Potential use of azithromycin alone and in combination with ivermectin in fighting against the symptoms of COVID-19.
Dr Rizwan Faisal, Syed Furqan Ali Shah, Mazhar Hussain
The Professional Medical Journal, doi:10.29309/tpmj/2021.28.05.5867
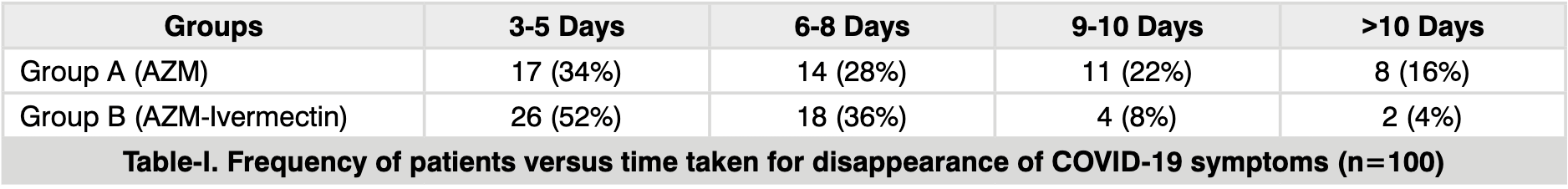
Material & Methods: Total patients included in the study were 100. Patients were divided into two groups by systematic random sampling: Group A: who received AZM (500mg once a day for 5 days), Group B: who received Ivermectin (12mg once a day for 5 days) and AZM (500mg once a day for 5 days). All the participants were informed to revisit hospital as soon as symptoms (at least two of the mentioned symptoms) like fever, fatigue, sore throat, cough, body aches/myalgia, anosmia/hyposmia, ageusia/hypogeusia and diarrhea disappears at least for 3 days (the actual day on which symptom(s) disappeared was noted). They were also advised to revisit hospital if they feel any inconvenience with the treatment or they notice worsening of the symptoms. The efficacy of the two regimens was based on the duration of disappearance of symptoms. RT-PCR was repeated after 15 days of the diagnosis and on day 21 who came positive on day 15. Results: In group A, the symptoms of 34% patients started to disappear during 3-5 days (mean±SD, 4.86 ± 0.42 days) following therapy, it disappeared during 6-8 days (7.18 ± 0.37) in 28% patients, 22% were symptom free during 9-10 days (10.12 ± 0.12), and 16% took ˃10 days to become symptom free (12 ± 0.26). Similarly, 52% of group B patient were relieved during 3-5 days (4.01 ± 0.32) of therapy, 36% were symptom free during 6-8 days (6.32 ± 0.14), symptoms of 8% disappeared during 9-10days (9.06 ± 0.25), and 4% took ˃10 days (11 ± 0.0) to become symptom free. Conclusion: Combination of ivermectin and azithromycin was more effective in making patients symptom free than azithromycin alone.
References
Ang, Lee, Choi, Zhang, Lee, Herbal medicine and pattern identification for treating COVID-19: A rapid review of guidelines, Integr Med Res
Campbell, Benz, Ivermectin: a review of efficacy and safety, J Vet Pharmacol Ther
Canga, Prieto, Liébana, Martínez, Vega et al., The pharmacokinetics and interactions of ivermectin in humans--a mini-review, AAPS J
Crump, Omura, Ivermectin, 'wonderdrug' from Japan: The human use perspective, Proceed Japan Acad Series B
Crump, Ōmura, Ivermectin, 'wonder drug' from Japan: The human use perspective, Proc Jpn Acad Ser B Phys Biol Sci
Faisal, Rehman, Bahadur, Shinwari, Problem based learning in comparison with lecture based learning among medical students, Journal of Pakistan Medical Association
Faisal, Shinwari, Izzat, Academic performance of day scholars versus boarders in pharmacology examinations of a medical school in Pakistan, J Pak Med Assoc
Götz, Magar, Dornfeld, Giese, Pohlmann et al., Influenza A viruses escape from MxA restriction at the expense of efficient nuclear vRNP import, Sci. Rep
Kircik, Rosso, Layton, Schauber, Over 25 years of clinical experience with ivermectin: An overview of safety for an increasing number of indications, J Drugs Dermatol
Laing, Devaney, Ivermectin-old drug, new tricks?, Trends Parasitol
Lu, Chen, Chang, Potential therapeutic agents against COVID-19: What we know so far, J Chin Med Assoc
Lu, Stratton, Tang, Outbreak of pneumonia of unknown etiology in Wuhan, China: The mystery and the miracle, J Med Virol
Lundberg, Pinkham, Baer, Amaya, Narayanan et al., Nuclear import and export inhibitors alter capsid protein distribution in mammalian cells and reduce Venezuelan Equine Encephalitis Virus replication, Antivir. Res
Sohrabi, Alsafi, Neill, World Health Organization declares global emergency: A review of the 2019 novel coronavirus (COVID-19), Int J Surg
Tay, Fraser, Chan, Moreland, Rathore et al., Nuclear localization of dengue virus (DENV) 1-4 non-structural protein 5; protection against all 4 DENV serotypes by the inhibitor Ivermectin Antivir, Res
Voorhis, Huijsduijnen, Profile, William, Campbell et al., Nobel Laureates in Physiology or Medicine, Proc Natl Acad Sci
Wagstaff, Rawlinson, Hearps, Jans, An Alpha Screen(R)-based assay for high-throughput screening for specific inhibitors of nuclear import, J. Biomol. Screen
DOI record:
{
"DOI": "10.29309/tpmj/2021.28.05.5867",
"ISSN": [
"2071-7733",
"1024-8919"
],
"URL": "http://dx.doi.org/10.29309/TPMJ/2021.28.05.5867",
"abstract": "<jats:p>Objectives: To compare efficacy of azithromycin alone and in combination with ivermectin against the duration of novel corona virus symptoms. Study Design: Cross-sectional study. Setting: Shah Care Hospital, Peshawar. Period: April 5, 2020 to May 30, 2020. Material & Methods: Total patients included in the study were 100. Patients were divided into two groups by systematic random sampling: Group A: who received AZM (500mg once a day for 5 days), Group B: who received Ivermectin (12mg once a day for 5 days) and AZM (500mg once a day for 5 days). All the participants were informed to revisit hospital as soon as symptoms (at least two of the mentioned symptoms) like fever, fatigue, sore throat, cough, body aches/myalgia, anosmia/hyposmia, ageusia/hypogeusia and diarrhea disappears at least for 3 days (the actual day on which symptom(s) disappeared was noted). They were also advised to revisit hospital if they feel any inconvenience with the treatment or they notice worsening of the symptoms. The efficacy of the two regimens was based on the duration of disappearance of symptoms. RT-PCR was repeated after 15 days of the diagnosis and on day 21 who came positive on day 15. Results: In group A, the symptoms of 34% patients started to disappear during 3-5 days (mean±SD, 4.86 ± 0.42 days) following therapy, it disappeared during 6-8 days (7.18 ± 0.37) in 28% patients, 22% were symptom free during 9-10 days (10.12 ± 0.12), and 16% took ˃10 days to become symptom free (12 ± 0.26). Similarly, 52% of group B patient were relieved during 3-5 days (4.01 ± 0.32) of therapy, 36% were symptom free during 6-8 days (6.32 ± 0.14), symptoms of 8% disappeared during 9-10days (9.06 ± 0.25), and 4% took ˃10 days (11 ± 0.0) to become symptom free. Conclusion: Combination of ivermectin and azithromycin was more effective in making patients symptom free than azithromycin alone.</jats:p>",
"author": [
{
"affiliation": [],
"family": "Faisal",
"given": "Rizwan",
"sequence": "first"
},
{
"affiliation": [],
"family": "Shah",
"given": "Syed Furqan Ali",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hussain",
"given": "Mazhar",
"sequence": "additional"
}
],
"container-title": "The Professional Medical Journal",
"container-title-short": "TPMJ",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2021,
5,
7
]
],
"date-time": "2021-05-07T09:05:31Z",
"timestamp": 1620378331000
},
"deposited": {
"date-parts": [
[
2021,
11,
9
]
],
"date-time": "2021-11-09T10:43:16Z",
"timestamp": 1636454596000
},
"indexed": {
"date-parts": [
[
2024,
3,
28
]
],
"date-time": "2024-03-28T16:46:36Z",
"timestamp": 1711644396823
},
"is-referenced-by-count": 9,
"issue": "05",
"issued": {
"date-parts": [
[
2021,
5,
10
]
]
},
"journal-issue": {
"issue": "05",
"published-online": {
"date-parts": [
[
2021,
5,
5
]
]
}
},
"member": "11864",
"original-title": [],
"page": "737-741",
"prefix": "10.29309",
"published": {
"date-parts": [
[
2021,
5,
10
]
]
},
"published-online": {
"date-parts": [
[
2021,
5,
10
]
]
},
"publisher": "Independent Medical Trust",
"reference-count": 0,
"references-count": 0,
"relation": {},
"resource": {
"primary": {
"URL": "http://theprofesional.com/index.php/tpmj/article/view/5867"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subtitle": [],
"title": "Potential use of azithromycin alone and in combination with ivermectin in fighting against the symptoms of COVID-19.",
"type": "journal-article",
"volume": "28"
}