Efficacy and safety of ivermectin in the treatment of mild to moderate COVID-19 infection: a randomized, double-blind, placebo-controlled trial
Anan Manomaipiboon, Kittisak Pholtawornkulchai, Sujaree Poopipatpab, Swangjit Suraamornkul, Jakravoot Maneerit, Wiroj Ruksakul, Uraporn Phumisantiphong, Thananda Trakarnvanich
Trials, doi:10.1186/s13063-022-06649-3
Background: The emergent outbreak of coronavirus disease , caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has emphasized the requirement for therapeutic opportunities to overcome this pandemic. Ivermectin is an antiparasitic drug that has shown effectiveness against various agents, including SARS-CoV-2. This study aimed to assess the efficacy of ivermectin treatment compared with the standard of care (SOC) among people with mild to moderate COVID-19 symptoms.
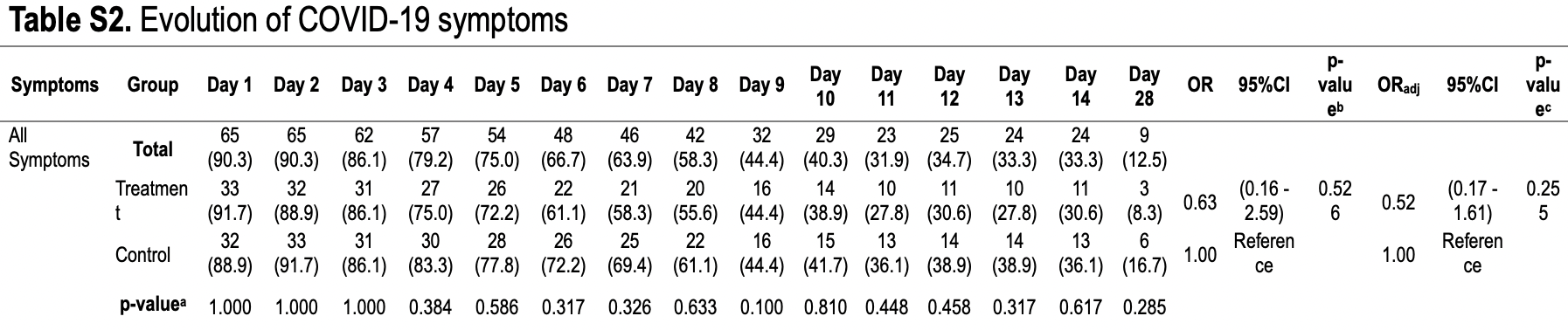
Methods: In this randomized, double-blind, placebo-controlled, single-center, parallel-arm, superiority trial among adult hospitalized patients with mild to moderate COVID-19, 72 patients (mean age 48.57 ± 14.80 years) were randomly assigned to either the ivermectin (n=36) or placebo (n=36) group, along with receiving standard care. We aimed to compare the negativity of reverse transcription polymerase chain reaction (RT-PCR) result at days 7 and 14 of enrolment as the primary outcome. The secondary outcomes were duration of hospitalization, frequency of clinical worsening, survival on day 28, and adverse events. Results: At days 7 and 14, no differences were observed in the proportion of PCR-positive patients (RR 0.97 at day 7 (p=0.759) and 0.95 at day 14 (p=0.813). No significant differences were found between the groups for any of the secondary endpoints, and no adverse events were reported.
Conclusion: No difference was found in the proportion of PCR-positive cases after treatment with ivermectin compared with standard care among patients with mild to moderate COVID-19 symptoms. However, early symptomatic recovery was observed without side effects. Trial registration: ClinicalTrials.gov NCT05076253. Registered on 8 October 2021, prospectively.
Supplementary Information The online version contains supplementary material available at https:// doi. org/ 10. 1186/ s13063-022-06649-3.
Additional file 1. Authors' contributions AM: supervised the project, had full access to the data in the study, and contributed to the study design. KP: contributed to data collection and conceived and designed the study. SS: collected and interpreted the data. JM: contributed in reviewing the design of the study and acquiring the data. WR: coordinated sample collection and oversaw data collection. UP: conducted and analyzed the laboratory results. TT: designed the study, analyzed, and interpreted the data and contributed towards the writing of the manuscript. All authors approve the final version of the manuscript for submission.
Declarations Ethics approval and consent to participate The study was conducted according to the guidelines of the Declaration of Helsinki and Good Clinical Practice Guidelines. The trial was approved by the Vajira Ethics Committee, approval no 171/64. Written informed consent was obtained from all subjects involved in the study.
Consent for publication Consent for publication is not applicable.
Competing interests The authors declare they have no competing interests.
Publisher's Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Ahmed, Karim, Ross, Hossain, Clemens et al., A five-day course of ivermectin for the treatment of COVID-19 may reduce the duration of illness, Int J Infect Dis,
doi:10.1016/j.ijid.2020.11.191Ahmed, Karim, Ross, Hossain, Clemens et al., A five-day course of ivermectin for the treatment of COVID-19 may reduce the duration of illness, Int J Infect Dis,
doi:10.1016/j.ijid.2020.11.191Alam, Murshed, Ebhiuyan, Saber, Alam et al., A case series of 100 COVID-19 positive patients treated with combination of ivermectin and doxycycline, J Bangladesh Coll Phys,
doi:10.3329/jbcps.v38i0.47512Bryant, Lawrie, Dowswell, Fordham, Mitchell et al., Ivermectin for prevention and treatment of COVID-19 infection: a systematic review, meta-analysis, and trial sequential analysis to inform clinical guidelines, Am J Ther,
doi:10.1097/MJT.0000000000001402Bukhari, Asghar, Perveen, Hayat, Mangat et al., Efficacy of ivermectin in COVID-19 patients with mild to moderate disease, medRxiv,
doi:10.1101/2021.02.02.21250840Camprubí, Almuedo-Riera, Martí-Soler, Soriano, Hurtado et al., Lack of efficacy of standard doses of ivermectin in severe COVID-19 patients, PLoS One,
doi:10.1371/journal.pone.0242184Chaccour, The effect of early treatment with ivermectin on viral load, symptoms and humoral response in patients with non-severe COVID-19: a pilot, double-blind, placebo-controlled, randomized clinical trial, EClinicalmedicine
Chachar, Khan, Asif, Tanveer, Khaqan et al., Effectiveness of ivermectin in SARS-CoV-2/COVID-19 patients, Int J Sci
Chen, Kubo, Ivermectin and its target molecules: shared and unique modulation mechanisms of ion channels and receptors by ivermectin, J Physiol,
doi:10.1113/JP275236Choudhary, Sharma, Potential use of hydroxychloroquine, ivermectin and azithromycin drugs in fighting COVID-19: trends, scope and relevance, New Microbes New Infect,
doi:10.1016/j.nmni.2020.100684Cortegiani, Ippolito, Greco, Granone, Protti et al., Rationale and evidence on the use of tocilizumab in COVID-19: a systematic review, Pulmonology,
doi:10.1016/j.pulmoe.2020.07.003Formiga, Leblanc, De Souza Rebouças, Farias, De Oliveira et al., Ivermectin: an award-winning drug with expected antiviral activity against COVID-19, J Control Release,
doi:10.1016/j.jconrel.2020.10.009Galan, Santos, Asato, Araújo, De Lima Moreira et al., Phase 2 randomized study on chloroquine, hydroxychloroquine or ivermectin in hospitalized patients with severe manifestations of SARS-CoV-2 infection, Pathog Glob Health,
doi:10.1080/20477724.2021.1890887Hashim, Controlled randomized clinical trial on using ivermectin with doxycycline for treating COVID-19 patients in Baghdad, Iraq,
doi:10.1101/2020.10.26.20219345Heidary, Gharebaghi, Ivermectin: a systematic review from antiviral effects to COVID-19 complementary regimen, J Antibiot,
doi:10.1038/s41429-020-0336-zKhan, Khan, Debnath, Nath, Mahtab et al., Ivermectin treatment may improve the prognosis of patients with COVID-19, Arch Bronconeumol,
doi:10.1016/j.arbres.2020.08.007Kory, Gu, Varon, Review of the emerging evidence demonstrating the efficacy of ivermectin in the prophylaxis and treatment of COVID-19, Am J Ther
Mahmud, Rahman, Alam, Ahmed, Kabir et al., Ivermectin in combination with doxycycline for treating COVID-19 symptoms: a randomized trial, J Int Med Res,
doi:10.1177/03000605211013550Nurullah, Demirtürk, Çetinkaya, Güner, Avcı et al., Evaluation of the effectiveness and safety of adding ivermectin to treatment in severe COVID-19 patients, BMC Infect Dis,
doi:10.1186/s12879-021-06104-9Rajter, Sherman, Fatteh, Vogel, Sacks et al., Use of ivermectin is associated with lower mortality in hospitalized patients with coronavirus disease 2019: the ivermectin in COVID nineteen study, Chest,
doi:10.1016/j.chest.2020.10.009Ravikirti, Pattadar, Raj, Agarwal, Biswas, Ivermectin as a potential treatment for mild to moderate COVID-19 -a double blind randomized placebo-controlled trial, medRxiv,
doi:10.1101/2021.01.05.21249310Roman, Burela, Pasupuleti, Piscoya, Vidal et al., Ivermectin for the treatment of COVID-19: a systematic review and metaanalysis of randomized controlled trials, Clin Infect Dis,
doi:10.1093/cid/ciab591Shahbaznejad, Davoudi, Eslami, Markowitz, Navaeifar et al., Effects of ivermectin in patients with COVID-19: a multicenter, double-blind, randomized, controlled clinical trial, Clin Ther,
doi:10.1016/j.clinthera.2021.04.007Yang, Atkinson, Wang, Lee, Bogoyevitch et al., The broad spectrum antiviral ivermectin targets the host nuclear transport importin α/β1 heterodimer, Antiviral Res,
doi:10.1016/j.antiviral.2020.104760DOI record:
{
"DOI": "10.1186/s13063-022-06649-3",
"ISSN": [
"1745-6215"
],
"URL": "http://dx.doi.org/10.1186/s13063-022-06649-3",
"abstract": "<jats:title>Abstract</jats:title><jats:sec>\n <jats:title>Background</jats:title>\n <jats:p>The emergent outbreak of coronavirus disease (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has emphasized the requirement for therapeutic opportunities to overcome this pandemic. Ivermectin is an antiparasitic drug that has shown effectiveness against various agents, including SARS-CoV-2. This study aimed to assess the efficacy of ivermectin treatment compared with the standard of care (SOC) among people with mild to moderate COVID-19 symptoms.</jats:p>\n </jats:sec><jats:sec>\n <jats:title>Methods</jats:title>\n <jats:p>In this randomized, double-blind, placebo-controlled, single-center, parallel-arm, superiority trial among adult hospitalized patients with mild to moderate COVID-19, 72 patients (mean age 48.57 ± 14.80 years) were randomly assigned to either the ivermectin (<jats:italic>n</jats:italic>=36) or placebo (<jats:italic>n</jats:italic>=36) group, along with receiving standard care. We aimed to compare the negativity of reverse transcription polymerase chain reaction (RT-PCR) result at days 7 and 14 of enrolment as the primary outcome. The secondary outcomes were duration of hospitalization, frequency of clinical worsening, survival on day 28, and adverse events.</jats:p>\n </jats:sec><jats:sec>\n <jats:title>Results</jats:title>\n <jats:p>At days 7 and 14, no differences were observed in the proportion of PCR-positive patients (RR 0.97 at day 7 (<jats:italic>p</jats:italic>=0.759) and 0.95 at day 14 (<jats:italic>p</jats:italic>=0.813). No significant differences were found between the groups for any of the secondary endpoints, and no adverse events were reported.</jats:p>\n </jats:sec><jats:sec>\n <jats:title>Conclusion</jats:title>\n <jats:p>No difference was found in the proportion of PCR-positive cases after treatment with ivermectin compared with standard care among patients with mild to moderate COVID-19 symptoms. However, early symptomatic recovery was observed without side effects.</jats:p>\n </jats:sec><jats:sec>\n <jats:title>Trial registration</jats:title>\n <jats:p>ClinicalTrials.gov NCT05076253. Registered on 8 October 2021, prospectively.</jats:p>\n </jats:sec>",
"alternative-id": [
"6649"
],
"article-number": "714",
"assertion": [
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Received",
"name": "received",
"order": 1,
"value": "23 February 2022"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Accepted",
"name": "accepted",
"order": 2,
"value": "7 August 2022"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "First Online",
"name": "first_online",
"order": 3,
"value": "26 August 2022"
},
{
"group": {
"label": "Declarations",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 1
},
{
"group": {
"label": "Ethics approval and consent to participate",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 2,
"value": "The study was conducted according to the guidelines of the Declaration of Helsinki and Good Clinical Practice Guidelines. The trial was approved by the Vajira Ethics Committee, approval no 171/64. Written informed consent was obtained from all subjects involved in the study."
},
{
"group": {
"label": "Consent for publication",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 3,
"value": "Consent for publication is not applicable."
},
{
"group": {
"label": "Competing interests",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 4,
"value": "The authors declare they have no competing interests."
}
],
"author": [
{
"affiliation": [],
"family": "Manomaipiboon",
"given": "Anan",
"sequence": "first"
},
{
"affiliation": [],
"family": "Pholtawornkulchai",
"given": "Kittisak",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Poopipatpab",
"given": "Sujaree",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Suraamornkul",
"given": "Swangjit",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Maneerit",
"given": "Jakravoot",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ruksakul",
"given": "Wiroj",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Phumisantiphong",
"given": "Uraporn",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-4374-251X",
"affiliation": [],
"authenticated-orcid": false,
"family": "Trakarnvanich",
"given": "Thananda",
"sequence": "additional"
}
],
"container-title": "Trials",
"container-title-short": "Trials",
"content-domain": {
"crossmark-restriction": false,
"domain": [
"link.springer.com"
]
},
"created": {
"date-parts": [
[
2022,
8,
26
]
],
"date-time": "2022-08-26T12:02:55Z",
"timestamp": 1661515375000
},
"deposited": {
"date-parts": [
[
2022,
8,
26
]
],
"date-time": "2022-08-26T12:19:00Z",
"timestamp": 1661516340000
},
"funder": [
{
"award": [
"171/64"
],
"name": "Navamindradhiraj University"
}
],
"indexed": {
"date-parts": [
[
2022,
8,
26
]
],
"date-time": "2022-08-26T12:43:06Z",
"timestamp": 1661517786061
},
"is-referenced-by-count": 0,
"issue": "1",
"issued": {
"date-parts": [
[
2022,
8,
26
]
]
},
"journal-issue": {
"issue": "1",
"published-online": {
"date-parts": [
[
2022,
12
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
8,
26
]
],
"date-time": "2022-08-26T00:00:00Z",
"timestamp": 1661472000000
}
},
{
"URL": "https://creativecommons.org/licenses/by/4.0",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
8,
26
]
],
"date-time": "2022-08-26T00:00:00Z",
"timestamp": 1661472000000
}
}
],
"link": [
{
"URL": "https://link.springer.com/content/pdf/10.1186/s13063-022-06649-3.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/article/10.1186/s13063-022-06649-3/fulltext.html",
"content-type": "text/html",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/content/pdf/10.1186/s13063-022-06649-3.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "297",
"original-title": [],
"prefix": "10.1186",
"published": {
"date-parts": [
[
2022,
8,
26
]
]
},
"published-online": {
"date-parts": [
[
2022,
8,
26
]
]
},
"publisher": "Springer Science and Business Media LLC",
"reference": [
{
"key": "6649_CR1",
"unstructured": "COVID-19 dashboard. 2021. https://coronavirus.jhu.edu/map.html. Accessed 20 Nov 2021."
},
{
"DOI": "10.1016/j.pt.2014.07.005",
"author": "S Omura",
"doi-asserted-by": "publisher",
"first-page": "445",
"journal-title": "Trends Parasitol",
"key": "6649_CR2",
"unstructured": "Omura S, Crump A. Ivermectin: panacea for resource-poor communities? Trends Parasitol. 2014;30:445–55. https://doi.org/10.1016/j.pt.2014.07.005.",
"volume": "30",
"year": "2014"
},
{
"DOI": "10.1016/j.antiviral.2020.104787",
"author": "L Caly",
"doi-asserted-by": "publisher",
"first-page": "104787",
"journal-title": "Antiviral Res",
"key": "6649_CR3",
"unstructured": "Caly L, Druce JD, Catton MG, Jans DA, Wagstaff KM. The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antiviral Res. 2020;178:104787. https://doi.org/10.1016/j.antiviral.2020.104787.",
"volume": "178",
"year": "2020"
},
{
"DOI": "10.1113/JP275236",
"author": "IS Chen",
"doi-asserted-by": "publisher",
"first-page": "1833",
"journal-title": "J Physiol",
"key": "6649_CR4",
"unstructured": "Chen IS, Kubo Y. Ivermectin and its target molecules: shared and unique modulation mechanisms of ion channels and receptors by ivermectin. J Physiol. 2018;596:1833–45. https://doi.org/10.1113/JP275236.",
"volume": "596",
"year": "2018"
},
{
"DOI": "10.3329/jbcps.v38i0.47512",
"author": "MT Alam",
"doi-asserted-by": "publisher",
"first-page": "10",
"journal-title": "J Bangladesh Coll Phys",
"key": "6649_CR5",
"unstructured": "Alam MT, Murshed R, Ebhiuyan E, Saber S, Alam RF, Robin RC. A case series of 100 COVID-19 positive patients treated with combination of ivermectin and doxycycline. J Bangladesh Coll Phys. 2020;38:10–5. https://doi.org/10.3329/jbcps.v38i0.47512.",
"volume": "38",
"year": "2020"
},
{
"DOI": "10.1097/MJT.0000000000001377",
"author": "P Kory",
"doi-asserted-by": "publisher",
"first-page": "e299",
"journal-title": "Am J Ther",
"key": "6649_CR6",
"unstructured": "Kory P, GU M, Varon J, et al.. Review of the emerging evidence demonstrating the efficacy of ivermectin in the prophylaxis and treatment of COVID-19. Am J Ther. 2021;28:e299–318.",
"volume": "28",
"year": "2021"
},
{
"DOI": "10.1016/j.arbres.2020.08.007",
"author": "MSI Khan",
"doi-asserted-by": "publisher",
"first-page": "828",
"journal-title": "Arch Bronconeumol",
"key": "6649_CR7",
"unstructured": "Khan MSI, Khan MSI, Debnath CR, Nath PN, Mahtab MA, Nabeka H, et al. Ivermectin treatment may improve the prognosis of patients with COVID-19. Arch Bronconeumol. 2020;56:828–30. https://doi.org/10.1016/j.arbres.2020.08.007.",
"volume": "56",
"year": "2020"
},
{
"DOI": "10.1016/j.chest.2020.10.009",
"author": "JC Rajter",
"doi-asserted-by": "publisher",
"first-page": "85",
"journal-title": "Chest",
"key": "6649_CR8",
"unstructured": "Rajter JC, Sherman MS, Fatteh N, Vogel F, Sacks J, Rajter JJ. Use of ivermectin is associated with lower mortality in hospitalized patients with coronavirus disease 2019: the ivermectin in COVID nineteen study. Chest. 2021;159:85–92. https://doi.org/10.1016/j.chest.2020.10.009.",
"volume": "159",
"year": "2021"
},
{
"DOI": "10.1016/j.jconrel.2020.10.009",
"author": "FR Formiga",
"doi-asserted-by": "publisher",
"first-page": "758",
"journal-title": "J Control Release",
"key": "6649_CR9",
"unstructured": "Formiga FR, Leblanc R, de Souza Rebouças J, Farias LP, de Oliveira RN, Pena L. Ivermectin: an award-winning drug with expected antiviral activity against COVID-19. J Control Release. 2021;329:758–61. https://doi.org/10.1016/j.jconrel.2020.10.009.",
"volume": "329",
"year": "2021"
},
{
"DOI": "10.1097/MJT.0000000000001402",
"author": "A Bryant",
"doi-asserted-by": "publisher",
"first-page": "e434",
"issue": "4",
"journal-title": "Am J Ther",
"key": "6649_CR10",
"unstructured": "Bryant A, Lawrie TA, Dowswell T, Fordham EJ, Mitchell S, Hill SR, et al. Ivermectin for prevention and treatment of COVID-19 infection: a systematic review, meta-analysis, and trial sequential analysis to inform clinical guidelines. Am J Ther. 2021;28(4):e434–60. https://doi.org/10.1097/MJT.0000000000001402.",
"volume": "28",
"year": "2021"
},
{
"author": "AZK Chachar",
"first-page": "31",
"journal-title": "Int J Sci",
"key": "6649_CR11",
"unstructured": "Chachar AZK, Khan KA, Asif M, Tanveer K, Khaqan A, Basri R. Effectiveness of ivermectin in SARS-CoV-2/COVID-19 patients. Int J Sci. 2020;9:31–5.",
"volume": "9",
"year": "2020"
},
{
"DOI": "10.1002/14651858.CD015017.pub2",
"author": "M Popp",
"doi-asserted-by": "publisher",
"first-page": "CD015017",
"issue": "7",
"journal-title": "Cochrane Database Syst Rev",
"key": "6649_CR12",
"unstructured": "Popp M, Stegemann M, Metzendorf MI, Gould S, Kranke P, Meybohm P, et al. Ivermectin for preventing and treating COVID-19. Cochrane Database Syst Rev. 2021;7(7):CD015017. https://doi.org/10.1002/14651858.CD015017.pub2 PMID: 34318930; PMCID: PMC8406455.",
"volume": "7",
"year": "2021"
},
{
"DOI": "10.1148/radiol.2020200490",
"doi-asserted-by": "publisher",
"key": "6649_CR13",
"unstructured": "Zu ZY, Jiang MD, Xu PP, Chen W, Ni QQ, Lu GM, Zhang LJ. Coronavirus Disease 2019 (COVID-19): A Perspective from China. Radiology. 2020;296(2):E15-E25. https://doi.org/10.1148/radiol.2020200490."
},
{
"DOI": "10.1101/2021.02.02.21250840 10.1016/j.ijid.2020.11.191",
"doi-asserted-by": "publisher",
"key": "6649_CR14",
"unstructured": "Bukhari KHS, Asghar A, Perveen N, Hayat A, Mangat SA, Kamil RB, et al. Efficacy of ivermectin in COVID-19 patients with mild to moderate disease. medRxiv 2021. https://doi.org/10.1101/2021.02.02.21250840 [Preprint 5 February 2021]. Ahmed S, Karim MM, Ross AG, Hossain MS, Clemens JD, Sumiya MK, et al. A five-day course of ivermectin for the treatment of COVID-19 may reduce the duration of illness. Int J Infect Dis. 2021;103:214–216. https://doi.org/10.1016/j.ijid.2020.11.191."
},
{
"DOI": "10.4055/cios.2014.6.1.103",
"author": "J Kim",
"doi-asserted-by": "publisher",
"first-page": "103",
"journal-title": "Clin Orthop Surg",
"key": "6649_CR15",
"unstructured": "Kim J, Shin W. How to do random allocation (randomization). Clin Orthop Surg. 2014;6:103–9. https://doi.org/10.4055/cios.2014.6.1.103.",
"volume": "6",
"year": "2014"
},
{
"DOI": "10.1080/20477724.2021.1890887",
"author": "LEB Galan",
"doi-asserted-by": "publisher",
"first-page": "235",
"journal-title": "Pathog Glob Health",
"key": "6649_CR16",
"unstructured": "Galan LEB, Santos NMD, Asato MS, Araújo JV, de Lima Moreira A, Araújo AMM, et al. Phase 2 randomized study on chloroquine, hydroxychloroquine or ivermectin in hospitalized patients with severe manifestations of SARS-CoV-2 infection. Pathog Glob Health. 2021;115:235–42. https://doi.org/10.1080/20477724.2021.1890887.",
"volume": "115",
"year": "2021"
},
{
"DOI": "10.1016/j.pulmoe.2020.07.003",
"author": "A Cortegiani",
"doi-asserted-by": "publisher",
"first-page": "52",
"journal-title": "Pulmonology",
"key": "6649_CR17",
"unstructured": "Cortegiani A, Ippolito M, Greco M, Granone V, Protti A, Gregoretti C, et al. Rationale and evidence on the use of tocilizumab in COVID-19: a systematic review. Pulmonology. 2021;27:52–66. https://doi.org/10.1016/j.pulmoe.2020.07.003.",
"volume": "27",
"year": "2021"
},
{
"key": "6649_CR18",
"unstructured": "US Food and Drug Administration. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-additional-oral-antiviral-treatment-covid-19-certain. Accessed 30 Nov 2021."
},
{
"DOI": "10.1016/j.antiviral.2020.104760",
"author": "SNY Yang",
"doi-asserted-by": "publisher",
"first-page": "104760",
"journal-title": "Antiviral Res",
"key": "6649_CR19",
"unstructured": "Yang SNY, Atkinson SC, Wang C, Lee A, Bogoyevitch MA, Borg NA, et al. The broad spectrum antiviral ivermectin targets the host nuclear transport importin α/β1 heterodimer. Antiviral Res. 2020;177:104760. https://doi.org/10.1016/j.antiviral.2020.104760.",
"volume": "177",
"year": "2020"
},
{
"DOI": "10.1093/cid/ciab591",
"doi-asserted-by": "publisher",
"key": "6649_CR20",
"unstructured": "Roman YM, Burela PA, Pasupuleti V, Piscoya A, Vidal JE, Hernandez AV. Ivermectin for the treatment of COVID-19: a systematic review and meta-analysis of randomized controlled trials. Clin Infect Dis. 2021;ciab591. https://doi.org/10.1093/cid/ciab591."
},
{
"DOI": "10.1177/03000605211013550",
"author": "R Mahmud",
"doi-asserted-by": "publisher",
"first-page": "300060521101355",
"journal-title": "J Int Med Res",
"key": "6649_CR21",
"unstructured": "Mahmud R, Rahman MM, Alam I, Ahmed KGU, Kabir AKMH, Sayeed SKJB, et al. Ivermectin in combination with doxycycline for treating COVID-19 symptoms: a randomized trial. J Int Med Res. 2021;49:3000605211013550. https://doi.org/10.1177/03000605211013550.",
"volume": "49",
"year": "2021"
},
{
"DOI": "10.1371/journal.pone.0242184",
"author": "D Camprubí",
"doi-asserted-by": "publisher",
"first-page": "e0242184",
"journal-title": "PLoS One",
"key": "6649_CR22",
"unstructured": "Camprubí D, Almuedo-Riera A, Martí-Soler H, Soriano A, Hurtado JC, Subirà C, et al. Lack of efficacy of standard doses of ivermectin in severe COVID-19 patients. PLoS One. 2020;15:e0242184. https://doi.org/10.1371/journal.pone.0242184.",
"volume": "15",
"year": "2020"
},
{
"DOI": "10.1016/j.nmni.2020.100684",
"author": "R Choudhary",
"doi-asserted-by": "publisher",
"first-page": "100684",
"journal-title": "New Microbes New Infect",
"key": "6649_CR23",
"unstructured": "Choudhary R, Sharma AK. Potential use of hydroxychloroquine, ivermectin and azithromycin drugs in fighting COVID-19: trends, scope and relevance. New Microbes New Infect. 2020;35:100684. https://doi.org/10.1016/j.nmni.2020.100684.",
"volume": "35",
"year": "2020"
},
{
"DOI": "10.1101/2020.10.26.20219345",
"doi-asserted-by": "publisher",
"key": "6649_CR24",
"unstructured": "Hashim HA, et al. Controlled randomized clinical trial on using ivermectin with doxycycline for treating COVID-19 patients in Baghdad, Iraq. medRxiv. 2020. https://doi.org/10.1101/2020.10.26.20219345 [Preprint 27 October 2020]."
},
{
"DOI": "10.1016/j.eclinm.2020.100720",
"author": "C Chaccour",
"doi-asserted-by": "publisher",
"first-page": "100720",
"journal-title": "EClinicalmedicine",
"key": "6649_CR25",
"unstructured": "Chaccour C, et al. The effect of early treatment with ivermectin on viral load, symptoms and humoral response in patients with non-severe COVID-19: a pilot, double-blind, placebo-controlled, randomized clinical trial. EClinicalmedicine. 2021;32:100720.",
"volume": "32",
"year": "2021"
},
{
"DOI": "10.1016/j.clinthera.2021.04.007",
"author": "L Shahbaznejad",
"doi-asserted-by": "publisher",
"first-page": "1007",
"journal-title": "Clin Ther",
"key": "6649_CR26",
"unstructured": "Shahbaznejad L, Davoudi A, Eslami G, Markowitz J, Navaeifar M, Hosseinzadeh F, et al. Effects of ivermectin in patients with COVID-19: a multicenter, double-blind, randomized, controlled clinical trial. Clin Ther. 2021;43:1007–19. https://doi.org/10.1016/j.clinthera.2021.04.007.",
"volume": "43",
"year": "2021"
},
{
"DOI": "10.1186/s12879-021-06104-9",
"author": "O Nurullah",
"doi-asserted-by": "publisher",
"first-page": "411",
"journal-title": "BMC Infect Dis",
"key": "6649_CR27",
"unstructured": "Nurullah O, Demirtürk N, Çetinkaya R, Güner R, Avcı I, Orhan S, et al. Evaluation of the effectiveness and safety of adding ivermectin to treatment in severe COVID-19 patients. BMC Infect Dis. 2021;21:411. https://doi.org/10.1186/s12879-021-06104-9.",
"volume": "21",
"year": "2021"
},
{
"DOI": "10.1016/j.ijid.2020.11.191",
"author": "S Ahmed",
"doi-asserted-by": "publisher",
"first-page": "214",
"journal-title": "Int J Infect Dis",
"key": "6649_CR28",
"unstructured": "Ahmed S, Karim MM, Ross AG, Hossain MS, Clemens JD, Sumiya MK, et al. A five-day course of ivermectin for the treatment of COVID-19 may reduce the duration of illness. Int J Infect Dis. 2021;103:214–6. https://doi.org/10.1016/j.ijid.2020.11.191.",
"volume": "103",
"year": "2021"
},
{
"DOI": "10.1101/2021.01.05.21249310",
"doi-asserted-by": "publisher",
"key": "6649_CR29",
"unstructured": "Ravikirti, Roy R, Pattadar C, Raj R, Agarwal N, Biswas B, et al. Ivermectin as a potential treatment for mild to moderate COVID-19 - a double blind randomized placebo-controlled trial. medRxiv. 2021. https://doi.org/10.1101/2021.01.05.21249310 [Preprint 9 January 2021]."
},
{
"DOI": "10.1038/s41429-020-0336-z",
"author": "F Heidary",
"doi-asserted-by": "publisher",
"first-page": "593",
"journal-title": "J Antibiot (Tokyo)",
"key": "6649_CR30",
"unstructured": "Heidary F, Gharebaghi R. Ivermectin: a systematic review from antiviral effects to COVID-19 complementary regimen. J Antibiot (Tokyo). 2020;73:593–602. https://doi.org/10.1038/s41429-020-0336-z.",
"volume": "73",
"year": "2020"
}
],
"reference-count": 30,
"references-count": 30,
"relation": {},
"resource": {
"primary": {
"URL": "https://trialsjournal.biomedcentral.com/articles/10.1186/s13063-022-06649-3"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Pharmacology (medical)",
"Medicine (miscellaneous)"
],
"subtitle": [],
"title": "Efficacy and safety of ivermectin in the treatment of mild to moderate COVID-19 infection: a randomized, double-blind, placebo-controlled trial",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1007/springer_crossmark_policy",
"volume": "23"
}