Results of a systematic review and meta-analysis of early studies on ivermectin in SARS-CoV-2 infection
Zsuzsanna Ragó, Barbara Tóth, Ágnes Szalenko-Tőkés, Zsolt Bella, Fanni Dembrovszky, Nelli Farkas, Szabolcs Kiss, Péter Hegyi, Mária Matuz, Noémi Tóth, Imre Hegedüs, Domokos Máthé, Dezső Csupor
GeroScience, doi:10.1007/s11357-023-00756-y
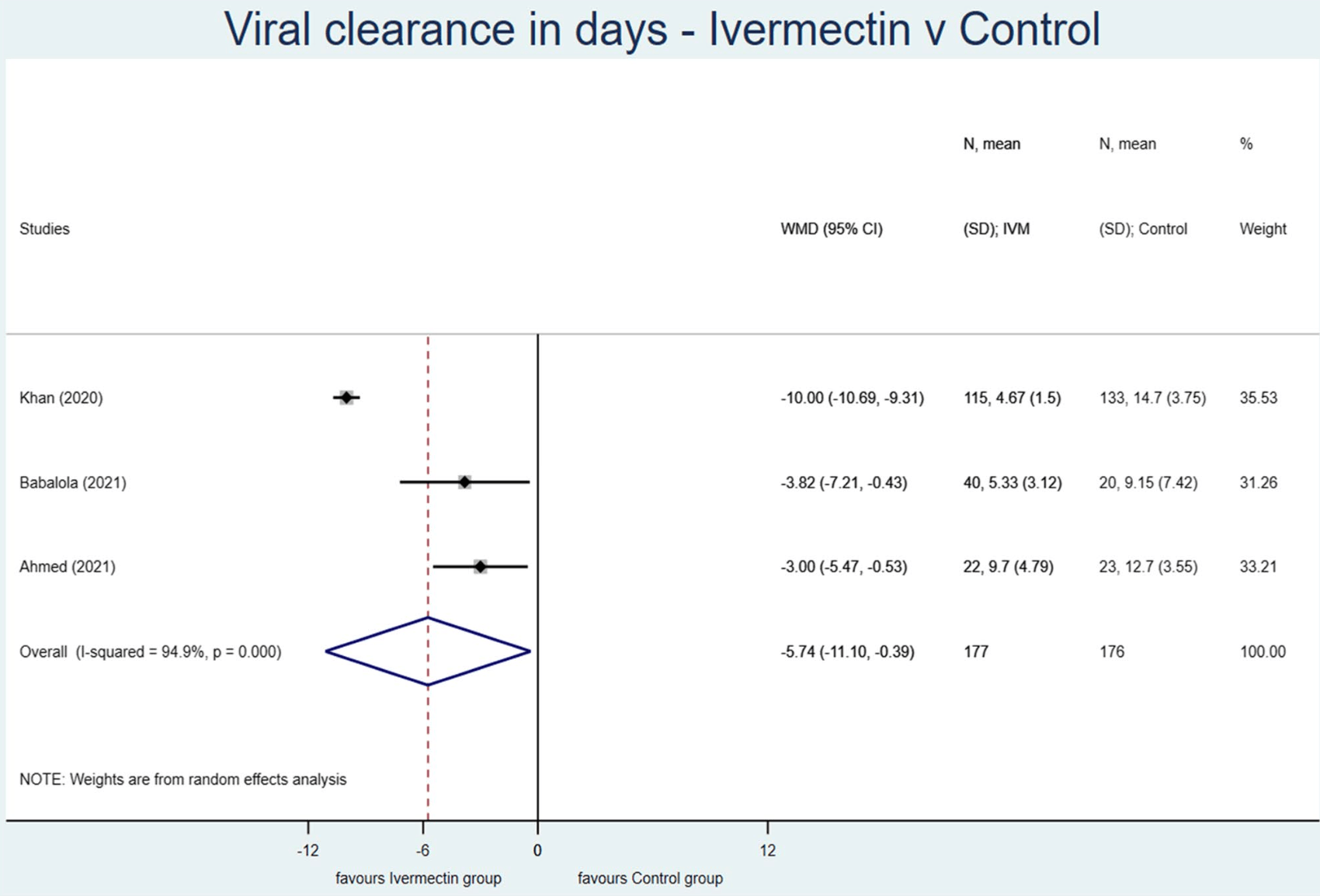
Ivermectin, an antiparasitic drug, has been repurposed for COVID-19 treatment during the SARS-CoV-2 pandemic. Although its antiviral efficacy was confirmed early in vitro and in preclinical studies, its clinical efficacy remained ambiguous. Our purpose was to assess the efficacy of ivermectin in terms of time to viral clearance based on the metaanalysis of available clinical trials at the closing date of the data search period, one year after the start of the pandemic. This meta-analysis was reported by following the PRISMA guidelines and by using the PICO format for formulating the question. The study protocol was registered on PROSPERO. Embase, MEDLINE (via PubMed), Cochrane Central Register of Controlled Trials (CENTRAL), bioRvix, and medRvix were searched for human studies of patients receiving ivermectin therapy with control groups. No language or publication status restrictions were applied. The search ended on 1/31/2021 exactly one year after WHO declared the public health emergency on novel coronavirus. The meta-analysis of three trials involving 382 patients revealed that the mean time to viral clearance was 5.74 days shorter in case of ivermectin treatment compared to the control groups [WMD = −5.74, 95% CI (−11.1, −0.39), p = 0.036]. Ivermectin has significantly reduced the time to viral clearance in mild to moderate COVID-19 diseases
Conflict of interest reported Abd-Elsalam et al. [36] 31 May
References
Abd-Elsalam, Noor, Badawi, Khalaf, Esmail et al., Clinical study evaluating the efficacy of ivermectin in COVID-19 treatment: A randomized controlled study, J Med Virol,
doi:10.1002/jmv.27122Ahmed, Karim, Ross, Hossain, Clemens et al., A five-day course of ivermectin for the treatment of COVID-19 may reduce the duration of illness, Int J Infect Dis
Arevalo, Pagotto, Pórfido, Daghero, Segovia et al., Ivermectin reduces in vivo coronavirus infection in a mouse experimental model, Scientific Rep
Babalola, Bode, Ajayi, Alakaloko, Akase et al., Ivermectin shows clinical benefits in mild to moderate COVID19: a randomized controlled double-blind, dose-response study in Lagos, QJM
Bartoszko, Siemieniuk, Kum, Qasim, Zeraatkar et al., Prophylaxis against covid-19: living systematic review and network meta-analysis
Biber, Harmelin, Ram, Shaham, Nemet, The effect of ivermectin on the viral load and culture viability in early treatment of nonhospitalized patients with mild COVID-19 -a doubleblind, randomized placebo-controlled trial, Int J Infect Dis,
doi:10.1016/j.ijid.2022.07.003Bryant, Lawrie, Dowswell, Fordham, Mitchell et al., Ivermectin for prevention and treatment of COVID-19 infection: a systematic review, meta-analysis, and trial sequential analysis to inform clinical guidelines, Am J Ther
Caly, Druce, Catton, Jans, Wagstaff, The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro, Antiviral res
Camprubí, Almuedo-Riera, Martí-Soler, Soriano, Hurtado et al., Lack of efficacy of standard doses of ivermectin in severe COVID-19 patients, PloS one
Chaccour, Casellas, Blanco-Di Matteo, Pineda, Fernandez-Montero et al., The effect of early treatment with ivermectin on viral load, symptoms and humoral response in patients with non-severe COVID-19: a pilot, double-blind, placebo-controlled, randomized clinical trial, EClinicalMed
Chandler, Serious neurological adverse events after ivermectin-do they occur beyond the indication of onchocerciasis?, Am J Trop Med Hyg
De Castro, Gregianin, Burger, Continuous highdose ivermectin appears to be safe in patients with acute myelogenous leukemia and could inform clinical repurposing for COVID-19 infection, Leuk Lymphoma,
doi:10.1080/10428194.2020.1786559De Melo, Lazarini, Larrous, Feige, Kornobis et al., Attenuation of clinical and immunological outcomes during SARS-CoV-2 infection by ivermectin, EMBO Mol Med,
doi:10.15252/emmm.202114122Draganov, Han, Bennett, Irvine, Lee, Ivermectin converts cold tumors hot and synergizes with immune checkpoint blockade for treatment of breast cancer, NPJ Breast Cancer,
doi:10.1038/s41523-021-00229-5Gonzalez, Gámez, Enciso, Maldonado, Palacios et al., Efficacy and safety of ivermectin and Hydroxychloroquine in patients with severe COVID-19: a randomized controlled trial, Infect Dis Rep,
doi:10.3390/idr14020020Gupta, Biswal, Panda, Ray, Rana, Binding mechanism and structural insights into the identified protein target of COVID-19 and importin-α with in-vitro effective drug ivermectin, J Biomol Struct Dyn
Guyatt, Oxman, Vist, Kunz, Falck-Ytter et al., GRADE: an emerging consensus on rating quality of evidence and strength of recommendations, Bmj
Guzzo, Furtek, Porras, Chen, Tipping et al., Safety, tolerability, and pharmacokinetics of escalating high doses of ivermectin in healthy adult subjects, The J of Clinical Pharma
Higgins, Thomas, Chandler, Cumpston, Li et al., Cochrane handbook for systematic reviews of interventions
Janabi, Effective anti-SARS-CoV-2 RNA dependent RNA polymerase drugs based on docking methods: the case of milbemycin, ivermectin, and baloxavir marboxil, Avicenna J Med Biotechnol
Jans, Wagstaff, Ivermectin as a broad-spectrum host-directed antiviral: the real deal?, Cells
Kerr, Cadegiani, Baldi, Lobo, Assagra et al., Corrected: Ivermectin Prophylaxis Used for COVID-19: A Citywide, Prospective, Observational Study of 223,128 Subjects Using Propensity Score Matching, Cureus,
doi:10.7759/cureus.c61Khan, Khan, Debnath, Nath, Mahtab et al., Ivermectin treatment may improve the prognosis of patients with COVID-19, Arch Bronconeumol
Kim, An, Kim, Hwang, Comparative efficacy and safety of pharmacological interventions for the treatment of COVID-19: a systematic review and network meta-analysis, PLoS med
Krolewiecki, Lifschitz, Moragas, Travacio, Valentini et al., Corrigendum to Antiviral effect of highdose ivermectin in adults with COVID-19: a proof-of-concept randomized trial, EClinicalMedicine,
doi:10.1016/j.eclinm.2021.100959Lehrer, Rheinstein, Ivermectin docks to the SARS-CoV-2 spike receptor-binding domain attached to ACE2, vivo
López-Medina, López, Hurtado, Dávalos, Ramirez et al., Effect oilf ivermectin on time to resolution of symptoms among adults with md COVID-19: a randomized clinical trial, JAMA,
doi:10.1001/jama.2021.3071Matsuyama, Kubli, Yoshinaga, Pfeffer, Mak, An aberrant STAT pathway is central to COVID-19, Cell Death & Diff
Mohan, Tiwari, Suri, Mittal, Patel et al., Single-dose oral ivermectin in mild and moderate COVID-19 (RIVET-COV): A single-centre randomized, placebo-controlled trial, J Infect Chemother,
doi:10.1016/j.jiac.2021.08.021Naggie, Boulware, Lindsell, Stewart, Gentile et al., Effect of ivermectin vs placebo on time to sustained recovery in outpatients with mild to moderate COVID-19: a randomized clinical trial, JAMA,
doi:10.1001/jama.2022.18590Navarro, Camprubí, Requena-Méndez, Buonfrate, Giorli et al., Safety of high-dose ivermectin: a systematic review and meta-analysis, J Antimicrob Chemother
Okumuş, Demirtürk, Çetinkaya, Güner, Avcı et al., Evaluation of the effectiveness and safety of adding ivermectin to treatment in severe COVID-19 patients, BMC Infect Dis
Padhy, Mohanty, Das, Meher, Therapeutic potential of ivermectin as add on treatment in COVID 19: a systematic review and meta-analysis: Ivermectin in COVID-19: A meta-analysis, J Pharm Pharm Sci
Rizzo, Ivermectin, antiviral properties and COVID-19: a possible new mechanism of action. Naunyn Schmiedebergs, Arch Pharmacol
Samaha, Mouawia, Fawaz, Hassan, Salami et al., Effects of a single dose of ivermectin on viral and clinical outcomes in asymptomatic SARS-CoV-2 infected subjects: a pilot clinical trial in Lebanon, Viruses,
doi:10.3390/v13060989Sterne, Hernán, Reeves, Savović, Berkman et al., ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions, Int J Epidemiol
Vallejos, Zoni, Bangher, Villamandos, Bobadilla et al., Ivermectin to prevent hospitalizations in patients with COVID-19 (IVERCOR-COVID19) a randomized, double-blind, placebo-controlled trial, BMC Infect Dis,
doi:10.1186/s12879-021-06348-5Wagstaff, Sivakumaran, Heaton, Harrich, Jans, Ivermectin is a specific inhibitor of importin α/β-mediated nuclear import able to inhibit replication of HIV-1 and dengue virus, Biochem J
Zein, Sulistiyana, Raffaelo, Pranata, Ivermectin and mortality in patients with COVID-19: a systematic review, meta-analysis, and meta-regression of randomized controlled trials, Diabetes Metab Syndr
Õmura, Crump, The life and times of ivermectin-a success story, Nat Rev Microbiol
DOI record:
{
"DOI": "10.1007/s11357-023-00756-y",
"ISSN": [
"2509-2715",
"2509-2723"
],
"URL": "http://dx.doi.org/10.1007/s11357-023-00756-y",
"abstract": "<jats:title>Abstract</jats:title><jats:p>Ivermectin, an antiparasitic drug, has been repurposed for COVID-19 treatment during the SARS-CoV-2 pandemic. Although its antiviral efficacy was confirmed early in vitro and in preclinical studies, its clinical efficacy remained ambiguous. Our purpose was to assess the efficacy of ivermectin in terms of time to viral clearance based on the meta-analysis of available clinical trials at the closing date of the data search period, one year after the start of the pandemic. This meta-analysis was reported by following the PRISMA guidelines and by using the PICO format for formulating the question. The study protocol was registered on PROSPERO. Embase, MEDLINE (via PubMed), Cochrane Central Register of Controlled Trials (CENTRAL), bioRvix, and medRvix were searched for human studies of patients receiving ivermectin therapy with control groups. No language or publication status restrictions were applied. The search ended on 1/31/2021 exactly one year after WHO declared the public health emergency on novel coronavirus. The meta-analysis of three trials involving 382 patients revealed that the mean time to viral clearance was 5.74 days shorter in case of ivermectin treatment compared to the control groups [WMD = −5.74, 95% CI (−11.1, −0.39), <jats:italic>p</jats:italic> = 0.036]. Ivermectin has significantly reduced the time to viral clearance in mild to moderate COVID-19 diseases compared to control groups. However, more eligible studies are needed for analysis to increase the quality of evidence of ivermectin use in COVID-19.</jats:p>",
"alternative-id": [
"756"
],
"assertion": [
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Received",
"name": "received",
"order": 1,
"value": "11 November 2022"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Accepted",
"name": "accepted",
"order": 2,
"value": "16 February 2023"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "First Online",
"name": "first_online",
"order": 3,
"value": "7 March 2023"
},
{
"group": {
"label": "Declarations",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 1
},
{
"group": {
"label": "Institutional review board statement",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 2,
"value": "Not applicable."
},
{
"group": {
"label": "Informed consent statement",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 3,
"value": "Not applicable."
},
{
"group": {
"label": "Competing interests",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 4,
"value": "The authors declare no competing interests."
}
],
"author": [
{
"affiliation": [],
"family": "Ragó",
"given": "Zsuzsanna",
"sequence": "first"
},
{
"affiliation": [],
"family": "Tóth",
"given": "Barbara",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Szalenko-Tőkés",
"given": "Ágnes",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bella",
"given": "Zsolt",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Dembrovszky",
"given": "Fanni",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Farkas",
"given": "Nelli",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kiss",
"given": "Szabolcs",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hegyi",
"given": "Péter",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Matuz",
"given": "Mária",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tóth",
"given": "Noémi",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hegedüs",
"given": "Imre",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-7343-0413",
"affiliation": [],
"authenticated-orcid": false,
"family": "Máthé",
"given": "Domokos",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Csupor",
"given": "Dezső",
"sequence": "additional"
}
],
"container-title": "GeroScience",
"container-title-short": "GeroScience",
"content-domain": {
"crossmark-restriction": false,
"domain": [
"link.springer.com"
]
},
"created": {
"date-parts": [
[
2023,
3,
7
]
],
"date-time": "2023-03-07T01:02:16Z",
"timestamp": 1678150936000
},
"deposited": {
"date-parts": [
[
2023,
3,
7
]
],
"date-time": "2023-03-07T01:08:14Z",
"timestamp": 1678151294000
},
"funder": [
{
"DOI": "10.13039/100010664",
"award": [
"No 739593: HCEMM"
],
"doi-asserted-by": "publisher",
"name": "H2020 Future and Emerging Technologies"
},
{
"DOI": "10.13039/501100015498",
"award": [
"2020.1.16-Jövő-2021-00013"
],
"doi-asserted-by": "publisher",
"name": "Innovációs és Technológiai Minisztérium"
},
{
"award": [
"Physical Virology Research Group"
],
"name": "MTA-TKI Grant"
},
{
"DOI": "10.13039/501100002332",
"doi-asserted-by": "crossref",
"name": "Semmelweis University"
}
],
"indexed": {
"date-parts": [
[
2023,
3,
7
]
],
"date-time": "2023-03-07T05:40:58Z",
"timestamp": 1678167658250
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2023,
3,
7
]
]
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
3,
7
]
],
"date-time": "2023-03-07T00:00:00Z",
"timestamp": 1678147200000
}
},
{
"URL": "https://creativecommons.org/licenses/by/4.0",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
3,
7
]
],
"date-time": "2023-03-07T00:00:00Z",
"timestamp": 1678147200000
}
}
],
"link": [
{
"URL": "https://link.springer.com/content/pdf/10.1007/s11357-023-00756-y.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/article/10.1007/s11357-023-00756-y/fulltext.html",
"content-type": "text/html",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/content/pdf/10.1007/s11357-023-00756-y.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "297",
"original-title": [],
"prefix": "10.1007",
"published": {
"date-parts": [
[
2023,
3,
7
]
]
},
"published-online": {
"date-parts": [
[
2023,
3,
7
]
]
},
"publisher": "Springer Science and Business Media LLC",
"reference": [
{
"DOI": "10.1016/S2213-2600(21)00270-8",
"author": "P Venkatesan",
"doi-asserted-by": "publisher",
"issue": "7",
"journal-title": "Lancet Respir Med.",
"key": "756_CR1",
"unstructured": "Venkatesan P. Repurposing drugs for treatment of COVID-19. Lancet Respir Med. 2021;9(7):e63. https://doi.org/10.1016/S2213-2600(21)00270-8.",
"volume": "9",
"year": "2021"
},
{
"DOI": "10.1038/nrmicro1048",
"author": "S Õmura",
"doi-asserted-by": "publisher",
"first-page": "984",
"issue": "12",
"journal-title": "Nat Rev Microbiol.",
"key": "756_CR2",
"unstructured": "Õmura S, Crump A. The life and times of ivermectin—a success story. Nat Rev Microbiol. 2004;2(12):984–9.",
"volume": "2",
"year": "2004"
},
{
"DOI": "10.21873/invivo.12134",
"author": "S Lehrer",
"doi-asserted-by": "publisher",
"first-page": "3023",
"issue": "5",
"journal-title": "in vivo.",
"key": "756_CR3",
"unstructured": "Lehrer S, Rheinstein PH. Ivermectin docks to the SARS-CoV-2 spike receptor-binding domain attached to ACE2. in vivo. 2020;34(5):3023–6.",
"volume": "34",
"year": "2020"
},
{
"DOI": "10.1042/BJ20120150",
"author": "KM Wagstaff",
"doi-asserted-by": "publisher",
"first-page": "851",
"issue": "3",
"journal-title": "Biochem J.",
"key": "756_CR4",
"unstructured": "Wagstaff KM, Sivakumaran H, Heaton SM, Harrich D, Jans DA. Ivermectin is a specific inhibitor of importin α/β-mediated nuclear import able to inhibit replication of HIV-1 and dengue virus. Biochem J. 2012;443(3):851–6.",
"volume": "443",
"year": "2012"
},
{
"author": "AHD Janabi",
"first-page": "246",
"issue": "4",
"journal-title": "Avicenna J Med Biotechnol.",
"key": "756_CR5",
"unstructured": "Janabi AHD. Effective anti-SARS-CoV-2 RNA dependent RNA polymerase drugs based on docking methods: the case of milbemycin, ivermectin, and baloxavir marboxil. Avicenna J Med Biotechnol. 2020;12(4):246.",
"volume": "12",
"year": "2020"
},
{
"DOI": "10.1080/07391102.2020.1839564",
"author": "PS Sen Gupta",
"doi-asserted-by": "publisher",
"first-page": "2217",
"issue": "5",
"journal-title": "J Biomol Struct Dyn.",
"key": "756_CR6",
"unstructured": "Sen Gupta PS, Biswal S, Panda SK, Ray AK, Rana MK. Binding mechanism and structural insights into the identified protein target of COVID-19 and importin-α with in-vitro effective drug ivermectin. J Biomol Struct Dyn. 2022;40(5):2217–26.",
"volume": "40",
"year": "2022"
},
{
"DOI": "10.1007/s00210-020-01902-5",
"author": "E Rizzo",
"doi-asserted-by": "publisher",
"first-page": "1153",
"issue": "7",
"journal-title": "Naunyn Schmiedebergs Arch Pharmacol.",
"key": "756_CR7",
"unstructured": "Rizzo E. Ivermectin, antiviral properties and COVID-19: a possible new mechanism of action. Naunyn Schmiedebergs Arch Pharmacol. 2020;393(7):1153–6.",
"volume": "393",
"year": "2020"
},
{
"DOI": "10.1038/s41418-020-00633-7",
"author": "T Matsuyama",
"doi-asserted-by": "publisher",
"first-page": "3209",
"issue": "12",
"journal-title": "Cell Death & Diff.",
"key": "756_CR8",
"unstructured": "Matsuyama T, Kubli SP, Yoshinaga SK, Pfeffer K, Mak TW. An aberrant STAT pathway is central to COVID-19. Cell Death & Diff. 2020;27(12):3209–25.",
"volume": "27",
"year": "2020"
},
{
"DOI": "10.1016/j.pt.2017.02.004",
"author": "R Laing",
"doi-asserted-by": "publisher",
"first-page": "463",
"issue": "6",
"journal-title": "Trends Parasitol.",
"key": "756_CR9",
"unstructured": "Laing R, Gillan V, Devaney E. Ivermectin - old drug, new tricks? Trends Parasitol. 2017;33(6):463–72. https://doi.org/10.1016/j.pt.2017.02.004.",
"volume": "33",
"year": "2017"
},
{
"DOI": "10.1038/s41523-021-00229-5",
"doi-asserted-by": "publisher",
"key": "756_CR10",
"unstructured": "Draganov D, Han Z, Rana A, Bennett N, Irvine DJ, Lee PP. Ivermectin converts cold tumors hot and synergizes with immune checkpoint blockade for treatment of breast cancer. NPJ Breast Cancer. 2021;7(1):22. https://doi.org/10.1038/s41523-021-00229-5."
},
{
"DOI": "10.1080/10428194.2020.1786559",
"author": "CG de Castro",
"doi-asserted-by": "publisher",
"first-page": "2536",
"issue": "10",
"journal-title": "Leuk Lymphoma.",
"key": "756_CR11",
"unstructured": "de Castro CG, Jr., Gregianin LJ, Burger JA. Continuous high-dose ivermectin appears to be safe in patients with acute myelogenous leukemia and could inform clinical repurposing for COVID-19 infection. Leuk Lymphoma. 2020;61(10):2536–7. https://doi.org/10.1080/10428194.2020.1786559.",
"volume": "61",
"year": "2020"
},
{
"DOI": "10.1038/s41598-021-86679-0",
"doi-asserted-by": "crossref",
"key": "756_CR12",
"unstructured": "Arevalo AP, Pagotto R, Pórfido JL, Daghero H, Segovia M, Yamasaki K, et al. Ivermectin reduces in vivo coronavirus infection in a mouse experimental model. Scientific Rep. 2021;11(1):1-12."
},
{
"DOI": "10.15252/emmm.202114122",
"author": "GD de Melo",
"doi-asserted-by": "publisher",
"issue": "8",
"journal-title": "EMBO Mol Med.",
"key": "756_CR13",
"unstructured": "de Melo GD, Lazarini F, Larrous F, Feige L, Kornobis E, Levallois S, et al. Attenuation of clinical and immunological outcomes during SARS-CoV-2 infection by ivermectin. EMBO Mol Med. 2021;13(8):e14122. https://doi.org/10.15252/emmm.202114122.",
"volume": "13",
"year": "2021"
},
{
"DOI": "10.3390/cells9092100",
"author": "DA Jans",
"doi-asserted-by": "publisher",
"first-page": "2100",
"issue": "9",
"journal-title": "Cells.",
"key": "756_CR14",
"unstructured": "Jans DA, Wagstaff KM. Ivermectin as a broad-spectrum host-directed antiviral: the real deal? Cells. 2020;9(9):2100.",
"volume": "9",
"year": "2020"
},
{
"DOI": "10.1016/j.antiviral.2020.104787",
"author": "L Caly",
"doi-asserted-by": "publisher",
"journal-title": "Antiviral res.",
"key": "756_CR15",
"unstructured": "Caly L, Druce JD, Catton MG, Jans DA, Wagstaff KM. The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antiviral res. 2020;178:104787.",
"volume": "178",
"year": "2020"
},
{
"DOI": "10.1177/009127002237994",
"author": "CA Guzzo",
"doi-asserted-by": "publisher",
"first-page": "1122",
"issue": "10",
"journal-title": "The J of Clinical Pharma.",
"key": "756_CR16",
"unstructured": "Guzzo CA, Furtek CI, Porras AG, Chen C, Tipping R, Clineschmidt CM, et al. Safety, tolerability, and pharmacokinetics of escalating high doses of ivermectin in healthy adult subjects. The J of Clinical Pharma. 2002;42(10):1122–33.",
"volume": "42",
"year": "2002"
},
{
"DOI": "10.1093/jac/dkz524",
"author": "M Navarro",
"doi-asserted-by": "publisher",
"first-page": "827",
"issue": "4",
"journal-title": "J Antimicrob Chemother.",
"key": "756_CR17",
"unstructured": "Navarro M, Camprubí D, Requena-Méndez A, Buonfrate D, Giorli G, Kamgno J, et al. Safety of high-dose ivermectin: a systematic review and meta-analysis. J Antimicrob Chemother. 2020;75(4):827–34.",
"volume": "75",
"year": "2020"
},
{
"DOI": "10.4269/ajtmh.17-0042",
"author": "RE Chandler",
"doi-asserted-by": "publisher",
"first-page": "382",
"issue": "2",
"journal-title": "Am J Trop Med Hyg.",
"key": "756_CR18",
"unstructured": "Chandler RE. Serious neurological adverse events after ivermectin—do they occur beyond the indication of onchocerciasis? Am J Trop Med Hyg. 2018;98(2):382.",
"volume": "98",
"year": "2018"
},
{
"key": "756_CR19",
"unstructured": "EMA advises against use of ivermectin for the prevention or treatment of COVID-19 outside randomised clinical trials. https://www.ema.europa.eu/en/news/ema-advises-against-use-ivermectin-prevention-treatment-COVID-19-outside-randomised-clinical-trials. (2021). Accessed."
},
{
"key": "756_CR20",
"unstructured": "Why you should not use ivermectin to treat or prevent COVID-19. https://www.fda.gov/consumers/consumer-updates/why-you-should-not-use-ivermectin-treat-or-prevent-COVID-19 (2021). Accessed."
},
{
"key": "756_CR21",
"unstructured": "Merck statement on ivermectin use during the COVID-19 pandemic. https://www.merck.com/news/merck-statement-on-ivermectin-use-during-the-COVID-19-pandemic/. (2021). Accessed."
},
{
"DOI": "10.1002/9781119536604",
"author": "JP Higgins",
"doi-asserted-by": "publisher",
"key": "756_CR22",
"unstructured": "Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane handbook for systematic reviews of interventions: John Wiley & Sons; 2019.",
"volume-title": "Cochrane handbook for systematic reviews of interventions",
"year": "2019"
},
{
"DOI": "10.1136/bmj.i4919",
"doi-asserted-by": "crossref",
"key": "756_CR23",
"unstructured": "Sterne J, Hernán M, Reeves B, Savović J, Berkman N, Viswanathan M. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions BMJ. 2016; 355: i4919. Int J Epidemiol. 2018;1."
},
{
"DOI": "10.1136/bmj.39489.470347.AD",
"author": "GH Guyatt",
"doi-asserted-by": "publisher",
"first-page": "924",
"issue": "7650",
"journal-title": "Bmj.",
"key": "756_CR24",
"unstructured": "Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. Bmj. 2008;336(7650):924–6.",
"volume": "336",
"year": "2008"
},
{
"DOI": "10.1371/journal.pone.0242184",
"author": "D Camprubí",
"doi-asserted-by": "publisher",
"issue": "11",
"journal-title": "PloS one.",
"key": "756_CR25",
"unstructured": "Camprubí D, Almuedo-Riera A, Martí-Soler H, Soriano A, Hurtado JC, Subirà C, et al. Lack of efficacy of standard doses of ivermectin in severe COVID-19 patients. PloS one. 2020;15(11):e0242184.",
"volume": "15",
"year": "2020"
},
{
"DOI": "10.1016/j.eclinm.2020.100720",
"author": "C Chaccour",
"doi-asserted-by": "publisher",
"journal-title": "EClinicalMed.",
"key": "756_CR26",
"unstructured": "Chaccour C, Casellas A, Blanco-Di Matteo A, Pineda I, Fernandez-Montero A, Ruiz-Castillo P, et al. The effect of early treatment with ivermectin on viral load, symptoms and humoral response in patients with non-severe COVID-19: a pilot, double-blind, placebo-controlled, randomized clinical trial. EClinicalMed. 2021;32:100720.",
"volume": "32",
"year": "2021"
},
{
"DOI": "10.1093/qjmed/hcab035",
"author": "OE Babalola",
"doi-asserted-by": "publisher",
"first-page": "780",
"issue": "11",
"journal-title": "QJM.",
"key": "756_CR27",
"unstructured": "Babalola OE, Bode CO, Ajayi AA, Alakaloko FM, Akase IE, Otrofanowei E, et al. Ivermectin shows clinical benefits in mild to moderate COVID19: a randomized controlled double-blind, dose-response study in Lagos. QJM. 2021;114(11):780–8.",
"volume": "114",
"year": "2021"
},
{
"DOI": "10.1016/j.ijid.2020.11.191",
"author": "S Ahmed",
"doi-asserted-by": "publisher",
"first-page": "214",
"journal-title": "Int J Infect Dis.",
"key": "756_CR28",
"unstructured": "Ahmed S, Karim MM, Ross AG, Hossain MS, Clemens JD, Sumiya MK, et al. A five-day course of ivermectin for the treatment of COVID-19 may reduce the duration of illness. Int J Infect Dis. 2021;103:214–6.",
"volume": "103",
"year": "2021"
},
{
"DOI": "10.1016/j.arbres.2020.08.007",
"author": "MSI Khan",
"doi-asserted-by": "publisher",
"first-page": "828",
"issue": "12",
"journal-title": "Arch Bronconeumol.",
"key": "756_CR29",
"unstructured": "Khan MSI, Khan MSI, Debnath CR, Nath PN, Al Mahtab M, Nabeka H, et al. Ivermectin treatment may improve the prognosis of patients with COVID-19. Arch Bronconeumol. 2020;56(12):828.",
"volume": "56",
"year": "2020"
},
{
"DOI": "10.1186/s12879-021-06104-9",
"author": "N Okumuş",
"doi-asserted-by": "publisher",
"first-page": "1",
"issue": "1",
"journal-title": "BMC Infect Dis.",
"key": "756_CR30",
"unstructured": "Okumuş N, Demirtürk N, Çetinkaya RA, Güner R, Avcı İY, Orhan S, et al. Evaluation of the effectiveness and safety of adding ivermectin to treatment in severe COVID-19 patients. BMC Infect Dis. 2021;21(1):1–11.",
"volume": "21",
"year": "2021"
},
{
"DOI": "10.18433/jpps31457",
"author": "BM Padhy",
"doi-asserted-by": "publisher",
"first-page": "462",
"journal-title": "J Pharm Pharm Sci.",
"key": "756_CR31",
"unstructured": "Padhy BM, Mohanty RR, Das S, Meher BR. Therapeutic potential of ivermectin as add on treatment in COVID 19: a systematic review and meta-analysis: Ivermectin in COVID-19: A meta-analysis. J Pharm Pharm Sci. 2020;23:462–9.",
"volume": "23",
"year": "2020"
},
{
"DOI": "10.1136/bmj.n949",
"doi-asserted-by": "crossref",
"key": "756_CR32",
"unstructured": "Bartoszko JJ, Siemieniuk RA, Kum E, Qasim A, Zeraatkar D, Ge L, et al. Prophylaxis against covid-19: living systematic review and network meta-analysis. bmj. 2021:373."
},
{
"DOI": "10.1016/j.dsx.2021.102186",
"author": "AFMZ Zein",
"doi-asserted-by": "publisher",
"issue": "4",
"journal-title": "Diabetes Metab Syndr.",
"key": "756_CR33",
"unstructured": "Zein AFMZ, Sulistiyana CS, Raffaelo WM, Pranata R. Ivermectin and mortality in patients with COVID-19: a systematic review, meta-analysis, and meta-regression of randomized controlled trials. Diabetes Metab Syndr. 2021;15(4):102186.",
"volume": "15",
"year": "2021"
},
{
"DOI": "10.1097/MJT.0000000000001402",
"author": "A Bryant",
"doi-asserted-by": "publisher",
"issue": "4",
"journal-title": "Am J Ther.",
"key": "756_CR34",
"unstructured": "Bryant A, Lawrie TA, Dowswell T, Fordham EJ, Mitchell S, Hill SR, et al. Ivermectin for prevention and treatment of COVID-19 infection: a systematic review, meta-analysis, and trial sequential analysis to inform clinical guidelines. Am J Ther. 2021;28(4):e434.",
"volume": "28",
"year": "2021"
},
{
"DOI": "10.1371/journal.pmed.1003501",
"author": "MS Kim",
"doi-asserted-by": "publisher",
"issue": "12",
"journal-title": "PLoS med.",
"key": "756_CR35",
"unstructured": "Kim MS, An MH, Kim WJ, Hwang T-H. Comparative efficacy and safety of pharmacological interventions for the treatment of COVID-19: a systematic review and network meta-analysis. PLoS med. 2020;17(12):e1003501.",
"volume": "17",
"year": "2020"
},
{
"DOI": "10.1002/jmv.27122",
"doi-asserted-by": "publisher",
"key": "756_CR36",
"unstructured": "Abd-Elsalam S, Noor RA, Badawi R, Khalaf M, Esmail ES, Soliman S, Abd El Ghafar MS, Elbahnasawy M, Moustafa EF, Hassany SM, Medhat MA, Ramadan HK, Eldeen MAS, Alboraie M, Cordie A, Esmat G. Clinical study evaluating the efficacy of ivermectin in COVID-19 treatment: A randomized controlled study. J Med Virol. 2021;93(10):5833–5838. https://doi.org/10.1002/jmv.27122."
},
{
"DOI": "10.1001/jama.2021.3071",
"author": "E López-Medina",
"doi-asserted-by": "publisher",
"first-page": "1426",
"issue": "14",
"journal-title": "JAMA.",
"key": "756_CR37",
"unstructured": "López-Medina E, López P, Hurtado IC, Dávalos DM, Ramirez O, Martínez E, Díazgranados JA, Oñate JM, Chavarriaga H, Herrera S, Parra B, Libreros G, Jaramillo R, Avendaño AC, Toro DF, Torres M, Lesmes MC, Rios CA, Caicedo I. Effect oilf ivermectin on time to resolution of symptoms among adults with md COVID-19: a randomized clinical trial. JAMA. 2021;325(14):1426–35. https://doi.org/10.1001/jama.2021.3071.",
"volume": "325",
"year": "2021"
},
{
"DOI": "10.1186/s12879-021-06348-5",
"author": "J Vallejos",
"doi-asserted-by": "publisher",
"first-page": "635",
"issue": "1",
"journal-title": "BMC Infect Dis.",
"key": "756_CR38",
"unstructured": "Vallejos J, Zoni R, Bangher M, Villamandos S, Bobadilla A, Plano F, Campias C, Chaparro Campias E, Medina MF, Achinelli F, Guglielmone HA, Ojeda J, Farizano Salazar D, Andino G, Kawerin P, Dellamea S, Aquino AC, Flores V, Martemucci CN, Martinez SM, Segovia JE, Reynoso PI, Sosa NC, Robledo ME, Guarrochena JM, Vernengo MM, Ruiz Diaz N, Meza E, Aguirre MG. Ivermectin to prevent hospitalizations in patients with COVID-19 (IVERCOR-COVID19) a randomized, double-blind, placebo-controlled trial. BMC Infect Dis. 2021;21(1):635. https://doi.org/10.1186/s12879-021-06348-5.",
"volume": "21",
"year": "2021"
},
{
"DOI": "10.1016/j.jiac.2021.08.021",
"author": "A Mohan",
"doi-asserted-by": "publisher",
"first-page": "1743",
"issue": "12",
"journal-title": "J Infect Chemother.",
"key": "756_CR39",
"unstructured": "Mohan A, Tiwari P, Suri TM, Mittal S, Patel A, Jain A, Velpandian T, Das US, Boppana TK, Pandey RM, Shelke SS, Singh AR, Bhatnagar S, Masih S, Mahajan S, Dwivedi T, Sahoo B, Pandit A, Bhopale S, Vig S, Gupta R, Madan K, Hadda V, Gupta N, Garg R, Meena VP, Guleria R. Single-dose oral ivermectin in mild and moderate COVID-19 (RIVET-COV): A single-centre randomized, placebo-controlled trial. J Infect Chemother. 2021;27(12):1743–9. https://doi.org/10.1016/j.jiac.2021.08.021.",
"volume": "27",
"year": "2021"
},
{
"DOI": "10.3390/v13060989",
"author": "AA Samaha",
"doi-asserted-by": "publisher",
"first-page": "989",
"issue": "6",
"journal-title": "Viruses.",
"key": "756_CR40",
"unstructured": "Samaha AA, Mouawia H, Fawaz M, Hassan H, Salami A, Bazzal AA, Saab HB, Al-Wakeel M, Alsaabi A, Chouman M, Moussawi MA, Ayoub H, Raad A, Hajjeh O, Eid AH, Raad H. Effects of a single dose of ivermectin on viral and clinical outcomes in asymptomatic SARS-CoV-2 infected subjects: a pilot clinical trial in Lebanon. Viruses. 2021;13(6):989. https://doi.org/10.3390/v13060989.",
"volume": "13",
"year": "2021"
},
{
"DOI": "10.3390/idr14020020",
"author": "JL Beltran Gonzalez",
"doi-asserted-by": "publisher",
"first-page": "160",
"issue": "2",
"journal-title": "Infect Dis Rep",
"key": "756_CR41",
"unstructured": "Beltran Gonzalez JL, González Gámez M, Mendoza Enciso EA, Esparza Maldonado RJ, Hernández Palacios D, Dueñas Campos S, Robles IO, Macías Guzmán MJ, García Díaz AL, Gutiérrez Peña CM, Martinez Medina L, Monroy Colin VA, Arreola Guerra JM. Efficacy and safety of ivermectin and Hydroxychloroquine in patients with severe COVID-19: a randomized controlled trial. Infect Dis Rep. 2022;14(2):160–8. https://doi.org/10.3390/idr14020020.",
"volume": "14",
"year": "2022"
},
{
"DOI": "10.1016/j.eclinm.2021.100959",
"doi-asserted-by": "publisher",
"key": "756_CR42",
"unstructured": "Krolewiecki A, Lifschitz A, Moragas M, Travacio M, Valentini R, Alonso DF, et al. Corrigendum to Antiviral effect of high-dose ivermectin in adults with COVID-19: a proof-of-concept randomized trial. EClinicalMedicine. 2021;37:100959. https://doi.org/10.1016/j.eclinm.2021.100959."
},
{
"DOI": "10.1016/j.ijid.2022.07.003",
"author": "A Biber",
"doi-asserted-by": "publisher",
"first-page": "733",
"journal-title": "Int J Infect Dis.",
"key": "756_CR43",
"unstructured": "Biber A, Harmelin G, Lev D, Ram L, Shaham A, Nemet I, et al. The effect of ivermectin on the viral load and culture viability in early treatment of nonhospitalized patients with mild COVID-19 - a double-blind, randomized placebo-controlled trial. Int J Infect Dis. 2022;122:733–40. https://doi.org/10.1016/j.ijid.2022.07.003.",
"volume": "122",
"year": "2022"
},
{
"DOI": "10.1001/jama.2022.18590",
"author": "S Naggie",
"doi-asserted-by": "publisher",
"first-page": "1595",
"issue": "16",
"journal-title": "JAMA.",
"key": "756_CR44",
"unstructured": "Naggie S, Boulware DR, Lindsell CJ, Stewart TG, Gentile N, Collins S, et al. Effect of ivermectin vs placebo on time to sustained recovery in outpatients with mild to moderate COVID-19: a randomized clinical trial. JAMA. 2022;328(16):1595–603. https://doi.org/10.1001/jama.2022.18590.",
"volume": "328",
"year": "2022"
},
{
"DOI": "10.7759/cureus.c61",
"doi-asserted-by": "publisher",
"key": "756_CR45",
"unstructured": "Kerr L, Cadegiani FA, Baldi F, Lobo RB, Assagra WLO, Proença FC, et al. Corrected: Ivermectin Prophylaxis Used for COVID-19: A Citywide, Prospective, Observational Study of 223,128 Subjects Using Propensity Score Matching. Cureus. 2022;14(3):c6. https://doi.org/10.7759/cureus.c61."
}
],
"reference-count": 45,
"references-count": 45,
"relation": {},
"resource": {
"primary": {
"URL": "https://link.springer.com/10.1007/s11357-023-00756-y"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Geriatrics and Gerontology",
"Aging"
],
"subtitle": [],
"title": "Results of a systematic review and meta-analysis of early studies on ivermectin in SARS-CoV-2 infection",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1007/springer_crossmark_policy"
}