Efficacy and safety of single-dose ivermectin in mild-to-moderate COVID-19: the double-blind, randomized, placebo-controlled CORVETTE-01 trial
Tatsuhiko Wada, Makoto Hibino, Hiromi Aono, Shunsuke Kyoda, Yosuke Iwadate, Eri Shishido, Keisuke Ikeda, Nana Kinoshita, Yasuki Matsuda, Sakiko Otani, Ryo Kameda, Kenta Matoba, Miwa Nonaka, Mika Maeda, Yuji Kumagai, Junya Ako, Masayoshi Shichiri, Katsuhiko Naoki, Masato Katagiri, Masashi Takaso, Masatsugu Iwamura, Kazuhiko Katayama, Takeshi Miyatsuka, Yasushi Orihashi, Kunihiro Yamaoka
Frontiers in Medicine, doi:10.3389/fmed.2023.1139046
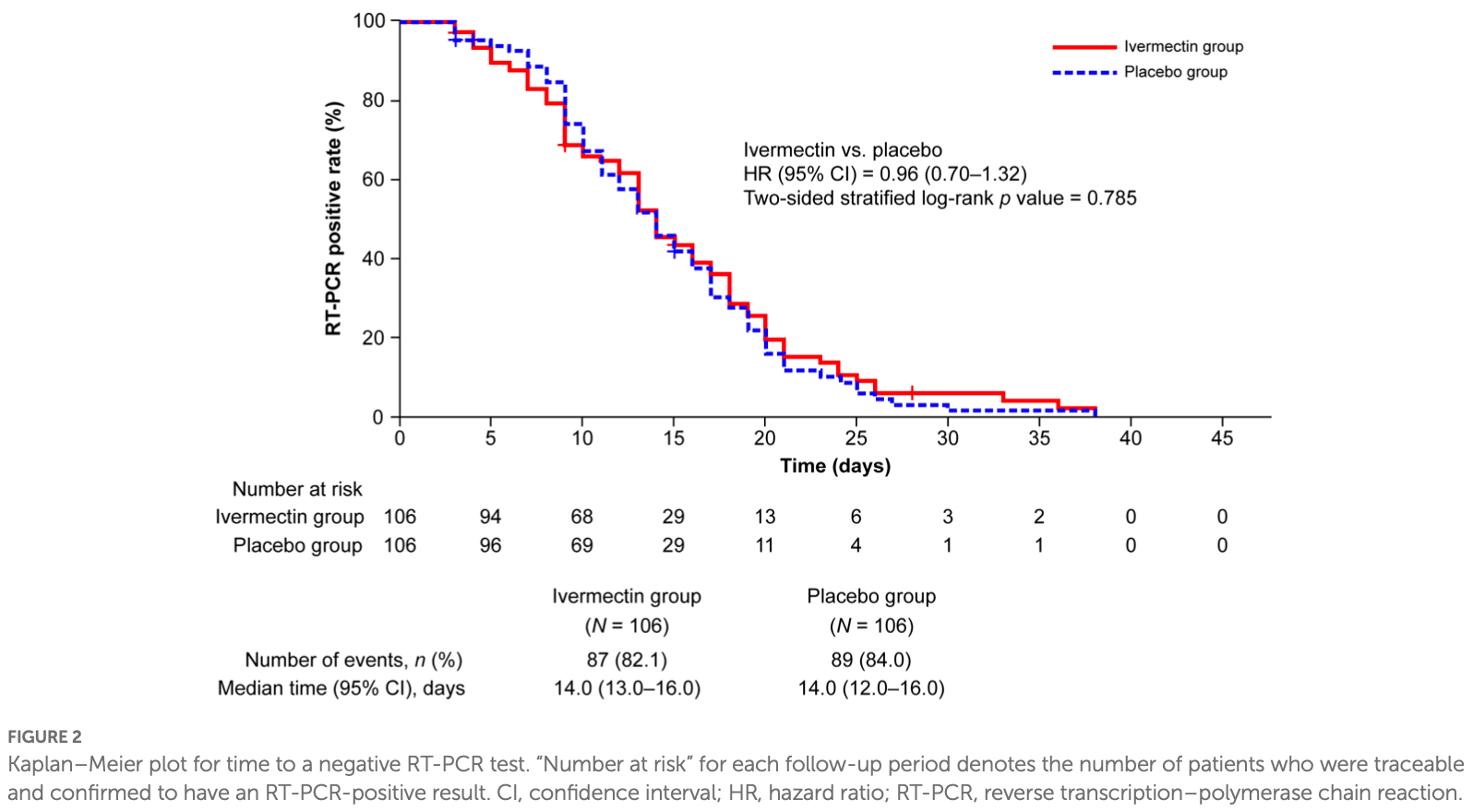
Background: To investigate whether ivermectin inhibits SARS-CoV-2 proliferation in patients with mild-to-moderate COVID-19 using time to a negative COVID-19 reverse transcription-polymerase chain reaction (RT-PCR) test. Methods: CORVETTE-01 was a double-blind, randomized, placebo-controlled study (August 2020-October 2021) conducted in Japan. Overall, 248 patients diagnosed with COVID-19 using RT-PCR were assessed for eligibility. A single oral dose of ivermectin (200 μg/kg) or placebo was administered under fasting. The primary outcome was time to a negative COVID-19 RT-PCR test result for SARS-CoV-2 nucleic acid, assessed using stratified log-rank test and Cox regression models. Results: Overall, 112 and 109 patients were randomized to ivermectin and placebo, respectively; 106 patients from each group were included in the full analysis set (male [%], mean age: 68.9%, 47.9 years [ivermectin]; 62.3%, 47.5 years [placebo]). No significant difference was observed in the occurrence of negative RT-PCR tests between the groups (hazard ratio, 0.96; 95% confidence interval [CI] 0.70-1.32; p = 0.785). Median (95% CI) time to a negative RT-PCR test was 14.0
Ethics statement The studies involving human participants were reviewed and approved by the IRB of Kitasato University Shirokane Campus, affiliation: Kitasato University. The patients/participants provided their written informed consent to participate in this study.
Author contributions KY contributed towards conceptualization and drafting the work. YK contributed towards conceptualization, drafting the work, and revising it critically for important intellectual content. MS, TM, JA, KN, MI, MT, MM, MK, and KK contributed towards conceptualization, drafting the work, and revising it critically for important intellectual content. MH, HA, MN, KI, ES, YI, NK, SK, SO, RK, KM, and YM contributed towards acquisition, analysis, or interpretation of data for the work, drafting the work, and revising it critically for important intellectual content. TW contributed towards conceptualization, acquisition, analysis, or interpretation of data for the work, and drafting the work. YO contributed towards the data analysis, drafting the work, and revising it critically for important intellectual content. All authors provide approval for publication of the content and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Conflict of interest The authors declare that the research was conducted in the absence of any commercial or financial relationships that could..
References
Ahmed, Karim, Ross, Hossain, Clemens et al., Ivermectin shows clinical benefits in mild to moderate COVID19: a randomized controlled double-blind, dose-response study in Lagos, Int J Infect Dis,
doi:10.1093/qjmed/hcab035Batiha, Alqahtani, Ilesanmi, Saati, El-Mleeh et al., Avermectin derivatives, pharmacokinetics, therapeutic and toxic dosages, mechanism of action, and their biological effects, Pharmaceuticals (Basel),
doi:10.3390/ph13080196Behera, Patro, Singh, Chandanshive, Ravikumar et al., Role of ivermectin in the prevention of SARS-CoV-2 infection among healthcare workers in India: a matched case-control study, PLoS One,
doi:10.1371/journal.pone.0247163Brookmeyer, Crowley, A confidence interval for the median survival time, Biometrics,
doi:10.2307/2530286Chaccour, Casellas, Blanco-Di Matteo, Pineda, Fernandez-Montero et al., The effect of early treatment with ivermectin on viral load, symptoms and humoral response in patients with non-severe COVID-19: a pilot, double-blind, placebo-controlled, randomized clinical trial, EClinicalMedcine,
doi:10.1016/j.eclinm.2020.100720Guzzo, Furtek, Porras, Chen, Tipping et al., Safety, tolerability, and pharmacokinetics of escalating high doses of ivermectin in healthy adult subjects, J Clin Pharmacol,
doi:10.1177/009127002237994Heidary, Gharebaghi, Ivermectin: a systematic review from antiviral effects to COVID-19 complementary regimen, J Antibiot,
doi:10.1038/s41429-020-0336-zHill, Mirchandani, Pilkington, Ivermectin for COVID-19: addressing potential bias and medical fraud, Open Forum Infect Dis,
doi:10.1093/ofid/ofab645Hu, Guo, Zhou, Shi, Characteristics of SARS-CoV-2 and COVID-19
Jermain, Hanafin, Cao, Lifschitz, Lanusse et al., Development of a minimal physiologically-based pharmacokinetic model to simulate lung exposure in humans following oral administration of ivermectin for COVID-19 drug repurposing, J Pharm Sci,
doi:10.1016/j.xphs.2020.08.024Koyama, Tokumasu, Katayama, Saito, Kudo et al., Cross-border transmissions of the Delta substrain AY.29 during Tokyo Olympic and Paralympic games, Front Microbiol,
doi:10.3389/fmicb.2022.883849Krolewiecki, Lifschitz, Moragas, Travacio, Valentini et al., Antiviral effect of high-dose ivermectin in adults with COVID-19: a proof-of-concept randomized trial, EClinicalMedicine,
doi:10.1016/j.eclinm.2021.100959Lifschitz, Virkel, Sallovitz, Sutra, Galtier et al., Comparative distribution of ivermectin and doramectin to parasite location tissues in cattle, Vet Parasitol,
doi:10.1016/S0304-4017(99)00175-2Lim, Hor, Tay, Jelani, Tan et al., Efficacy of ivermectin treatment on disease progression among adults with mild to moderate COVID-19 and comorbidities: the I-TECH randomized clinical trial, JAMA Intern Med,
doi:10.1001/jamainternmed.2022.0189Merck, Over 30 Years: The Mectizan ® Donation Program
Mittal, Mittal, Inhaled route and anti-inflammatory action of ivermectin: do they hold promise in fighting against COVID-19? Med Hypotheses,
doi:10.1016/j.mehy.2020.110364Mohan, Tiwari, Suri, Mittal, Patel et al., Single-dose oral ivermectin in mild and moderate COVID-19 (RIVET-COV): a single-centre randomized, placebo-controlled trial, J Infect Chemother,
doi:10.1016/j.jiac.2021.08.021Momekov, Momekova, Ivermectin as a potential COVID-19 treatment from the pharmacokinetic point of view: antiviral levels are not likely attainable with known dosing regimens, Biotechnol Equip,
doi:10.1080/13102818.2020.1775118Morgenstern, Redondo, Olavarria, Rondon, Roca et al., Ivermectin as a SARS-CoV-2 pre-exposure prophylaxis method in healthcare workers: a propensity score-matched retrospective cohort study, Cureus,
doi:10.7759/cureus.17455Navarro, Camprubi, Requena-Méndez, Buonfrate, Giorli et al., Safety of high-dose ivermectin: a systematic review and meta-analysis, J Antimicrob Chemother,
doi:10.1093/jac/dkz524Ozer, Goksu, Ulker, Balderas, Mahdi, Effectiveness and safety of ivermectin in COVID-19 patients: a prospective study at a safety-net hospital, Cochrane Database Syst Rev,
doi:10.1002/14651858.CD015017Rajter, Sherman, Fatteh, Vogel, Sacks et al., Use of ivermectin is associated with lower mortality in hospitalized patients with coronavirus disease 2019: the ivermectin in COVID nineteen study, Chest,
doi:10.1016/j.chest.2020.10.009Ravikirti, Pattadar, Raj, Agarwal, Biswas, Evaluation of ivermectin as a potential treatment for mild to moderate COVID-19: a double-blind randomized placebo controlled trial in eastern India, J Pharm Sci,
doi:10.18433/jpps32105Reis, Silva, Silva, Thabane, Milagres et al., Effect of early treatment with ivermectin among patients with Covid-19, N Engl J Med,
doi:10.1056/NEJMoa2115869Schmith, Zhou, Lohmer, The approved dose of ivermectin alone is not the ideal dose for the treatment of COVID-19, Clin Pharmacol Ther,
doi:10.1002/cpt.1889Schulz, Altman, Moher Dfor the CONSORT Group. Statement: updated guidelines for reporting parallel group randomised trials, Clin Epidemiol,
doi:10.1016/j.jclinepi.2010.02.005Wang, Levi, Ellis, Hill, Minimum manufacturing costs, national prices, and estimated global availability of new repurposed therapies for coronavirus disease, Open Forum Infect Dis,
doi:10.1093/ofid/ofab581DOI record:
{
"DOI": "10.3389/fmed.2023.1139046",
"ISSN": [
"2296-858X"
],
"URL": "http://dx.doi.org/10.3389/fmed.2023.1139046",
"abstract": "<jats:sec><jats:title>Background</jats:title><jats:p>To investigate whether ivermectin inhibits SARS-CoV-2 proliferation in patients with mild-to-moderate COVID-19 using time to a negative COVID-19 reverse transcription-polymerase chain reaction (RT-PCR) test.</jats:p></jats:sec><jats:sec><jats:title>Methods</jats:title><jats:p>CORVETTE-01 was a double-blind, randomized, placebo-controlled study (August 2020–October 2021) conducted in Japan. Overall, 248 patients diagnosed with COVID-19 using RT-PCR were assessed for eligibility. A single oral dose of ivermectin (200 μg/kg) or placebo was administered under fasting. The primary outcome was time to a negative COVID-19 RT-PCR test result for SARS-CoV-2 nucleic acid, assessed using stratified log-rank test and Cox regression models.</jats:p></jats:sec><jats:sec><jats:title>Results</jats:title><jats:p>Overall, 112 and 109 patients were randomized to ivermectin and placebo, respectively; 106 patients from each group were included in the full analysis set (male [%], mean age: 68.9%, 47.9 years [ivermectin]; 62.3%, 47.5 years [placebo]). No significant difference was observed in the occurrence of negative RT-PCR tests between the groups (hazard ratio, 0.96; 95% confidence interval [CI] 0.70–1.32; <jats:italic>p</jats:italic> = 0.785). Median (95% CI) time to a negative RT-PCR test was 14.0 (13.0–16.0) and 14.0 (12.0–16.0) days for ivermectin and placebo, respectively; 82.1% and 84% of patients achieved negative RT-PCR tests, respectively.</jats:p></jats:sec><jats:sec><jats:title>Conclusion</jats:title><jats:p>In patients with COVID-19, single-dose ivermectin was ineffective in decreasing the time to a negative RT-PCR test.</jats:p></jats:sec><jats:sec><jats:title>Clinical Trial Registration</jats:title><jats:p><jats:ext-link>ClinicalTrials.gov</jats:ext-link>, NCT04703205.</jats:p></jats:sec>",
"alternative-id": [
"10.3389/fmed.2023.1139046"
],
"author": [
{
"affiliation": [],
"family": "Wada",
"given": "Tatsuhiko",
"sequence": "first"
},
{
"affiliation": [],
"family": "Hibino",
"given": "Makoto",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Aono",
"given": "Hiromi",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kyoda",
"given": "Shunsuke",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Iwadate",
"given": "Yosuke",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Shishido",
"given": "Eri",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ikeda",
"given": "Keisuke",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kinoshita",
"given": "Nana",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Matsuda",
"given": "Yasuki",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Otani",
"given": "Sakiko",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kameda",
"given": "Ryo",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Matoba",
"given": "Kenta",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Nonaka",
"given": "Miwa",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Maeda",
"given": "Mika",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kumagai",
"given": "Yuji",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ako",
"given": "Junya",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Shichiri",
"given": "Masayoshi",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Naoki",
"given": "Katsuhiko",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Katagiri",
"given": "Masato",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Takaso",
"given": "Masashi",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Iwamura",
"given": "Masatsugu",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Katayama",
"given": "Kazuhiko",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Miyatsuka",
"given": "Takeshi",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Orihashi",
"given": "Yasushi",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Yamaoka",
"given": "Kunihiro",
"sequence": "additional"
},
{
"affiliation": [],
"name": "for the CORVETTE-01 Study Group",
"sequence": "additional"
}
],
"container-title": "Frontiers in Medicine",
"container-title-short": "Front. Med.",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"frontiersin.org"
]
},
"created": {
"date-parts": [
[
2023,
5,
22
]
],
"date-time": "2023-05-22T12:56:07Z",
"timestamp": 1684760167000
},
"deposited": {
"date-parts": [
[
2023,
5,
22
]
],
"date-time": "2023-05-22T12:56:12Z",
"timestamp": 1684760172000
},
"funder": [
{
"DOI": "10.13039/100009619",
"doi-asserted-by": "publisher",
"name": "Japan Agency for Medical Research and Development"
},
{
"DOI": "10.13039/501100007830",
"doi-asserted-by": "publisher",
"name": "Kitasato University"
}
],
"indexed": {
"date-parts": [
[
2023,
5,
23
]
],
"date-time": "2023-05-23T04:27:17Z",
"timestamp": 1684816037878
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2023,
5,
22
]
]
},
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
5,
22
]
],
"date-time": "2023-05-22T00:00:00Z",
"timestamp": 1684713600000
}
}
],
"link": [
{
"URL": "https://www.frontiersin.org/articles/10.3389/fmed.2023.1139046/full",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "1965",
"original-title": [],
"prefix": "10.3389",
"published": {
"date-parts": [
[
2023,
5,
22
]
]
},
"published-online": {
"date-parts": [
[
2023,
5,
22
]
]
},
"publisher": "Frontiers Media SA",
"reference": [
{
"DOI": "10.1038/s41579-020-00459-7",
"article-title": "Characteristics of SARS-CoV-2 and COVID-19",
"author": "Hu",
"doi-asserted-by": "publisher",
"first-page": "141",
"journal-title": "Nat Rev Microbiol",
"key": "ref1",
"volume": "19",
"year": "2021"
},
{
"DOI": "10.1016/j.metop.2021.100121",
"article-title": "Drug repurposing in COVID-19: a review with past, present and future",
"author": "Srivastava",
"doi-asserted-by": "publisher",
"first-page": "100121",
"journal-title": "Metabol Open",
"key": "ref2",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.1093/ofid/ofab581",
"article-title": "Minimum manufacturing costs, national prices, and estimated global availability of new repurposed therapies for coronavirus disease 2019",
"author": "Wang",
"doi-asserted-by": "publisher",
"first-page": "ofab581",
"journal-title": "Open Forum Infect Dis",
"key": "ref3",
"volume": "9",
"year": "2021"
},
{
"key": "ref4",
"year": "2022"
},
{
"DOI": "10.3390/ph13080196",
"article-title": "Avermectin derivatives, pharmacokinetics, therapeutic and toxic dosages, mechanism of action, and their biological effects",
"author": "El-Saber Batiha",
"doi-asserted-by": "publisher",
"first-page": "196",
"journal-title": "Pharmaceuticals (Basel)",
"key": "ref5",
"volume": "13",
"year": "2020"
},
{
"DOI": "10.1016/j.antiviral.2020.104787",
"article-title": "The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro",
"author": "Caly",
"doi-asserted-by": "publisher",
"first-page": "104787",
"journal-title": "Antivir Res",
"key": "ref6",
"volume": "178",
"year": "2020"
},
{
"DOI": "10.1080/13102818.2020.1775118",
"article-title": "Ivermectin as a potential COVID-19 treatment from the pharmacokinetic point of view: antiviral levels are not likely attainable with known dosing regimens",
"author": "Momekov",
"doi-asserted-by": "publisher",
"first-page": "469",
"journal-title": "Biotechnol Equip",
"key": "ref7",
"volume": "34",
"year": "2020"
},
{
"DOI": "10.1016/j.jiac.2021.08.021",
"article-title": "Single-dose oral ivermectin in mild and moderate COVID-19 (RIVET-COV): a single-centre randomized, placebo-controlled trial",
"author": "Mohan",
"doi-asserted-by": "publisher",
"first-page": "1743",
"journal-title": "J Infect Chemother",
"key": "ref8",
"volume": "27",
"year": "2021"
},
{
"DOI": "10.1038/s41429-020-0336-z",
"article-title": "Ivermectin: a systematic review from antiviral effects to COVID-19 complementary regimen",
"author": "Heidary",
"doi-asserted-by": "publisher",
"first-page": "593",
"journal-title": "J Antibiot (Tokyo)",
"key": "ref9",
"volume": "73",
"year": "2020"
},
{
"DOI": "10.1016/S0304-4017(99)00175-2",
"article-title": "Comparative distribution of ivermectin and doramectin to parasite location tissues in cattle",
"author": "Lifschitz",
"doi-asserted-by": "publisher",
"first-page": "327",
"journal-title": "Vet Parasitol",
"key": "ref10",
"volume": "87",
"year": "2000"
},
{
"DOI": "10.1371/journal.pone.0247163",
"article-title": "Role of ivermectin in the prevention of SARS-CoV-2 infection among healthcare workers in India: a matched case-control study",
"author": "Behera",
"doi-asserted-by": "publisher",
"first-page": "e0247163",
"journal-title": "PLoS One",
"key": "ref11",
"volume": "16",
"year": "2021"
},
{
"DOI": "10.7759/cureus.17455",
"article-title": "Ivermectin as a SARS-CoV-2 pre-exposure prophylaxis method in healthcare workers: a propensity score-matched retrospective cohort study",
"author": "Morgenstern",
"doi-asserted-by": "publisher",
"first-page": "e17455",
"journal-title": "Cureus",
"key": "ref12",
"volume": "13",
"year": "2021"
},
{
"DOI": "10.1016/j.chest.2020.10.009",
"article-title": "Use of ivermectin is associated with lower mortality in hospitalized patients with coronavirus disease 2019: the ivermectin in COVID nineteen study",
"author": "Rajter",
"doi-asserted-by": "publisher",
"first-page": "85",
"journal-title": "Chest",
"key": "ref13",
"volume": "159",
"year": "2021"
},
{
"DOI": "10.1002/jmv.27469",
"article-title": "Effectiveness and safety of ivermectin in COVID-19 patients: a prospective study at a safety-net hospital",
"author": "Ozer",
"doi-asserted-by": "publisher",
"first-page": "1473",
"journal-title": "J Med Virol",
"key": "ref14",
"volume": "94",
"year": "2022"
},
{
"DOI": "10.1002/14651858.CD015017",
"article-title": "Ivermectin for preventing and treating COVID-19",
"author": "Popp",
"doi-asserted-by": "publisher",
"first-page": "Cd015017",
"journal-title": "Cochrane Database Syst Rev",
"key": "ref15",
"volume": "7",
"year": "2021"
},
{
"DOI": "10.1093/ofid/ofab645",
"article-title": "Ivermectin for COVID-19: addressing potential bias and medical fraud",
"author": "Hill",
"doi-asserted-by": "publisher",
"first-page": "ofab645",
"journal-title": "Open Forum Infect Dis",
"key": "ref16",
"volume": "9",
"year": "2022"
},
{
"DOI": "10.1001/jamainternmed.2022.0189",
"article-title": "Efficacy of ivermectin treatment on disease progression among adults with mild to moderate COVID-19 and comorbidities: the I-TECH randomized clinical trial",
"author": "Lim",
"doi-asserted-by": "publisher",
"first-page": "426",
"journal-title": "JAMA Intern Med",
"key": "ref17",
"volume": "182",
"year": "2022"
},
{
"DOI": "10.1016/j.jclinepi.2010.02.005",
"article-title": "Statement: updated guidelines for reporting parallel group randomised trials",
"author": "Schulz",
"doi-asserted-by": "publisher",
"first-page": "834",
"journal-title": "Clin Epidemiol",
"key": "ref18",
"volume": "63",
"year": "2010"
},
{
"DOI": "10.2307/2530286",
"article-title": "A confidence interval for the median survival time",
"author": "Brookmeyer",
"doi-asserted-by": "publisher",
"first-page": "29",
"journal-title": "Biometrics",
"key": "ref19",
"volume": "38",
"year": "1982"
},
{
"DOI": "10.3389/fmicb.2022.883849",
"article-title": "Cross-border transmissions of the Delta substrain AY.29 during Tokyo Olympic and Paralympic games",
"author": "Koyama",
"doi-asserted-by": "publisher",
"first-page": "883849",
"journal-title": "Front Microbiol",
"key": "ref20",
"volume": "13",
"year": "2022"
},
{
"DOI": "10.18433/jpps32105",
"article-title": "Evaluation of ivermectin as a potential treatment for mild to moderate COVID-19: a double-blind randomized placebo controlled trial in eastern India",
"author": "Ravikirti",
"doi-asserted-by": "publisher",
"first-page": "343",
"journal-title": "J Pharm Sci",
"key": "ref21",
"volume": "24",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2115869",
"article-title": "Effect of early treatment with ivermectin among patients with Covid-19",
"author": "Reis",
"doi-asserted-by": "publisher",
"first-page": "1721",
"journal-title": "N Engl J Med",
"key": "ref22",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.1016/j.eclinm.2020.100720",
"article-title": "The effect of early treatment with ivermectin on viral load, symptoms and humoral response in patients with non-severe COVID-19: a pilot, double-blind, placebo-controlled, randomized clinical trial",
"author": "Chaccour",
"doi-asserted-by": "publisher",
"first-page": "100720",
"journal-title": "EClinicalMedcine",
"key": "ref23",
"volume": "32",
"year": "2021"
},
{
"DOI": "10.1016/j.ijid.2020.11.191",
"article-title": "A five-day course of ivermectin for the treatment of COVID-19 may reduce the duration of illness",
"author": "Ahmed",
"doi-asserted-by": "publisher",
"first-page": "214",
"journal-title": "Int J Infect Dis",
"key": "ref24",
"volume": "103",
"year": "2021"
},
{
"DOI": "10.1177/03000605211013550",
"article-title": "Ivermectin in combination with doxycycline for treating COVID-19 symptoms: a randomized trial",
"author": "Mahmud",
"doi-asserted-by": "publisher",
"first-page": "030006052110135",
"journal-title": "J Int Med Res",
"key": "ref25",
"volume": "49",
"year": "2021"
},
{
"DOI": "10.1093/qjmed/hcab035",
"article-title": "Ivermectin shows clinical benefits in mild to moderate COVID19: a randomized controlled double-blind, dose-response study in Lagos",
"author": "Babalola",
"doi-asserted-by": "publisher",
"first-page": "780",
"journal-title": "QJM",
"key": "ref26",
"volume": "114",
"year": "2022"
},
{
"DOI": "10.1016/j.eclinm.2021.100959",
"article-title": "Antiviral effect of high-dose ivermectin in adults with COVID-19: a proof-of-concept randomized trial",
"author": "Krolewiecki",
"doi-asserted-by": "publisher",
"first-page": "100959",
"journal-title": "EClinicalMedicine",
"key": "ref27",
"volume": "37",
"year": "2021"
},
{
"DOI": "10.1002/cpt.1889",
"article-title": "The approved dose of ivermectin alone is not the ideal dose for the treatment of COVID-19",
"author": "Schmith",
"doi-asserted-by": "publisher",
"first-page": "762",
"journal-title": "Clin Pharmacol Ther",
"key": "ref28",
"volume": "108",
"year": "2020"
},
{
"DOI": "10.1016/j.xphs.2020.08.024",
"article-title": "Development of a minimal physiologically-based pharmacokinetic model to simulate lung exposure in humans following oral administration of ivermectin for COVID-19 drug repurposing",
"author": "Jermain",
"doi-asserted-by": "publisher",
"first-page": "3574",
"journal-title": "J Pharm Sci",
"key": "ref29",
"volume": "109",
"year": "2020"
},
{
"DOI": "10.1093/jac/dkz524",
"article-title": "Safety of high-dose ivermectin: a systematic review and meta-analysis",
"author": "Navarro",
"doi-asserted-by": "publisher",
"first-page": "827",
"journal-title": "J Antimicrob Chemother",
"key": "ref30",
"volume": "75",
"year": "2020"
},
{
"DOI": "10.1177/009127002237994",
"article-title": "Safety, tolerability, and pharmacokinetics of escalating high doses of ivermectin in healthy adult subjects",
"author": "Guzzo",
"doi-asserted-by": "publisher",
"first-page": "1122",
"journal-title": "J Clin Pharmacol",
"key": "ref31",
"volume": "42",
"year": "2002"
},
{
"DOI": "10.1016/j.mehy.2020.110364",
"article-title": "Inhaled route and anti-inflammatory action of ivermectin: do they hold promise in fighting against COVID-19?",
"author": "Mittal",
"doi-asserted-by": "publisher",
"first-page": "110364",
"journal-title": "Med Hypotheses",
"key": "ref32",
"volume": "146",
"year": "2021"
},
{
"key": "ref33",
"year": "2022"
}
],
"reference-count": 33,
"references-count": 33,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.frontiersin.org/articles/10.3389/fmed.2023.1139046/full"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"General Medicine"
],
"subtitle": [],
"title": "Efficacy and safety of single-dose ivermectin in mild-to-moderate COVID-19: the double-blind, randomized, placebo-controlled CORVETTE-01 trial",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.3389/crossmark-policy",
"volume": "10"
}