZ, PPM. All authors read and approved he final manuscript.
Declarations Ethics approval and consent to participate Not applicable, as this is a systematic review.
Consent for publication Not applicable.
Competing interests The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article. • fast, convenient online submission • thorough peer review by experienced researchers in your field • rapid publication on..
References
Abd-Elsalam, Noor, Badawi, Clinical study evaluating the efficacy of ivermectin in COVID-19 treatment: a randomized controlled study, J Med Virol,
doi:10.1002/jmv.27122Ahmed, Karim, Ross, A five-day course of ivermectin for the treatment of COVID-19 may reduce the duration of illness, Int J Infect Dis
Araya, Kraemer, Burdiles, Herrera, Castillo et al., The living overview of evidence database (LOVE) may be more efficient than a traditional search of systematic reviews and randomized trials
Axfors, Schmitt, Janiaud, Mortality outcomes with hydroxychloroquine and chloroquine in COVID-19 from an international collaborative meta-analysis of randomized trials, Nat Commun,
doi:10.1038/s41467-021-22446-zBabalola, Bode, Ajayi, Alakaloko, Akase et al., Ivermectin shows clinical benefits in mild to moderate COVID19: a randomized controlled double-blind, dose-response study in Lagos, QJM,
doi:10.1093/qjmed/hcab035Bhimraj, Morgan, Shumaker, Infectious diseases Society of America guidelines on the treatment and management of patients with COVID-19, Clin Infect Dis,
doi:10.1093/cid/ciaa478Biber, Mandelboim, Harmelin, Favorable outcome on viral load and culture viability using Ivermectin in early treatment of nonhospitalized patients with mild COVID-19-a double-blind, randomized placebo-controlled trial,
doi:10.1101/2021.05.31.21258081v1Bryant, Lawrie, Dowswell, Ivermectin for prevention and treatment of COVID-19 infection: a systematic review, meta-analysis, and trial sequential analysis to inform clinical guidelines, Am J Ther,
doi:10.1097/MJT.0000000000001402Buonfrate, Chesini, Martini, Roncaglioni, Fernandez et al., High-dose ivermectin for early treatment of COVID-19 (COVER study): a randomised, double-blind, multicentre, phase II, dosefinding, proof-of-concept clinical trial, Int J Antimicrob Agents,
doi:10.1016/j.ijantimicag.2021.106516Caly, Druce, Catton, The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro, Antiviral Res Elsevier
Campillo, Boussinesq, Bertout, Faillie, Chesnais, Serious adverse reactions associated with ivermectin: a systematic pharmacovigilance study in sub-Saharan Africa and in the rest of the World, PLoS Negl Trop Dis,
doi:10.1371/journal.pntd.0009354Chaccour, Casellas, Blanco-Di Matteo, The effect of early treatment with ivermectin on viral load, symptoms and humoral response in patients with non-severe COVID-19: a pilot, double-blind, placebocontrolled, randomized clinical trial, EClinicalMedicine,
doi:10.1016/j.eclinm.2020.100720Chachar, Khan, Asif, Tanveer, Khaqan et al., Effectiveness of ivermectin in SARS-COV-2/COVID-10 patients, Intern J Sci,
doi:10.18483/ijSci.2378Chowdhury, Shahbaz, Karim, Islam, Dan et al., A comparative study on ivermectin-doxycycline and hydroxychloroquineazithromycin therapy on COVID-19 patients, Eurasian J Med Oncol
Cochrane, None
Communit, Living systematic reviews
Elgazzar, Eltaweel, Youssef, Efficacy and safety of ivermectin for treatment and prophylaxis of COVID-19 pandemic, Res,
doi:10.21203/rs.3.rs-100956/v3Faisal, Shah, Hussain, Potential use of azithromycin alone and in combination with ivermectin in fighting against the symptoms of COVID-19, Professional Med J,
doi:10.29309/TPMJ/2021.28.05.5867Furlan, Caramelli, The regrettable story of the "Covid Kit" and the "Early Treatment of Covid-19, Lancet Reg Health Am,
doi:10.1016/j.lana.2021.100089Galan, Santos, Asato, Phase 2 randomized study on chloroquine, hydroxychloroquine or ivermectin in hospitalized patients with severe manifestations of SARS-CoV-2 infection, Pathog Glob Health
George, Moorthy, Kulkarni, Single dose of ivermectin is not useful in patients with hematological disorders and COVID-19 illness: a phase II B open labelled randomized controlled trial, Indian J Hematol Blood Transfus
Ghazy, Almaghraby, Shaaban, A systematic review and meta-analysis on chloroquine and hydroxychloroquine as monotherapy or combined with azithromycin in COVID-19 treatment, Sci Rep,
doi:10.1038/s41598-020-77748-xGonzalez, Gámez, Enciso, Maldonado, Palacios et al., Efficacy and safety of ivermectin and hydroxychloroquine in patients with severe COVID-19: a randomized controlled trial, Infect Dis Rep,
doi:10.3390/idr14020020Hazan, Gunaratne, Dolai, Clancy, Mccullough et al., Effectiveness of ivermectin-based multidrug therapy in severely hypoxic, ambulatory COVID-19 patients, Future Microbiol,
doi:10.2217/fmb-2022-0014Heidary, Gharebaghi, Ivermectin: a systematic review from antiviral effects to COVID-19 complementary regimen, J Antibiot,
doi:10.1038/s41429-020-0336-zHiggins, Lasserson, Chandler, Standards for the conduct and reporting of new Cochrane Intervention Reviews, reporting of protocols and the planning, conduct and reporting of updates
Higgins, Thomas, Chandler, Cochrane Handbook for Systematic Reviews of Interventions version
Hill, Garratt, Levi, Meta-analysis of randomized trials of ivermectin to treat SARS-CoV-2 infection, Open Forum Infect Dis,
doi:10.1093/ofid/ofab358Izcovich, Peiris, Ragusa, Tortosa, Rada et al., Bias as a source of inconsistency in ivermectin trials for COVID-19: a systematic review. Ivermectin's suggested benefits are mainly based on potentially biased results, J Clin Epidemiol
Kinobe, Owens, A systematic review of experimental evidence for antiviral effects of ivermectin and an in silico analysis of ivermectin's possible mode of action against SARS-CoV-2, Fundam Clin Pharmacol,
doi:10.1111/fcp.12644Kishoria, Mathur, Parmar, Ivermectin as adjuvant to hydroxychloroquine in patients resistant to standard treatment for SARS-CoV-2: results of an open-label randomized clinical study, Paripex Indian J Res
Krolewiecki, Lifschitz, Moragas, Travacio, Valentini et al., Corrigendum to antiviral effect of high-dose ivermectin in adults with COVID-19: a proof-of-concept randomized trial, EClinicalMedicine,
doi:10.1016/j.eclinm.2021.101119Lawrence, Meyerowitz-Katz, Heathers, The lesson of ivermectin: meta-analyses based on summary data alone are inherently unreliable, Nat Med,
doi:10.1038/s41591-021-01535-yLehrer, Rheinstein, Ivermectin docks to the SARS-CoV-2 spike receptor-binding domain attached to ACE2, Vivo,
doi:10.21873/invivo.12134Lim, Hor, Tay, Jelani, Tan et al., Efficacy of ivermectin treatment on disease progression among adults with mild to moderate COVID-19 and comorbidities: the I-TECH randomized clinical trial, JAMA Intern Med
López-Medina, López, Hurtado, Effect of ivermectin on time to resolution of symptoms among adults with mild COVID-19: a randomized clinical trial, JAMA,
doi:10.1001/jama.2021.3071Manomaipiboon, Pholtawornkulchai, Pupipatpab, Suraamornkul, Maneerit et al., COVID-19 infection: a randomized, double blind, placebo, controlled trial,
doi:10.21203/rs.3.rs-1290999/v1Meneses, Commentary about open-label randomized controlled study of ivermectin in mild to moderate COVID-19, IMC J Med Sci
Mohan, Tiwari, Suri, Single-dose oral ivermectin in mild and moderate COVID-19 (RIVET-COV): a single-centre randomized, placebocontrolled trial, J Infect Chemother
Niaee, Namdar, Allami, Ivermectin as an adjunct treatment for hospitalized adult COVID-19 patients: a randomized multi-center clinical trial, Asian Pac J Trop Med
Okumuş, Demirtürk, Çetinkaya, Evaluation of the effectiveness and safety of adding ivermectin to treatment in severe COVID-19 patients, BMC Infect Dis,
doi:10.1186/s12879-021-06104-9Page, Mckenzie, Bossuyt, Boutron, Hoffmann et al., The PRISMA 2020 statement: an updated guideline for reporting systematic reviews, BMJ,
doi:10.1136/bmj.n71Podder, Chowdhury, Sina, Haque, Outcome of ivermectin treated mild to moderate COVID-19 cases: a single-centre, open-label, randomised controlled study, IMC J Med Sci
Popp, Stegemann, Metzendorf, Gould, Kranke et al., Ivermectin for preventing and treating COVID-19, Cochrane Database Syst Rev,
doi:10.1002/14651858.CD015017Porta, Bornstein, Coye, Acute chloroquine and hydroxychloroquine toxicity: a review for emergency clinicians, Am J Emerg Med,
doi:10.1016/j.ajem.2020.07.030Prokop, Van Everdingen, Van Rees, Vt, COVID-19 Standardized Reporting Working Group of the Dutch Radiological Society. CO-RADS: a categorical CT assessment scheme for patients suspected of having COVID-19-definition and evaluation, Radiology
Quincho-Lopez, Benites-Ibarra, Gomez, Quijano-Escate, Taype-Rondan, Self-medication practices to prevent or manage COVID-19: a systematic review, PLoS ONE,
doi:10.1371/journal.pone.0259317Ravikirti, Pattadar, Raj, Agarwal, Biswas et al., Evaluation of ivermectin as a potential treatment for mild to moderate COVID-19: a double-blind randomized placebo controlled trial in Eastern India, J Pharm Pharm Sci
Reis, Silva, Silva, Thabane, Effect of early treatment with ivermectin among patients with COVID-19, N Engl J Med,
doi:10.1056/NEJMoa2115869Rocha, Cid-Lopez, Venegas-Lopez, Gómez-Mendes, Sánches-Ortiz, Ivermectin compared with placebo in the clinical evolution of Mexican patients with asymptomatic and mild COVID-19: a randomized clinical trial, Res Square,
doi:10.21203/rs.3.rs-1640339/v1Samaha, Mouawia, Fawaz, Effects of a single dose of ivermectin on viral and clinical outcomes in asymptomatic SARS-CoV-2 infected subjects: a pilot clinical trial in Lebanon, Viruses,
doi:10.3390/v13112154Schmith, Zhou, Lohmer, The Approved dose of ivermectin alone is not the ideal dose for the treatment of COVID-19, Am Soc Clin Pharmacol Thera,
doi:10.1002/cpt.1889Schünemann, Vist, Higgins, Chapter 15: Interpreting results and drawing conclusions
Seet, Quek, Ooi, Positive impact of oral hydroxychloroquine and povidone-iodine throat spray for COVID-19 prophylaxis: an open-label randomized trial, Int J Infect Dis,
doi:10.1016/j.ijid.2021.04.035Senado, Senado Notícias. Fabricante de ivermectina lucrou à custa de vidas, acusam senadores da CPI
Shahbaznejad, Davoudi, Eslami, Effects of ivermectin in patients with COVID-19: a multicenter, double-blind, randomized controlled clinical trial, Clin Thera
Shouman, Hegazy, Nafae, Use of ivermectin as a potential chemoprophylaxis for COVID-19 in Egypt: a randomised clinical trial, J Clin Diagn Res,
doi:10.7860/JCDR/2020/46795.0000Siemieniuk, Bartoszko, Ge, Drug treatments for covid-19: living systematic review and network meta-analysis, BMJ,
doi:10.1136/bmj.m2980Skipper, Pastick, Engen, Hydroxychloroquine in nonhospitalized adults with early COVID-19: a randomized trial, Ann Intern Med,
doi:10.7326/M20-4207Stefany, Almeida, Fonseca Almeida ; Burki, group which conducted data extraction; and Professor Maria Auxiliadora Parreiras Martins,
doi:10.1016/S2589-7500(20)30227-2Sterne, Savović, Page, RoB 2: a revised tool for assessing risk of bias in randomized trials, BMJ,
doi:10.1136/bmj.l4898Temple, Hoang, Hendrickson, Toxic effects from ivermectin use associated with prevention and treatment, N Engl J Med,
doi:10.1056/NEJMc2114907Vallejos, Zoni, Bangher, Ivermectin to prevent hospitalizations in patients with COVID-19 (IVERCOR-COVID19) a randomized, double-blind, placebo-controlled trial, BMC Infect Dis,
doi:10.1186/s12879-021-06348-5Zhang, Wen, Yin, Efficacy of COVID-19 treatments: a Bayesian network meta-analysis of randomized controlled trials, Front Public Health,
doi:10.3389/fpubh.2021.729559DOI record:
{
"DOI": "10.1186/s12879-022-07589-8",
"ISSN": [
"1471-2334"
],
"URL": "http://dx.doi.org/10.1186/s12879-022-07589-8",
"abstract": "<jats:title>Abstract</jats:title><jats:sec>\n <jats:title>Background</jats:title>\n <jats:p>The role of ivermectin in the treatment of COVID-19 is still under debate, yet the drug has been widely used in some parts of the world, as shown by impressive market data. The available body of evidence may have changed over the last months, as studies have been retracted and “standards of care” (SOC) used in control groups have changed with rapidly evolving knowledge on COVID-19. This review aims to summarize and critically appraise the evidence of randomized controlled trials (RCTs) of ivermectin, assessing clinical outcomes in COVID-19 patients.</jats:p>\n </jats:sec><jats:sec>\n <jats:title>Methods</jats:title>\n <jats:p>RCTs evaluating the effects of ivermectin in adult patients with COVID-19 were searched through June 22, 2022, in four databases, L.OVE platform, clinical trial registries and pre-prints platforms. Primary endpoints included all-cause mortality and invasive ventilation requirement. Secondary endpoint was the occurrence of adverse events. Risk of bias was evaluated using the Cochrane Risk of Bias 2.0 tool. Meta-analysis included only studies which compared ivermectin to placebo or SOC. Random-effects were used to pool the risk ratios (RRs) of individual trials. The quality of evidence was evaluated using GRADE. The protocol was register in PROSPERO (CRD42021257471).</jats:p>\n </jats:sec><jats:sec>\n <jats:title>Results</jats:title>\n <jats:p>Twenty-five RCTs fulfilled inclusion criteria (n = 6310). Of those, 14 compared ivermectin with placebo, in night ivermectin associated with SOC was compared to SOC and two studies compared ivermectin to an active comparator. Most RCTs had some concerns or high risk of bias, mostly due to lack of concealment of the randomization sequence and allocation, lack of blinding and high number of missing cases. Ivermectin did not show an effect in reducing mortality (RR = 0.76; 95%CI: 0.52–1.11) or mechanical ventilation (RR = 0.74; 95%CI: 0.48–1.16). This effect was consistent when comparing ivermectin vs. placebo, and ivermectin associated with SOC vs. SOC, as well as in sensitivity analysis. Additionally, there was very low quality of evidence regarding adverse effects (RR = 1.07; 95%CI: 0.84–1.35).</jats:p>\n </jats:sec><jats:sec>\n <jats:title>Conclusions</jats:title>\n <jats:p>The evidence suggests that ivermectin does not reduce mortality risk and the risk of mechanical ventilation requirement. Although we did not observe an increase in the risk of adverse effects, the evidence is very uncertain regarding this endpoint.</jats:p>\n </jats:sec>",
"alternative-id": [
"7589"
],
"article-number": "639",
"assertion": [
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Received",
"name": "received",
"order": 1,
"value": "13 January 2022"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Accepted",
"name": "accepted",
"order": 2,
"value": "5 July 2022"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "First Online",
"name": "first_online",
"order": 3,
"value": "23 July 2022"
},
{
"group": {
"label": "Declarations",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 1
},
{
"group": {
"label": "Ethics approval and consent to participate",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 2,
"value": "Not applicable, as this is a systematic review."
},
{
"group": {
"label": "Consent for publication",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 3,
"value": "Not applicable<i>.</i>"
},
{
"group": {
"label": "Competing interests",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 4,
"value": "The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article."
}
],
"author": [
{
"ORCID": "http://orcid.org/0000-0003-4278-3771",
"affiliation": [],
"authenticated-orcid": false,
"family": "Marcolino",
"given": "Milena Soriano",
"sequence": "first"
},
{
"ORCID": "http://orcid.org/0000-0002-1722-5703",
"affiliation": [],
"authenticated-orcid": false,
"family": "Meira",
"given": "Karina Cardoso",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-0487-0500",
"affiliation": [],
"authenticated-orcid": false,
"family": "Guimarães",
"given": "Nathalia Sernizon",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-5757-7255",
"affiliation": [],
"authenticated-orcid": false,
"family": "Motta",
"given": "Paula Perdigão",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-9058-2803",
"affiliation": [],
"authenticated-orcid": false,
"family": "Chagas",
"given": "Victor Schulthais",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kelles",
"given": "Silvana Márcia Bruschi",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-0845-5814",
"affiliation": [],
"authenticated-orcid": false,
"family": "de Sá",
"given": "Laura Caetano",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-2666-7092",
"affiliation": [],
"authenticated-orcid": false,
"family": "Valacio",
"given": "Reginaldo Aparecido",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-2851-2011",
"affiliation": [],
"authenticated-orcid": false,
"family": "Ziegelmann",
"given": "Patrícia Klarmann",
"sequence": "additional"
}
],
"container-title": "BMC Infectious Diseases",
"container-title-short": "BMC Infect Dis",
"content-domain": {
"crossmark-restriction": false,
"domain": [
"link.springer.com"
]
},
"created": {
"date-parts": [
[
2022,
7,
23
]
],
"date-time": "2022-07-23T10:03:17Z",
"timestamp": 1658570597000
},
"deposited": {
"date-parts": [
[
2022,
7,
23
]
],
"date-time": "2022-07-23T10:04:21Z",
"timestamp": 1658570661000
},
"funder": [
{
"DOI": "10.13039/501100004901",
"award": [
"APQ-01154-21"
],
"doi-asserted-by": "publisher",
"name": "Fundação de Amparo à Pesquisa do Estado de Minas Gerais"
},
{
"DOI": "10.13039/501100003593",
"award": [
"310561/2021-3",
"465518/2014-1"
],
"doi-asserted-by": "publisher",
"name": "Conselho Nacional de Desenvolvimento Científico e Tecnológico"
}
],
"indexed": {
"date-parts": [
[
2022,
11,
30
]
],
"date-time": "2022-11-30T09:29:30Z",
"timestamp": 1669800570802
},
"is-referenced-by-count": 4,
"issue": "1",
"issued": {
"date-parts": [
[
2022,
7,
23
]
]
},
"journal-issue": {
"issue": "1",
"published-print": {
"date-parts": [
[
2022,
12
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
7,
23
]
],
"date-time": "2022-07-23T00:00:00Z",
"timestamp": 1658534400000
}
},
{
"URL": "https://creativecommons.org/licenses/by/4.0",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
7,
23
]
],
"date-time": "2022-07-23T00:00:00Z",
"timestamp": 1658534400000
}
}
],
"link": [
{
"URL": "https://link.springer.com/content/pdf/10.1186/s12879-022-07589-8.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/article/10.1186/s12879-022-07589-8/fulltext.html",
"content-type": "text/html",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/content/pdf/10.1186/s12879-022-07589-8.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "297",
"original-title": [],
"prefix": "10.1186",
"published": {
"date-parts": [
[
2022,
7,
23
]
]
},
"published-online": {
"date-parts": [
[
2022,
7,
23
]
]
},
"published-print": {
"date-parts": [
[
2022,
12
]
]
},
"publisher": "Springer Science and Business Media LLC",
"reference": [
{
"DOI": "10.1016/S2589-7500(20)30227-2",
"author": "T Burki",
"doi-asserted-by": "publisher",
"journal-title": "Lancet Digit Health",
"key": "7589_CR1",
"unstructured": "Burki T. The online anti-vaccine movement in the age of COVID-19. Lancet Digit Health. 2020. https://doi.org/10.1016/S2589-7500(20)30227-2.",
"year": "2020"
},
{
"key": "7589_CR2",
"unstructured": "World Health Organization. Tracking SARS-CoV-2 variants. World Health Organization International Website. 2021. https://www.who.int/en/activities/tracking-SARS-CoV-2-variants. Accessed 04 Jan 2022."
},
{
"DOI": "10.1136/bmjopen-2021-055781",
"author": "M Somerville",
"doi-asserted-by": "publisher",
"journal-title": "BMJ Open",
"key": "7589_CR3",
"unstructured": "Somerville M, Curran JA, Dol J, et al. Public health implications of SARS-CoV-2 variants of concern: a rapid scoping review. BMJ Open. 2021. https://doi.org/10.1136/bmjopen-2021-055781.",
"year": "2021"
},
{
"DOI": "10.1136/bmj.m3379",
"author": "A Agarwal",
"doi-asserted-by": "publisher",
"journal-title": "BMJ",
"key": "7589_CR4",
"unstructured": "Agarwal A, Rochwerg B, Lamontagne F, et al. A living WHO guideline on drugs for COVID-19. BMJ. 2020. https://doi.org/10.1136/bmj.m3379.",
"year": "2020"
},
{
"DOI": "10.1038/s41598-020-77748-x",
"doi-asserted-by": "publisher",
"key": "7589_CR5",
"unstructured": "Ghazy RM, Almaghraby A, Shaaban R et al. A systematic review and meta-analysis on chloroquine and hydroxychloroquine as monotherapy or combined with azithromycin in COVID-19 treatment. Sci Rep. 2020; 10. https://doi.org/10.1038/s41598-020-77748-x."
},
{
"DOI": "10.7326/M20-4207",
"author": "CP Skipper",
"doi-asserted-by": "publisher",
"journal-title": "Ann Intern Med",
"key": "7589_CR6",
"unstructured": "Skipper CP, Pastick KA, Engen NW, et al. Hydroxychloroquine in nonhospitalized adults with early COVID-19: a randomized trial. Ann Intern Med. 2021. https://doi.org/10.7326/M20-4207.",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2115869",
"author": "G Reis",
"doi-asserted-by": "publisher",
"journal-title": "N Engl J Med",
"key": "7589_CR7",
"unstructured": "Reis G, Silva EASM, Silva DCM, Thabane L, et al. Effect of early treatment with ivermectin among patients with COVID-19. N Engl J Med. 2022. https://doi.org/10.1056/NEJMoa2115869.",
"year": "2022"
},
{
"DOI": "10.1016/j.lana.2021.100089",
"author": "L Furlan",
"doi-asserted-by": "publisher",
"journal-title": "Lancet Reg Health Am",
"key": "7589_CR8",
"unstructured": "Furlan L, Caramelli B. The regrettable story of the “Covid Kit” and the “Early Treatment of Covid-19” in Brazil. Lancet Reg Health Am. 2021. https://doi.org/10.1016/j.lana.2021.100089.",
"year": "2021"
},
{
"DOI": "10.1038/d41586-020-02958-2",
"author": "ER Mega",
"doi-asserted-by": "publisher",
"journal-title": "Nature",
"key": "7589_CR9",
"unstructured": "Mega ER. Latin America’s embrace of an unproven COVID treatment is hindering drug trials. Nature. 2020. https://doi.org/10.1038/d41586-020-02958-2.",
"year": "2020"
},
{
"DOI": "10.1016/j.antiviral.2020.104787",
"author": "L Caly",
"doi-asserted-by": "publisher",
"first-page": "104787",
"journal-title": "Antiviral Res Elsevier",
"key": "7589_CR10",
"unstructured": "Caly L, Druce JD, Catton MG, et al. The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antiviral Res Elsevier. 2020;178:104787.",
"volume": "178",
"year": "2020"
},
{
"DOI": "10.1002/cpt.1889",
"author": "VD Schmith",
"doi-asserted-by": "publisher",
"journal-title": "Am Soc Clin Pharmacol Thera",
"key": "7589_CR11",
"unstructured": "Schmith VD, Zhou JJ, Lohmer LRL. The Approved dose of ivermectin alone is not the ideal dose for the treatment of COVID-19. Am Soc Clin Pharmacol Thera. 2020. https://doi.org/10.1002/cpt.1889.",
"year": "2020"
},
{
"DOI": "10.1111/fcp.12644",
"author": "RT Kinobe",
"doi-asserted-by": "publisher",
"journal-title": "Fundam Clin Pharmacol",
"key": "7589_CR12",
"unstructured": "Kinobe RT, Owens L. A systematic review of experimental evidence for antiviral effects of ivermectin and an in silico analysis of ivermectin’s possible mode of action against SARS-CoV-2. Fundam Clin Pharmacol. 2021. https://doi.org/10.1111/fcp.12644.",
"year": "2021"
},
{
"DOI": "10.21873/invivo.12134",
"author": "S Lehrer",
"doi-asserted-by": "publisher",
"journal-title": "In Vivo",
"key": "7589_CR13",
"unstructured": "Lehrer S, Rheinstein PH. Ivermectin docks to the SARS-CoV-2 spike receptor-binding domain attached to ACE2. In Vivo. 2020. https://doi.org/10.21873/invivo.12134.",
"year": "2020"
},
{
"DOI": "10.1038/s41429-020-0336-z",
"author": "F Heidary",
"doi-asserted-by": "publisher",
"journal-title": "J Antibiot",
"key": "7589_CR14",
"unstructured": "Heidary F, Gharebaghi R. Ivermectin: a systematic review from antiviral effects to COVID-19 complementary regimen. J Antibiot. 2020. https://doi.org/10.1038/s41429-020-0336-z.",
"year": "2020"
},
{
"DOI": "10.3389/fpubh.2021.729559",
"author": "C Zhang",
"doi-asserted-by": "publisher",
"journal-title": "Front Public Health",
"key": "7589_CR15",
"unstructured": "Zhang C, Jin H, Wen YF, Yin G. Efficacy of COVID-19 treatments: a Bayesian network meta-analysis of randomized controlled trials. Front Public Health. 2021. https://doi.org/10.3389/fpubh.2021.729559.",
"year": "2021"
},
{
"DOI": "10.1016/j.ijid.2020.11.191",
"author": "S Ahmed",
"doi-asserted-by": "publisher",
"first-page": "214",
"journal-title": "Int J Infect Dis",
"key": "7589_CR16",
"unstructured": "Ahmed S, Karim MM, Ross AG, et al. A five-day course of ivermectin for the treatment of COVID-19 may reduce the duration of illness. Int J Infect Dis. 2021;103:214–6.",
"volume": "103",
"year": "2021"
},
{
"DOI": "10.1093/ofid/ofab358",
"author": "A Hill",
"doi-asserted-by": "publisher",
"journal-title": "Open Forum Infect Dis",
"key": "7589_CR17",
"unstructured": "Hill A, Garratt A, Levi J, et al. Meta-analysis of randomized trials of ivermectin to treat SARS-CoV-2 infection. Open Forum Infect Dis. 2021. https://doi.org/10.1093/ofid/ofab358.",
"year": "2021"
},
{
"DOI": "10.1186/s12879-021-06348-5",
"author": "J Vallejos",
"doi-asserted-by": "publisher",
"journal-title": "BMC Infect Dis",
"key": "7589_CR18",
"unstructured": "Vallejos J, Zoni R, Bangher M, et al. Ivermectin to prevent hospitalizations in patients with COVID-19 (IVERCOR-COVID19) a randomized, double-blind, placebo-controlled trial. BMC Infect Dis. 2021. https://doi.org/10.1186/s12879-021-06348-5.",
"year": "2021"
},
{
"DOI": "10.1001/jama.2021.3071",
"author": "E López-Medina",
"doi-asserted-by": "publisher",
"journal-title": "JAMA",
"key": "7589_CR19",
"unstructured": "López-Medina E, López P, Hurtado IC, et al. Effect of ivermectin on time to resolution of symptoms among adults with mild COVID-19: a randomized clinical trial. JAMA. 2021. https://doi.org/10.1001/jama.2021.3071.",
"year": "2021"
},
{
"DOI": "10.1038/s41591-021-01535-y",
"doi-asserted-by": "publisher",
"key": "7589_CR20",
"unstructured": "Lawrence JM, Meyerowitz-Katz G, Heathers JAJ, et al. The lesson of ivermectin: meta-analyses based on summary data alone are inherently unreliable. Nat Med. 2021; 27. https://doi.org/10.1038/s41591-021-01535-y."
},
{
"DOI": "10.21203/rs.3.rs-100956/v3",
"author": "A Elgazzar",
"doi-asserted-by": "publisher",
"journal-title": "Res Square",
"key": "7589_CR21",
"unstructured": "Elgazzar A, Eltaweel A, Youssef S, et al. Efficacy and safety of ivermectin for treatment and prophylaxis of COVID-19 pandemic. Res Square. 2020. https://doi.org/10.21203/rs.3.rs-100956/v3.",
"year": "2020"
},
{
"DOI": "10.1038/d41586-021-02081-w",
"author": "S Reardon",
"doi-asserted-by": "publisher",
"journal-title": "Nature",
"key": "7589_CR22",
"unstructured": "Reardon S. Flawed ivermectin preprint highlights challenges of COVID drug studies. Nature. 2021. https://doi.org/10.1038/d41586-021-02081-w.",
"year": "2021"
},
{
"DOI": "10.3390/v13112154",
"author": "AA Samaha",
"doi-asserted-by": "publisher",
"journal-title": "Viruses",
"key": "7589_CR23",
"unstructured": "Samaha AA, Mouawia H, Fawaz M, et al. Effects of a single dose of ivermectin on viral and clinical outcomes in asymptomatic SARS-CoV-2 infected subjects: a pilot clinical trial in Lebanon. Viruses. 2021. https://doi.org/10.3390/v13112154.",
"year": "2021"
},
{
"DOI": "10.1016/j.toxrep.2021.03.003",
"author": "H Pott-Junior",
"doi-asserted-by": "publisher",
"journal-title": "Toxicol Rep",
"key": "7589_CR24",
"unstructured": "Pott-Junior H, Paoliello MMB, Miguel AQC, et al. Use of ivermectin in the treatment of COVID-19: a pilot trial. Toxicol Rep. 2021. https://doi.org/10.1016/j.toxrep.2021.03.003.",
"year": "2021"
},
{
"key": "7589_CR25",
"unstructured": "BBC Reality. Ivermectin: how false science created a COVID ‘miracle’drug. 2021. http://www.bbc.com/news/health-58170809. Accessed 04 Jan 2022."
},
{
"DOI": "10.1056/NEJMc2114907",
"author": "C Temple",
"doi-asserted-by": "publisher",
"journal-title": "N Engl J Med",
"key": "7589_CR26",
"unstructured": "Temple C, Hoang R, Hendrickson RG. Toxic effects from ivermectin use associated with prevention and treatment. N Engl J Med. 2021. https://doi.org/10.1056/NEJMc2114907.",
"year": "2021"
},
{
"DOI": "10.1097/MJT.0000000000001402",
"author": "A Bryant",
"doi-asserted-by": "publisher",
"journal-title": "Am J Ther",
"key": "7589_CR27",
"unstructured": "Bryant A, Lawrie T, Dowswell T, et al. Ivermectin for prevention and treatment of COVID-19 infection: a systematic review, meta-analysis, and trial sequential analysis to inform clinical guidelines. Am J Ther. 2021. https://doi.org/10.1097/MJT.0000000000001402.",
"year": "2021"
},
{
"DOI": "10.1136/bmj.m2980",
"author": "RA Siemieniuk",
"doi-asserted-by": "publisher",
"journal-title": "BMJ",
"key": "7589_CR28",
"unstructured": "Siemieniuk RA, Bartoszko JJ, Ge L, et al. Drug treatments for covid-19: living systematic review and network meta-analysis. BMJ. 2020. https://doi.org/10.1136/bmj.m2980.",
"year": "2020"
},
{
"DOI": "10.1002/14651858.CD015017",
"author": "M Popp",
"doi-asserted-by": "publisher",
"journal-title": "Cochrane Database Syst Rev",
"key": "7589_CR29",
"unstructured": "Popp M, Stegemann M, Metzendorf MI, Gould S, Kranke P, Meybohm P, et al. Ivermectin for preventing and treating COVID-19. Cochrane Database Syst Rev. 2021. https://doi.org/10.1002/14651858.CD015017.",
"year": "2021"
},
{
"DOI": "10.1093/cid/ciaa478",
"author": "A Bhimraj",
"doi-asserted-by": "publisher",
"journal-title": "Clin Infect Dis",
"key": "7589_CR30",
"unstructured": "Bhimraj A, Morgan RL, Shumaker AH, et al. Infectious diseases Society of America guidelines on the treatment and management of patients with COVID-19. Clin Infect Dis. 2020. https://doi.org/10.1093/cid/ciaa478.",
"year": "2020"
},
{
"key": "7589_CR31",
"unstructured": "Higgins JPT, Thomas J, Chandler J, et al. Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). Cochrane. 2021. www.training.cochrane.org/handbook. Access 04 Jan 2022."
},
{
"DOI": "10.1136/bmj.n71",
"author": "MJ Page",
"doi-asserted-by": "publisher",
"journal-title": "BMJ",
"key": "7589_CR32",
"unstructured": "Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021. https://doi.org/10.1136/bmj.n71.",
"year": "2021"
},
{
"key": "7589_CR33",
"unstructured": "Higgins J, Lasserson T, Chandler J, et al. Standards for the conduct and reporting of new Cochrane Intervention Reviews, reporting of protocols and the planning, conduct and reporting of updates (version Feb 2022). Cochrane. 2022. https://community.cochrane.org/mecir-manual. Accessed 04 Jan 2022."
},
{
"DOI": "10.1186/s13643-016-0384-4",
"author": "M Ouzzani",
"doi-asserted-by": "publisher",
"first-page": "210",
"journal-title": "Syst Rev",
"key": "7589_CR34",
"unstructured": "Ouzzani M, Hammady H, Fedorowicz Z, et al. Rayyan—a web and mobile app for systematic reviews. Syst Rev. 2016;5:210. https://doi.org/10.1186/s13643-016-0384-4.",
"volume": "5",
"year": "2016"
},
{
"DOI": "10.1136/bmj.l4898",
"author": "JAC Sterne",
"doi-asserted-by": "publisher",
"journal-title": "BMJ",
"key": "7589_CR35",
"unstructured": "Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomized trials. BMJ. 2019. https://doi.org/10.1136/bmj.l4898.",
"year": "2019"
},
{
"key": "7589_CR36",
"unstructured": "Schünemann HJ, Vist GE, Higgins JPT, et al. Chapter 15: Interpreting results and drawing conclusions. In: Higgins JPT, Thomas J, Chandler J, editors. Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). Cochrane; 2021. Chapter 15."
},
{
"DOI": "10.1136/bmj.39489.470347.AD",
"author": "GH Guyatt",
"doi-asserted-by": "publisher",
"journal-title": "BMJ",
"key": "7589_CR37",
"unstructured": "Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008. https://doi.org/10.1136/bmj.39489.470347.AD.",
"year": "2008"
},
{
"DOI": "10.1038/s41467-021-22446-z",
"doi-asserted-by": "publisher",
"key": "7589_CR38",
"unstructured": "Axfors C, Schmitt AM, Janiaud P, et al. Mortality outcomes with hydroxychloroquine and chloroquine in COVID-19 from an international collaborative meta-analysis of randomized trials. Nat Commun. 2021; 12. https://doi.org/10.1038/s41467-021-22446-z."
},
{
"DOI": "10.1016/j.ajem.2020.07.030",
"author": "A Della Porta",
"doi-asserted-by": "publisher",
"journal-title": "Am J Emerg Med",
"key": "7589_CR39",
"unstructured": "Della Porta A, Bornstein K, Coye A, et al. Acute chloroquine and hydroxychloroquine toxicity: a review for emergency clinicians. Am J Emerg Med. 2020. https://doi.org/10.1016/j.ajem.2020.07.030.",
"year": "2020"
},
{
"DOI": "10.1016/s0895-4356(01)00377-8",
"author": "JA Sterne",
"doi-asserted-by": "publisher",
"journal-title": "J Clin Epidemiol",
"key": "7589_CR40",
"unstructured": "Sterne JA, Egger M. Funnel plots for detecting bias in meta-analysis: guidelines on choice of axis. J Clin Epidemiol. 2001. https://doi.org/10.1016/s0895-4356(01)00377-8.",
"year": "2001"
},
{
"DOI": "10.1111/j.0006-341x.2000.00455.x",
"author": "S Duval",
"doi-asserted-by": "publisher",
"journal-title": "Biometrics",
"key": "7589_CR41",
"unstructured": "Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000. https://doi.org/10.1111/j.0006-341x.2000.00455.x.",
"year": "2000"
},
{
"DOI": "10.1016/j.ijid.2021.04.035",
"author": "RCS Seet",
"doi-asserted-by": "publisher",
"first-page": "314",
"journal-title": "Int J Infect Dis",
"key": "7589_CR42",
"unstructured": "Seet RCS, Quek AML, Ooi DSQ, et al. Positive impact of oral hydroxychloroquine and povidone-iodine throat spray for COVID-19 prophylaxis: an open-label randomized trial. Int J Infect Dis. 2021;106:314–22. https://doi.org/10.1016/j.ijid.2021.04.035.",
"volume": "106",
"year": "2021"
},
{
"DOI": "10.7860/JCDR/2020/46795.0000",
"author": "WM Shouman",
"doi-asserted-by": "publisher",
"journal-title": "J Clin Diagn Res",
"key": "7589_CR43",
"unstructured": "Shouman WM, Hegazy AA, Nafae RM, et al. Use of ivermectin as a potential chemoprophylaxis for COVID-19 in Egypt: a randomised clinical trial. J Clin Diagn Res. 2020. https://doi.org/10.7860/JCDR/2020/46795.0000.",
"year": "2020"
},
{
"DOI": "10.1101/2020.07.07.20145979v1",
"doi-asserted-by": "publisher",
"key": "7589_CR44",
"unstructured": "Gorial FI, Mashhadani S, Sayaly HM, et al. Effectiveness of ivermectin as add-on therapy in COVID-19 management (Pilot Trial). Posted July 08 2020. medRxiv. Preprint. Available at https://doi.org/10.1101/2020.07.07.20145979v1."
},
{
"DOI": "10.2217/fmb-2022-0014",
"author": "S Hazan",
"doi-asserted-by": "publisher",
"first-page": "339",
"journal-title": "Future Microbiol",
"key": "7589_CR45",
"unstructured": "Hazan S, Dave S, Gunaratne AW, Dolai S, Clancy RL, McCullough PA, Borody TJ. Effectiveness of ivermectin-based multidrug therapy in severely hypoxic, ambulatory COVID-19 patients. Future Microbiol. 2022;17:339–50. https://doi.org/10.2217/fmb-2022-0014.",
"volume": "17",
"year": "2022"
},
{
"author": "B George",
"first-page": "1",
"journal-title": "Indian J Hematol Blood Transfus",
"key": "7589_CR46",
"unstructured": "George B, Moorthy M, Kulkarni U, et al. Single dose of ivermectin is not useful in patients with hematological disorders and COVID-19 illness: a phase II B open labelled randomized controlled trial. Indian J Hematol Blood Transfus. 2022;27:1–8.",
"volume": "27",
"year": "2022"
},
{
"key": "7589_CR47",
"unstructured": "Kishoria N, Mathur SL, Parmar V, et al. Ivermectin as adjuvant to hydroxychloroquine in patients resistant to standard treatment for SARS-CoV-2: results of an open-label randomized clinical study. Paripex Indian J Res. 2020; 9(8):4801859. Available online:https://www.worldwidejournals.com/paripex/recent_issues_pdf/2020/August/ivermectin-as-adjuvant-to-hydroxycholoroquine-in-patients-resistant-to-standard-treatment-for-sarscov2-results-of-an-openlabel-randomized-clinical-study_August_2020_1597492974_4801859.pdf. [Accessed 04 Jan 2022]."
},
{
"DOI": "10.1101/2021.05.31.21258081v1",
"author": "A Biber",
"doi-asserted-by": "publisher",
"journal-title": "medRxiv",
"key": "7589_CR48",
"unstructured": "Biber A, Mandelboim M, Harmelin G, et al. Favorable outcome on viral load and culture viability using Ivermectin in early treatment of non-hospitalized patients with mild COVID-19—a double-blind, randomized placebo-controlled trial. medRxiv. 2021. https://doi.org/10.1101/2021.05.31.21258081v1.",
"year": "2021"
},
{
"DOI": "10.1101/2021.02.02.21250840v1",
"author": "KHS Bukhari",
"doi-asserted-by": "publisher",
"journal-title": "MedRxiv",
"key": "7589_CR49",
"unstructured": "Bukhari KHS, Asghar A, Perveen N, et al. Efficacy of ivermectin in COVID-19 patients with mild to moderate disease. MedRxiv. 2021. https://doi.org/10.1101/2021.02.02.21250840v1.",
"year": "2021"
},
{
"DOI": "10.21203/rs.3.rs-1290999/v1",
"doi-asserted-by": "publisher",
"key": "7589_CR50",
"unstructured": "Manomaipiboon A, Pholtawornkulchai K, Pupipatpab S, Suraamornkul S, Maneerit J, Ruksakul W et al. Efficacy and safety of ivermectin in the treatment of mild-to-moderate COVID-19 infection: a randomized, double blind, placebo, controlled trial. https://doi.org/10.21203/rs.3.rs-1290999/v1. https://www.researchsquare.com/article/rs-1290999/v1. [Accessed 04 Jan 2022]."
},
{
"DOI": "10.3390/idr14020020",
"author": "JL Beltran Gonzalez",
"doi-asserted-by": "publisher",
"first-page": "160",
"issue": "2",
"journal-title": "Infect Dis Rep",
"key": "7589_CR51",
"unstructured": "Beltran Gonzalez JL, González Gámez M, Mendoza Enciso EA, Esparza Maldonado RJ, Hernández Palacios D, Dueñas Campos S, et al. Efficacy and safety of ivermectin and hydroxychloroquine in patients with severe COVID-19: a randomized controlled trial. Infect Dis Rep. 2022;14(2):160–8. https://doi.org/10.3390/idr14020020.",
"volume": "14",
"year": "2022"
},
{
"DOI": "10.1101/2022.06.10.22276252",
"author": "S Naggie",
"doi-asserted-by": "publisher",
"journal-title": "MedRxiv",
"key": "7589_CR52",
"unstructured": "Naggie S. Ivermectin for treatment of mild-to-moderate COVID-19 in the outpatient setting: a decentralized, placebo-controlled randomized, platform clinical trial. MedRxiv. 2022. https://doi.org/10.1101/2022.06.10.22276252.",
"year": "2022"
},
{
"DOI": "10.21203/rs.3.rs-1640339/v1",
"author": "C La Rocha",
"doi-asserted-by": "publisher",
"journal-title": "Res Square",
"key": "7589_CR53",
"unstructured": "La Rocha C, Cid-Lopez MA, Venegas-Lopez BI, Gómez-Mendes SC, Sánches-Ortiz A. Ivermectin compared with placebo in the clinical evolution of Mexican patients with asymptomatic and mild COVID-19: a randomized clinical trial. Res Square. 2022. https://doi.org/10.21203/rs.3.rs-1640339/v1.",
"year": "2022"
},
{
"DOI": "10.1093/qjmed/hcab035",
"author": "OE Babalola",
"doi-asserted-by": "publisher",
"first-page": "780",
"issue": "11",
"journal-title": "QJM",
"key": "7589_CR54",
"unstructured": "Babalola OE, Bode CO, Ajayi AA, Alakaloko FM, Akase IE, Otrofanowei E, et al. Ivermectin shows clinical benefits in mild to moderate COVID19: a randomized controlled double-blind, dose-response study in Lagos. QJM. 2022;114(11):780–8. https://doi.org/10.1093/qjmed/hcab035.",
"volume": "114",
"year": "2022"
},
{
"DOI": "10.29309/TPMJ/2021.28.05.5867",
"author": "R Faisal",
"doi-asserted-by": "publisher",
"first-page": "737",
"issue": "05",
"journal-title": "Professional Med J.",
"key": "7589_CR55",
"unstructured": "Faisal R, Shah SFA, Hussain M. Potential use of azithromycin alone and in combination with ivermectin in fighting against the symptoms of COVID-19. Professional Med J. 2021;28(05):737. https://doi.org/10.29309/TPMJ/2021.28.05.5867.",
"volume": "28",
"year": "2021"
},
{
"DOI": "10.3329/imcjms.v14i2.52826",
"doi-asserted-by": "crossref",
"key": "7589_CR56",
"unstructured": "Podder CS, Chowdhury N, Sina MI, Haque WMMU. Outcome of ivermectin treated mild to moderate COVID-19 cases: a single-centre, open-label, randomised controlled study. IMC J Med Sci. 2021; 14(2):11–8. https://www.banglajol.info/index.php/IMCJMS/article/view/52826. Accessed 04 Jan 2022."
},
{
"DOI": "10.1016/j.ijantimicag.2021.106516",
"author": "D Buonfrate",
"doi-asserted-by": "publisher",
"issue": "2",
"journal-title": "Int J Antimicrob Agents",
"key": "7589_CR57",
"unstructured": "Buonfrate D, Chesini F, Martini D, Roncaglioni MC, Ojeda Fernandez ML, Alvisi MF, et al. High-dose ivermectin for early treatment of COVID-19 (COVER study): a randomised, double-blind, multicentre, phase II, dose-finding, proof-of-concept clinical trial. Int J Antimicrob Agents. 2022;59(2): 106516. https://doi.org/10.1016/j.ijantimicag.2021.106516.",
"volume": "59",
"year": "2022"
},
{
"DOI": "10.1002/jmv.27122",
"author": "S Abd-Elsalam",
"doi-asserted-by": "publisher",
"first-page": "5833",
"issue": "10",
"journal-title": "J Med Virol",
"key": "7589_CR58",
"unstructured": "Abd-Elsalam S, Noor RA, Badawi R, et al. Clinical study evaluating the efficacy of ivermectin in COVID-19 treatment: a randomized controlled study. J Med Virol. 2021;93(10):5833–8. https://doi.org/10.1002/jmv.27122.",
"volume": "93",
"year": "2021"
},
{
"DOI": "10.1016/j.eclinm.2020.100720",
"author": "C Chaccour",
"doi-asserted-by": "publisher",
"journal-title": "EClinicalMedicine",
"key": "7589_CR59",
"unstructured": "Chaccour C, Casellas A, Blanco-Di Matteo A, et al. The effect of early treatment with ivermectin on viral load, symptoms and humoral response in patients with non-severe COVID-19: a pilot, double-blind, placebo-controlled, randomized clinical trial. EClinicalMedicine. 2021;32: 100720. https://doi.org/10.1016/j.eclinm.2020.100720.",
"volume": "32",
"year": "2021"
},
{
"DOI": "10.1186/s12879-021-06104-9",
"author": "N Okumuş",
"doi-asserted-by": "publisher",
"first-page": "411",
"journal-title": "BMC Infect Dis",
"key": "7589_CR60",
"unstructured": "Okumuş N, Demirtürk N, Çetinkaya RA, et al. Evaluation of the effectiveness and safety of adding ivermectin to treatment in severe COVID-19 patients. BMC Infect Dis. 2021;21:411. https://doi.org/10.1186/s12879-021-06104-9.",
"volume": "21",
"year": "2021"
},
{
"DOI": "10.1016/j.eclinm.2021.101119",
"author": "A Krolewiecki",
"doi-asserted-by": "publisher",
"journal-title": "EClinicalMedicine",
"key": "7589_CR61",
"unstructured": "Krolewiecki A, Lifschitz A, Moragas M, Travacio M, Valentini R, Alonso DF, et al. Corrigendum to antiviral effect of high-dose ivermectin in adults with COVID-19: a proof-of-concept randomized trial [EClinicalMedicine 37 (2021) 100,959]”. EClinicalMedicine. 2021;39: 101119. https://doi.org/10.1016/j.eclinm.2021.101119.",
"volume": "39",
"year": "2021"
},
{
"DOI": "10.1016/j.jiac.2021.08.021",
"author": "A Mohan",
"doi-asserted-by": "publisher",
"first-page": "1743",
"issue": "12",
"journal-title": "J Infect Chemother",
"key": "7589_CR62",
"unstructured": "Mohan A, Tiwari P, Suri TM, et al. Single-dose oral ivermectin in mild and moderate COVID-19 (RIVET-COV): a single-centre randomized, placebo-controlled trial. J Infect Chemother. 2021;27(12):1743–9.",
"volume": "27",
"year": "2021"
},
{
"DOI": "10.1001/jamainternmed.2022.0189",
"author": "SCL Lim",
"doi-asserted-by": "publisher",
"first-page": "426",
"issue": "4",
"journal-title": "JAMA Intern Med",
"key": "7589_CR63",
"unstructured": "Lim SCL, Hor CP, Tay KH, Mat Jelani A, Tan WH, Ker HB, Chow TS, Zaid M, Cheah WK, Lim HH, Khalid KE, Cheng JT, Mohd Unit H, An N, Nasruddin AB, Low LL, Khoo SWR, Loh JH, Zaidan NZ, Ab Wahab S, Song LH, Koh HM, King TL, Lai NM, Chidambaram SK, Peariasamy KM, I-TECH Study Group. Efficacy of ivermectin treatment on disease progression among adults with mild to moderate COVID-19 and comorbidities: the I-TECH randomized clinical trial. JAMA Intern Med. 2022;182(4):426–35.",
"volume": "182",
"year": "2022"
},
{
"DOI": "10.4103/1995-7645.318304",
"author": "MS Niaee",
"doi-asserted-by": "publisher",
"first-page": "266",
"journal-title": "Asian Pac J Trop Med",
"key": "7589_CR64",
"unstructured": "Niaee MS, Namdar P, Allami A, et al. Ivermectin as an adjunct treatment for hospitalized adult COVID-19 patients: a randomized multi-center clinical trial. Asian Pac J Trop Med. 2021;14:266–73.",
"volume": "14",
"year": "2021"
},
{
"DOI": "10.18433/jpps32105",
"doi-asserted-by": "crossref",
"key": "7589_CR65",
"unstructured": "Ravikirti, Roy R, Pattadar C, Raj R, Agarwal N, Biswas B, Manjhi PK, et al. Evaluation of ivermectin as a potential treatment for mild to moderate COVID-19: a double-blind randomized placebo controlled trial in Eastern India. J Pharm Pharm Sci. 2021; 24:343–350."
},
{
"DOI": "10.1371/journal.pone.0259317",
"author": "A Quincho-Lopez",
"doi-asserted-by": "publisher",
"issue": "11",
"journal-title": "PLoS ONE",
"key": "7589_CR66",
"unstructured": "Quincho-Lopez A, Benites-Ibarra CA, Hilario-Gomez MM, Quijano-Escate R, Taype-Rondan A. Self-medication practices to prevent or manage COVID-19: a systematic review. PLoS ONE. 2021;16(11): e0259317. https://doi.org/10.1371/journal.pone.0259317.",
"volume": "16",
"year": "2021"
},
{
"DOI": "10.1590/0102-311X00028721",
"author": "LZ Machado",
"doi-asserted-by": "publisher",
"issue": "4",
"journal-title": "Cadernos de Saúde Públ",
"key": "7589_CR67",
"unstructured": "Machado LZ, Marcon CEM. Carta às Editoras sobre o artigo de Melo et al. Cadernos de Saúde Públ. 2021;37(4): e00028721. https://doi.org/10.1590/0102-311X00028721.",
"volume": "37",
"year": "2021"
},
{
"key": "7589_CR68",
"unstructured": "Cpipandemia-CPI Da Pandemia-Atividade Legislativa-Senado. Federal. https://legis.senado.leg.br/comissoes/comissao?codcol=2441. Accessed 04 Jan 2022."
},
{
"key": "7589_CR69",
"unstructured": "Agência Senado. Senado Notícias. Fabricante de ivermectina lucrou à custa de vidas, acusam senadores da CPI.2021. https://www12.senado.leg.br/noticias/materias/2021/08/11/fabricante-de-ivermectina-lucrou-a-custa-de-vidas-acusam-senadores-da-cpi. Accessed 04 Jan 2022."
},
{
"key": "7589_CR70",
"unstructured": "Cochrane Communit. Living systematic reviews. https://community.cochrane.org/review-production/production-resources/living-systematic-reviews. Accessed 04 Jan 2021."
},
{
"key": "7589_CR71",
"unstructured": "Pan American Health Organization-PAHO. Ongoing living update of COVID-19 therapeutic options: summary of evidence. Rapid review, https://iris.paho.org/handle/10665.2/52719. Accessed 28 Jun 2022."
},
{
"DOI": "10.1016/j.jclinepi.2021.12.018",
"author": "A Izcovich",
"doi-asserted-by": "publisher",
"first-page": "43",
"journal-title": "J Clin Epidemiol",
"key": "7589_CR72",
"unstructured": "Izcovich A, Peiris S, Ragusa M, Tortosa F, Rada G, Aldighieri S, Reveiz L. Bias as a source of inconsistency in ivermectin trials for COVID-19: a systematic review. Ivermectin’s suggested benefits are mainly based on potentially biased results. J Clin Epidemiol. 2022;144:43–55.",
"volume": "144",
"year": "2022"
},
{
"author": "AMM Chowdhury",
"first-page": "63",
"issue": "1",
"journal-title": "Eurasian J Med Oncol.",
"key": "7589_CR73",
"unstructured": "Chowdhury AMM, Shahbaz M, Karim MR, Islam J, Dan G, He SX. A comparative study on ivermectin-doxycycline and hydroxychloroquine-azithromycin therapy on COVID-19 patients. Eurasian J Med Oncol. 2021;5(1):63–70.",
"volume": "5",
"year": "2021"
},
{
"DOI": "10.1002/9780470743386",
"doi-asserted-by": "publisher",
"key": "7589_CR74",
"unstructured": "Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. Introduction to meta-analysis. Wiley. ISBN: 978-0-470-05724-7. https://doi.org/10.1002/9780470743386. Accessed 04 Feb 2022."
},
{
"DOI": "10.1007/s40278-020-79019-x",
"doi-asserted-by": "publisher",
"key": "7589_CR75",
"unstructured": "Chloroquine and hydroxychloroquine increase risk of death in COVID-19. Reactions Weekly. 2020; 1806(1):1. https://doi.org/10.1007/s40278-020-79019-x."
},
{
"DOI": "10.3329/imcjms.v14i2.52833",
"author": "G Meneses",
"doi-asserted-by": "publisher",
"first-page": "67",
"issue": "2",
"journal-title": "IMC J Med Sci",
"key": "7589_CR76",
"unstructured": "Meneses G. Commentary about open-label randomized controlled study of ivermectin in mild to moderate COVID-19. IMC J Med Sci. 2021;14(2):67–8.",
"volume": "14",
"year": "2021"
},
{
"key": "7589_CR77",
"unstructured": "Discover the largest evidence database on COVID-19. Epistemonikos Foundation. https://www.mcmasterforum.org/docs/default-source/covidend/meeting-documents/partner-meetings/covid-end_tc_2020-06-11_5_covid_love_epistemonikos.pdf?sfvrsn=542d56d5_3. Accessed 04 Jan 2022."
},
{
"key": "7589_CR78",
"unstructured": "Araya V, Kraemer P, Burdiles P, Herrera P, Castillo C, Sepulveda D, et al. The living overview of evidence database (LOVE) may be more efficient than a traditional search of systematic reviews and randomized trials. https://abstracts.cochrane.org/2019-santiago/living-overview-evidence-database-love-may-be-more-efficient-traditional-search. Accessed 04 Jan 2022."
},
{
"DOI": "10.1371/journal.pntd.0009354",
"author": "JT Campillo",
"doi-asserted-by": "publisher",
"issue": "4",
"journal-title": "PLoS Negl Trop Dis",
"key": "7589_CR79",
"unstructured": "Campillo JT, Boussinesq M, Bertout S, Faillie JL, Chesnais CB. Serious adverse reactions associated with ivermectin: a systematic pharmacovigilance study in sub-Saharan Africa and in the rest of the World. PLoS Negl Trop Dis. 2021;15(4): e0009354. https://doi.org/10.1371/journal.pntd.0009354.",
"volume": "15",
"year": "2021"
},
{
"DOI": "10.18483/ijSci.2378",
"author": "AZK Chachar",
"doi-asserted-by": "publisher",
"first-page": "31",
"journal-title": "Intern J Sci.",
"key": "7589_CR80",
"unstructured": "Chachar AZK, Khan KA, Asif M, Tanveer K, Khaqan A, Basri R. Effectiveness of ivermectin in SARS-COV-2/COVID-10 patients. Intern J Sci. 2020;9:31. https://doi.org/10.18483/ijSci.2378.",
"volume": "9",
"year": "2020"
},
{
"DOI": "10.1016/j.clinthera.2021.04.007",
"author": "L Shahbaznejad",
"doi-asserted-by": "publisher",
"first-page": "1007",
"issue": "6",
"journal-title": "Clin Thera",
"key": "7589_CR81",
"unstructured": "Shahbaznejad L, Davoudi A, Eslami G, et al. Effects of ivermectin in patients with COVID-19: a multicenter, double-blind, randomized controlled clinical trial. Clin Thera. 2021;43(6):1007–19.",
"volume": "43",
"year": "2021"
},
{
"DOI": "10.1080/20477724.2021.1890887",
"author": "LEB Galan",
"doi-asserted-by": "publisher",
"first-page": "235",
"issue": "4",
"journal-title": "Pathog Glob Health",
"key": "7589_CR82",
"unstructured": "Galan LEB, Santos NMD, Asato MS, et al. Phase 2 randomized study on chloroquine, hydroxychloroquine or ivermectin in hospitalized patients with severe manifestations of SARS-CoV-2 infection. Pathog Glob Health. 2021;115(4):235–42.",
"volume": "115",
"year": "2021"
},
{
"DOI": "10.1148/radiol.2020201473",
"author": "M Prokop",
"doi-asserted-by": "publisher",
"first-page": "97",
"issue": "2",
"journal-title": "Radiology",
"key": "7589_CR83",
"unstructured": "Prokop M, van Everdingen W, van Rees VT. COVID-19 Standardized Reporting Working Group of the Dutch Radiological Society. CO-RADS: a categorical CT assessment scheme for patients suspected of having COVID-19-definition and evaluation. Radiology. 2020;296(2):97–104.",
"volume": "296",
"year": "2020"
}
],
"reference-count": 83,
"references-count": 83,
"relation": {},
"resource": {
"primary": {
"URL": "https://bmcinfectdis.biomedcentral.com/articles/10.1186/s12879-022-07589-8"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Infectious Diseases"
],
"subtitle": [],
"title": "Systematic review and meta-analysis of ivermectin for treatment of COVID-19: evidence beyond the hype",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1007/springer_crossmark_policy",
"volume": "22"
}