RETRACTED: Use of ivermectin in the treatment of Covid-19: A pilot trial
Henrique Pott-Junior, Mˆonica Maria Bastos Paoliello, Alice De Queiroz Constantino Miguel, Anderson Ferreira Da Cunha, Caio Cesar De Melo Freire, F´abio Fernandes Neves, Lucimar Retto Da Silva De Av´o, Meliza Goi Roscani, Sigrid De Sousa Dos Santos, Silvana Gama Florêncio Chach´a
Toxicology Reports, doi:10.1016/j.toxrep.2021.03.003
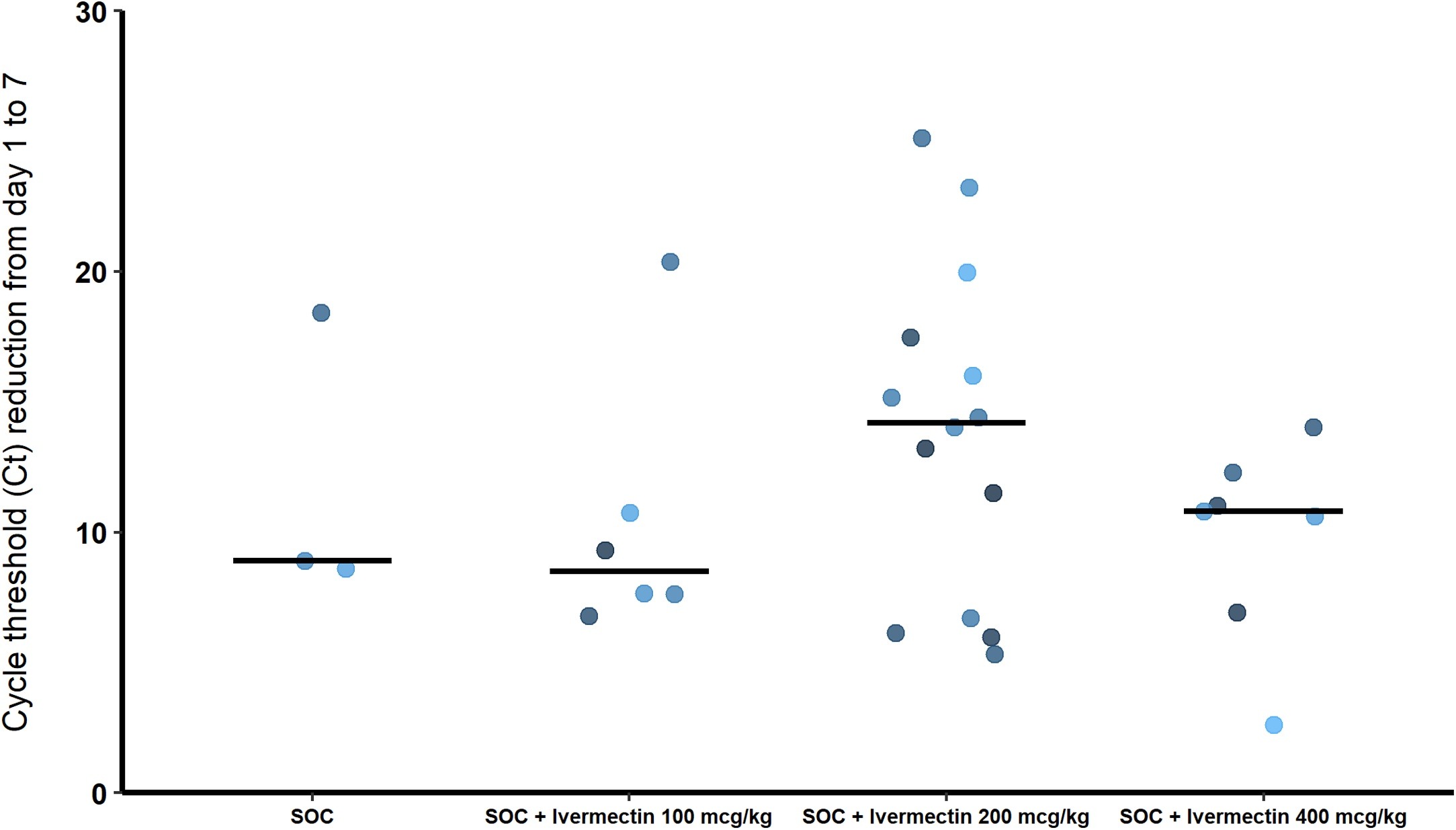
In this randomized open-label trial pilot study we assessed the antiviral effects and safety of various doses of ivermectin in patients with mild clinical symptoms of COVID-19. Methods: Patients were randomly assigned to receive standard of care (SOC) treatment at hospital admission; SOC plus ivermectin 100 mcg/kg; SOC plus ivermectin 200 mcg/kg; or SOC plus ivermectin 400 mcg/kg. The primary assessed endpoint was the proportion of patients who achieved two consecutive negative SARS-CoV-2 RT PCR tests within 7 days of the start of the dosing period. This study was registered at ClinicalTrials.gov (NCT04431466). Results: A total of 32 patients were enrolled and randomized to treatment. SOC treatment together with ivermectin did not result in any serious adverse events. All patients exhibited a reduction in SARS-CoV-2 viral load within 7 days; however, those who received ivermectin had a more consistent decrease as compared to the SOC alone group, characterized by a shorter time for obtaining two consecutive negative SARS-CoV-2 RT PCR tests. Conclusions: Ivermectin is safe in patients with SARS-CoV-2, reducing symptomatology and the SARS-CoV-2 viral load. This antiviral effect appears to depend on the dose used, and if confirmed in future studies, it suggests that ivermectin may be a useful adjuvant to the SOC treatment in patients with mild COVID-19 symptoms.
CRediT authorship contribution statement Henrique
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
Ahmed, Karim, Ross, A five-day course of ivermectin for the treatment of COVID-19 may reduce the duration of illness, Int. J. Infect. Dis,
doi:10.1016/j.ijid.2020.11.191Alexandris, Lagoumintzis, Chasapis, Nicotinic cholinergic system and COVID-19: in silico evaluation of nicotinic acetylcholine receptor agonists as potential therapeutic interventions, Toxicol. Rep,
doi:10.1016/j.toxrep.2020.12.013Banerjee, Nandy, Dalai, Ahmed, The Battle against COVID 19 pandemic: what we need to know Before we "test fire" ivermectin, Drug Res. (Stuttg),
doi:10.1055/a-1185-8913Behera, Patro, Singh, Role of ivermectin in the prevention of SARS-CoV-2 infection among healthcare workers in India: a matched case-control study, PLoS One,
doi:10.1371/journal.pone.0247163Control, Prevention. CDC 2019-Novel Coronavirus (2019-Ncov) Real-Time RT-PCR Diagnostic Panel
Fajnzylber, Regan, Coxen, SARS-CoV-2 viral load is associated with increased disease severity and mortality, Nat. Commun,
doi:10.1038/s41467-020-19057-5Gonzalez Canga, Sahagun Prieto, Diez Liebana, Martinez, Sierra et al., The pharmacokinetics and interactions of ivermectin in humans-a mini-review, AAPS J,
doi:10.1208/s12248-007-9000-9Lagoumintzis, Chasapis, Alexandris, Nicotinic cholinergic system and COVID-19: in silico identification of interactions between alpha7 nicotinic acetylcholine receptor and the cryptic epitopes of SARS-Co-v and SARS-CoV-2 spike glycoproteins, Food Chem. Toxicol,
doi:10.1016/j.fct.2021.112009Lehrer, Rheinstein, Ivermectin docks to the SARS-CoV-2 spike receptorbinding domain attached to ACE2, Vivo,
doi:10.21873/invivo.12134Padhy, Mohanty, Das, Meher, Therapeutic potential of ivermectin as add on treatment in COVID 19: a systematic review and meta-analysis, J. Pharm. Pharm. Sci,
doi:10.18433/jpps31457Pena-Silva, Duffull, Steer, Jaramillo-Rincon, Gwee et al., Pharmacokinetic considerations on the repurposing of ivermectin for treatment of COVID-19, Br. J. Clin. Pharmacol,
doi:10.1111/bcp.14476Rajter, Sherman, Fatteh, Vogel, Sacks et al., Use of ivermectin Is associated with Lower mortality in hospitalized patients with coronavirus disease 2019: the ICON study, Chest,
doi:10.1016/j.chest.2020.10.009Sen Gupta, Biswal, Panda, Ray, Rana, Binding mechanism and structural insights into the identified protein target of COVID-19 and importinalpha with in-vitro effective drug ivermectin, J. Biomol. Struct. Dyn,
doi:10.1080/07391102.2020.1839564Siddiqui, Jahan, Ashraf, Current status and strategic possibilities on potential use of combinational drug therapy against COVID-19 caused by SARS-CoV-2, J. Biomol. Struct. Dyn,
doi:10.1080/07391102.2020.1802345Telbisz, Ambrus, Mozner, Interactions of potential anti-COVID-19 compounds with multispecific ABC and OATP drug transporters, Pharmaceutics,
doi:10.3390/pharmaceutics13010081Zhang, Song, Ci, Ivermectin inhibits LPS-induced production of inflammatory cytokines and improves LPS-induced survival in mice, Inflamm. Res,
doi:10.1007/s00011-008-8007-8DOI record:
{
"DOI": "10.1016/j.toxrep.2021.03.003",
"ISSN": [
"2214-7500"
],
"URL": "http://dx.doi.org/10.1016/j.toxrep.2021.03.003",
"alternative-id": [
"S2214750021000445"
],
"assertion": [
{
"label": "This article is maintained by",
"name": "publisher",
"value": "Elsevier"
},
{
"label": "Article Title",
"name": "articletitle",
"value": "RETRACTED: Use of ivermectin in the treatment of Covid-19: A pilot trial"
},
{
"label": "Journal Title",
"name": "journaltitle",
"value": "Toxicology Reports"
},
{
"label": "CrossRef DOI link to publisher maintained version",
"name": "articlelink",
"value": "https://doi.org/10.1016/j.toxrep.2021.03.003"
},
{
"label": "Content Type",
"name": "content_type",
"value": "article"
},
{
"label": "Copyright",
"name": "copyright",
"value": "© 2021 The Authors. Published by Elsevier B.V."
}
],
"author": [
{
"affiliation": [],
"family": "Pott-Junior",
"given": "Henrique",
"sequence": "first"
},
{
"affiliation": [],
"family": "Paoliello",
"given": "Mˆonica Maria Bastos",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Miguel",
"given": "Alice de Queiroz Constantino",
"sequence": "additional"
},
{
"affiliation": [],
"family": "da Cunha",
"given": "Anderson Ferreira",
"sequence": "additional"
},
{
"affiliation": [],
"family": "de Melo Freire",
"given": "Caio Cesar",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Neves",
"given": "F´abio Fernandes",
"sequence": "additional"
},
{
"affiliation": [],
"family": "da Silva de Av´o",
"given": "Lucimar Retto",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Roscani",
"given": "Meliza Goi",
"sequence": "additional"
},
{
"affiliation": [],
"family": "dos Santos",
"given": "Sigrid De Sousa",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Chach´a",
"given": "Silvana Gama Florêncio",
"sequence": "additional"
}
],
"container-title": "Toxicology Reports",
"container-title-short": "Toxicology Reports",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"elsevier.com",
"sciencedirect.com"
]
},
"created": {
"date-parts": [
[
2021,
3,
10
]
],
"date-time": "2021-03-10T02:22:06Z",
"timestamp": 1615342926000
},
"deposited": {
"date-parts": [
[
2022,
4,
29
]
],
"date-time": "2022-04-29T13:47:28Z",
"timestamp": 1651240048000
},
"indexed": {
"date-parts": [
[
2024,
4,
8
]
],
"date-time": "2024-04-08T20:34:19Z",
"timestamp": 1712608459861
},
"is-referenced-by-count": 32,
"issued": {
"date-parts": [
[
2021
]
]
},
"language": "en",
"license": [
{
"URL": "https://www.elsevier.com/tdm/userlicense/1.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
1,
1
]
],
"date-time": "2021-01-01T00:00:00Z",
"timestamp": 1609459200000
}
},
{
"URL": "http://creativecommons.org/licenses/by-nc-nd/4.0/",
"content-version": "vor",
"delay-in-days": 66,
"start": {
"date-parts": [
[
2021,
3,
8
]
],
"date-time": "2021-03-08T00:00:00Z",
"timestamp": 1615161600000
}
}
],
"link": [
{
"URL": "https://api.elsevier.com/content/article/PII:S2214750021000445?httpAccept=text/xml",
"content-type": "text/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://api.elsevier.com/content/article/PII:S2214750021000445?httpAccept=text/plain",
"content-type": "text/plain",
"content-version": "vor",
"intended-application": "text-mining"
}
],
"member": "78",
"original-title": [],
"page": "505-510",
"prefix": "10.1016",
"published": {
"date-parts": [
[
2021
]
]
},
"published-print": {
"date-parts": [
[
2021
]
]
},
"publisher": "Elsevier BV",
"reference": [
{
"article-title": "The Battle against COVID 19 pandemic: what we need to know Before we \"test fire\" ivermectin",
"author": "Banerjee",
"first-page": "337",
"issue": "August (8)",
"journal-title": "Drug Res. (Stuttg)",
"key": "10.1016/j.toxrep.2021.03.003_bib0005",
"volume": "70",
"year": "2020"
},
{
"DOI": "10.21873/invivo.12134",
"article-title": "Ivermectin docks to the SARS-CoV-2 spike receptor-binding domain attached to ACE2",
"author": "Lehrer",
"doi-asserted-by": "crossref",
"first-page": "3023",
"issue": "September-October(5)",
"journal-title": "In Vivo",
"key": "10.1016/j.toxrep.2021.03.003_bib0010",
"volume": "34",
"year": "2020"
},
{
"article-title": "Binding mechanism and structural insights into the identified protein target of COVID-19 and importin-alpha with in-vitro effective drug ivermectin",
"author": "Sen Gupta",
"first-page": "1",
"journal-title": "J. Biomol. Struct. Dyn.",
"key": "10.1016/j.toxrep.2021.03.003_bib0015",
"volume": "28",
"year": "2020"
},
{
"DOI": "10.1080/07391102.2020.1852117",
"article-title": "Current status and strategic possibilities on potential use of combinational drug therapy against COVID-19 caused by SARS-CoV-2",
"author": "Siddiqui",
"doi-asserted-by": "crossref",
"first-page": "1",
"journal-title": "J. Biomol. Struct. Dyn.",
"key": "10.1016/j.toxrep.2021.03.003_bib0020",
"volume": "5",
"year": "2020"
},
{
"DOI": "10.1016/j.antiviral.2020.104787",
"article-title": "The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro",
"author": "Caly",
"doi-asserted-by": "crossref",
"journal-title": "Antiviral Res",
"key": "10.1016/j.toxrep.2021.03.003_bib0025",
"volume": "178",
"year": "2020"
},
{
"article-title": "Pharmacokinetic considerations on the repurposing of ivermectin for treatment of COVID-19",
"author": "Pena-Silva",
"journal-title": "Br. J. Clin. Pharmacol.",
"key": "10.1016/j.toxrep.2021.03.003_bib0030",
"volume": "17",
"year": "2020"
},
{
"key": "10.1016/j.toxrep.2021.03.003_bib0035",
"series-title": "Control C-CfD, Prevention. CDC 2019-Novel Coronavirus (2019-Ncov) Real-Time RT-PCR Diagnostic Panel",
"year": "2020"
},
{
"DOI": "10.1038/s41467-020-19057-5",
"article-title": "SARS-CoV-2 viral load is associated with increased disease severity and mortality",
"author": "Fajnzylber",
"doi-asserted-by": "crossref",
"first-page": "5493",
"issue": "October (1)",
"journal-title": "Nat. Commun.",
"key": "10.1016/j.toxrep.2021.03.003_bib0040",
"volume": "11",
"year": "2020"
},
{
"DOI": "10.1038/s41591-020-0869-5",
"article-title": "Temporal dynamics in viral shedding and transmissibility of COVID-19",
"author": "He",
"doi-asserted-by": "crossref",
"first-page": "672",
"issue": "May(5)",
"journal-title": "Nat. Med.",
"key": "10.1016/j.toxrep.2021.03.003_bib0045",
"volume": "26",
"year": "2020"
},
{
"DOI": "10.1016/j.jinf.2020.06.067",
"article-title": "SARS-CoV-2 detection, viral load and infectivity over the course of an infection",
"author": "Walsh",
"doi-asserted-by": "crossref",
"first-page": "357",
"issue": "September (3)",
"journal-title": "J. Infect.",
"key": "10.1016/j.toxrep.2021.03.003_bib0050",
"volume": "81",
"year": "2020"
},
{
"DOI": "10.1208/s12248-007-9000-9",
"article-title": "The pharmacokinetics and interactions of ivermectin in humans--a mini-review",
"author": "Gonzalez Canga",
"doi-asserted-by": "crossref",
"first-page": "42",
"issue": "1",
"journal-title": "AAPS J",
"key": "10.1016/j.toxrep.2021.03.003_bib0055",
"volume": "10",
"year": "2008"
},
{
"article-title": "Interactions of potential anti-COVID-19 compounds with multispecific ABC and OATP drug transporters",
"author": "Telbisz",
"issue": "January 9",
"journal-title": "Pharmaceutics",
"key": "10.1016/j.toxrep.2021.03.003_bib0060",
"volume": "13",
"year": "2021"
},
{
"DOI": "10.1016/j.toxrep.2020.12.013",
"article-title": "Nicotinic cholinergic system and COVID-19: in silico evaluation of nicotinic acetylcholine receptor agonists as potential therapeutic interventions",
"author": "Alexandris",
"doi-asserted-by": "crossref",
"first-page": "73",
"journal-title": "Toxicol. Rep.",
"key": "10.1016/j.toxrep.2021.03.003_bib0065",
"volume": "8",
"year": "2021"
},
{
"article-title": "Nicotinic cholinergic system and COVID-19: in silico identification of interactions between alpha7 nicotinic acetylcholine receptor and the cryptic epitopes of SARS-Co-v and SARS-CoV-2 spike glycoproteins",
"author": "Lagoumintzis",
"issue": "January 24",
"journal-title": "Food Chem. Toxicol.",
"key": "10.1016/j.toxrep.2021.03.003_bib0070",
"volume": "149",
"year": "2021"
},
{
"DOI": "10.1007/s00011-011-0307-8",
"article-title": "Anti-inflammatory effects of ivermectin in mouse model of allergic asthma",
"author": "Yan",
"doi-asserted-by": "crossref",
"first-page": "589",
"issue": "June (6)",
"journal-title": "Inflamm. Res.",
"key": "10.1016/j.toxrep.2021.03.003_bib0075",
"volume": "60",
"year": "2011"
},
{
"DOI": "10.1007/s00011-008-8007-8",
"article-title": "Ivermectin inhibits LPS-induced production of inflammatory cytokines and improves LPS-induced survival in mice",
"author": "Zhang",
"doi-asserted-by": "crossref",
"first-page": "524",
"issue": "November (11)",
"journal-title": "Inflamm. Res.",
"key": "10.1016/j.toxrep.2021.03.003_bib0080",
"volume": "57",
"year": "2008"
},
{
"DOI": "10.1016/j.onehlt.2020.100148",
"article-title": "COVID-19 and the rush for self-medication and self-dosing with ivermectin: a word of caution",
"author": "Molento",
"doi-asserted-by": "crossref",
"journal-title": "One Health",
"key": "10.1016/j.toxrep.2021.03.003_bib0085",
"volume": "10",
"year": "2020"
},
{
"DOI": "10.1371/journal.pone.0242184",
"article-title": "Lack of efficacy of standard doses of ivermectin in severe COVID-19 patients",
"author": "Camprubi",
"doi-asserted-by": "crossref",
"issue": "11",
"journal-title": "PLoS One",
"key": "10.1016/j.toxrep.2021.03.003_bib0090",
"volume": "15",
"year": "2020"
},
{
"article-title": "Use of ivermectin Is associated with Lower mortality in hospitalized patients with coronavirus disease 2019: the ICON study",
"author": "Rajter",
"issue": "October 13",
"journal-title": "Chest",
"key": "10.1016/j.toxrep.2021.03.003_bib0095",
"year": "2020"
},
{
"article-title": "A five-day course of ivermectin for the treatment of COVID-19 may reduce the duration of illness",
"author": "Ahmed",
"first-page": "214",
"issue": "December 2",
"journal-title": "Int. J. Infect. Dis.",
"key": "10.1016/j.toxrep.2021.03.003_bib0100",
"volume": "103",
"year": "2020"
},
{
"DOI": "10.18433/jpps31457",
"article-title": "Therapeutic potential of ivermectin as add on treatment in COVID 19: a systematic review and meta-analysis",
"author": "Padhy",
"doi-asserted-by": "crossref",
"first-page": "462",
"journal-title": "J. Pharm. Pharm. Sci.",
"key": "10.1016/j.toxrep.2021.03.003_bib0105",
"volume": "23",
"year": "2020"
},
{
"DOI": "10.1371/journal.pone.0247163",
"article-title": "Role of ivermectin in the prevention of SARS-CoV-2 infection among healthcare workers in India: a matched case-control study",
"author": "Behera",
"doi-asserted-by": "crossref",
"issue": "2",
"journal-title": "PLoS One",
"key": "10.1016/j.toxrep.2021.03.003_bib0110",
"volume": "16",
"year": "2021"
}
],
"reference-count": 22,
"references-count": 22,
"relation": {},
"resource": {
"primary": {
"URL": "https://linkinghub.elsevier.com/retrieve/pii/S2214750021000445"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Health, Toxicology and Mutagenesis",
"Toxicology"
],
"subtitle": [],
"title": "RETRACTED: Use of ivermectin in the treatment of Covid-19: A pilot trial",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1016/elsevier_cm_policy",
"update-to": [
{
"DOI": "10.1016/j.toxrep.2021.03.003",
"label": "Retraction",
"type": "retraction",
"updated": {
"date-parts": [
[
2021,
1,
1
]
],
"date-time": "2021-01-01T00:00:00Z",
"timestamp": 1609459200000
}
}
],
"volume": "8"
}