Comparison of Trials Using Ivermectin for COVID-19 Between Regions With High and Low Prevalence of Strongyloidiasis
MD Avi Bitterman, BS Caitlin Pestana Martins, MD Ahuva Cices, Makarand Prasad Nadendla
JAMA Network Open, doi:10.1001/jamanetworkopen.2022.3079
IMPORTANCE A widely cited meta-analysis of randomized clinical trials has claimed ivermectin as an effective treatment for prevention of mortality in COVID-19. However, an unrecognized interaction variable with the relative risk (RR) of mortality may substantially change the appropriate interpretation of this analysis.
OBJECTIVE To evaluate the association between regional prevalence of strongyloidiasis and ivermectin trial results for the outcome of mortality by testing the hypothesis that strongyloidiasis prevalence interacts with the RR of mortality. DATA SOURCES Original meta-analysis as well as a manual review of all references in a dedicated ivermectin trial database (c19ivermectin) from January 1, 2019, to November 6, 2021. STUDY SELECTION Randomized clinical trials using ivermectin as a treatment for COVID-19 and reporting the outcome of mortality. Studies were excluded in the event of publications revealing suspected trial fraud and/or randomization failure.
DATA EXTRACTION AND SYNTHESIS Study characteristics and RR estimates were extracted from each source. Estimates were pooled using random-effects meta-analysis. Differences by strongyloidiasis prevalence were estimated using subgroup meta-analysis and meta-regression. The Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guideline was followed.
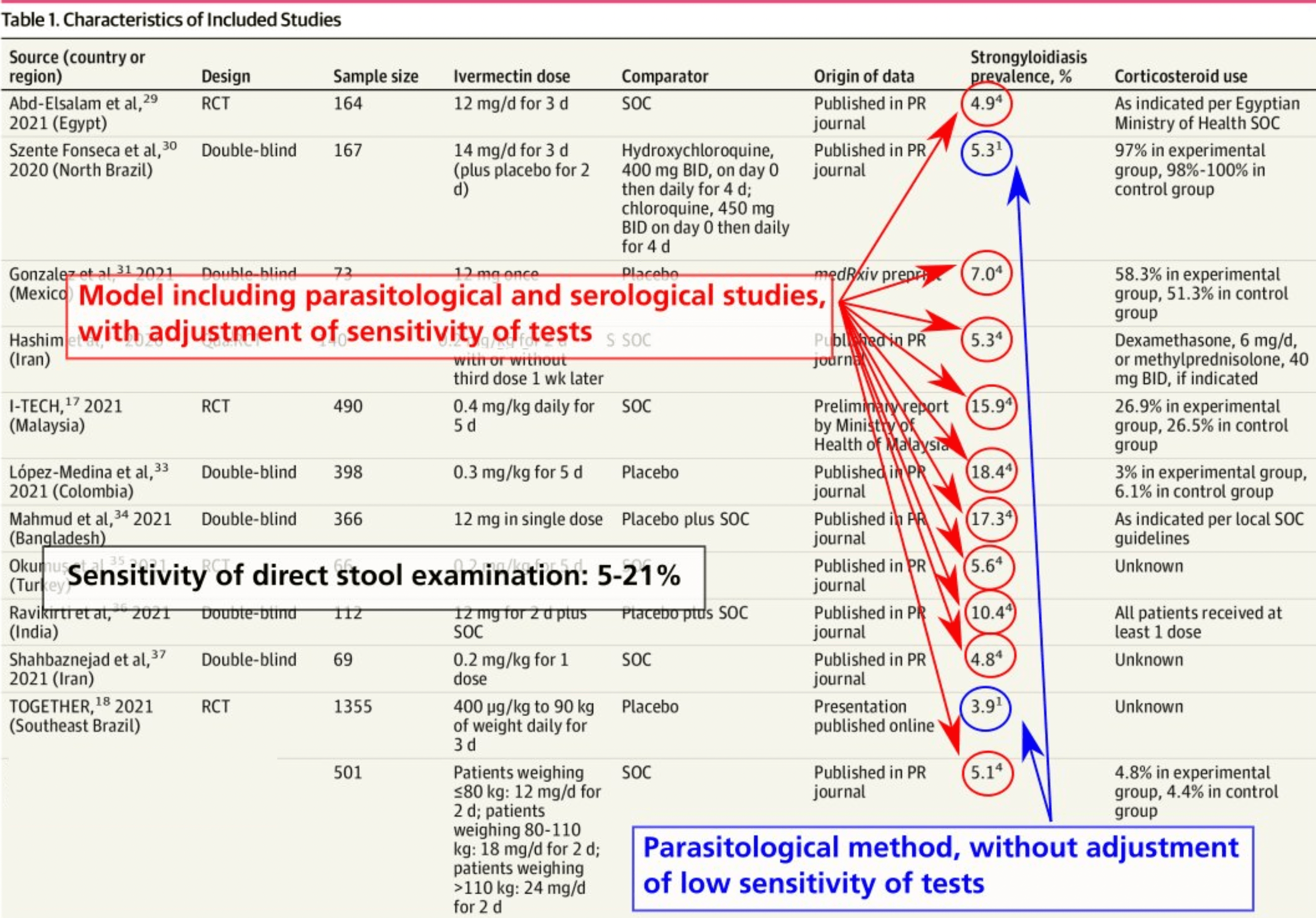
MAIN OUTCOMES AND MEASURES Relative risk of mortality in ivermectin trials in regions of high vs low strongyloidiasis prevalence and correlation coefficient of meta-regression analysis between RR of mortality and regional prevalence of strongyloidiasis. RESULTS A total of 12 trials comprising 3901 patients were included in the analysis. Four trials (33%) took place in regions of high strongyloidiasis prevalence and 8 (67%) trials took place in regions of low strongyloidiasis prevalence. Ivermectin trials that took place in areas of low regional strongyloidiasis prevalence were not associated with a statistically significant decreased risk of mortality (RR, 0.84 [95% CI, 0.60-1.18]; P = .31). By contrast, ivermectin trials that took place in areas of high regional strongyloidiasis prevalence were associated with a significantly decreased risk of mortality (RR, 0.25 [95% CI, 0.09-0.70]; P = .008). Testing for subgroup differences revealed a significant difference between the results of groups with low and high strongyloidiasis prevalence (χ 2 1 = 4.79; P = .03). The estimate for τ 2 (the variance of the study effect sizes) was 0 (95% CI, Key Points Question Does prevalence of strongyloidiasis interact with the relative risk (RR) of mortality in ivermectin trials for the treatment of COVID-19? Findings In this meta-analysis of 12 randomized clinical trials involving 3901 patients, favorable mortality results were limited to trials in high-prevalence regions, with no evidence that ivermectin had a mortality benefit in low-prevalence regions. Metaregression found an association between the regional prevalence of..
Source
Risk of bias by item a
Random sequence generation Allocation concealment
Blinding of participants and personnel
Blinding of outcome assessment
Incomplete outcome data Selective reporting Other bias Abd-Elsalam et al, 29 were not clear for all trials, precluding the inclusion of this variable in the regression model. Third, low event counts in the trials may make the results less reliable. Fourth, varying trial recruitment across urban and rural populations (where strongyloidiasis prevalences often differ) may diminish the reliability of strongyloidiasis trial prevalence estimates. Despite these limitations, the findings warrant concern for ivermectin trials for the treatment of COVID-19 that are not designed to address this interaction.
Conclusions In this meta-analysis of 12 trials comprising 3901 patients, strongyloidiasis prevalence was found to interact with the RR of mortality when ivermectin was used as a treatment for COVID-
References
Abd-Elsalam, Noor, Badawi, Risk of hospitalization for COVID-19 outpatients treated with various drug regimens in Brazil: comparative analysis, Travel Med Infect Dis,
doi:10.1016/j.tmaid.2020.101906Bryant, Lawrie, Dowswell, Ivermectin for prevention and treatment of COVID-19 infection: a systematic review, meta-analysis, and trial sequential analysis to inform clinical guidelines, Am J Ther,
doi:10.1097/MJT.0000000000001402Chan, Kennedy, Nelson, Fatal Strongyloides hyperinfection syndrome in an immunocompetent adult with review of the literature, Intern Med J,
doi:10.1111/imj.13940Core, R: A Language and Environment for Statistical Computing
Foundation, for Statistical Computing
Ganesh, Cruz, Strongyloidiasis: a multifaceted disease, Gastroenterol Hepatol
General, Health, Kenyataan Akhbar KPK 3 November 2021-Hasil Dapatan Kajian Keberkesanan Rawatan Ivermectin Untuk Pesakit COVID-19 Berisiko Tinggi (I-TECH Study
Geri, Rabbat, Mayaux, Strongyloides stercoralis hyperinfection syndrome: a case series and a review of the literature, Infection,
doi:10.1007/s15010-015-0799-1Gonzalez, Gámez, Enciso, Efficacy and safety of ivermectin and hydroxychloroquine in patients with severe COVID-19: a randomized controlled trial. medRxiv,
doi:10.1101/2021.02.18.21252037Gopalakrishna, Nair, Conti, Parasitic necrotizing pneumonia in an immunocompetent patient in United States, J Community Hosp Intern Med Perspect,
doi:10.1080/20009666.2020.1824333Harrer, Cuijpers, Furukawa, Ebert, Doing Meta-Analysis With R: A Hands-On Guide
Hashim, Maulood, Rasheed, Fatak, Kabah et al., Controlled randomized clinical trial on using ivermectin with doxycycline for treating COVID-19 patients in Baghdad, Iraq. medRxiv,
doi:10.1101/2020.10.26.20219345Henriquez-Camacho, Gotuzzo, Echevarria, Sensitivity Analysis Excluding Trials With High Risk of Bias Due to Randomization Protocols (Sensitivity Analysis Meta-regression) eFigure 5. Funnel Plot Assessing Publication Bias,
doi:10.1002/14651858.CD007745.pub3Higgins, Thomas, Chandler, JAMA Network Open | Infectious Diseases Trials of Ivermectin for COVID-19 Between Regions With High and Low Prevalence of Strongyloidiasis JAMA Network Open
Kassalik, Mönkemüller, Strongyloides stercoralis hyperinfection syndrome and disseminated disease, Gastroenterol Hepatol
Lawrence, Meyerowitz-Katz, Heathers, Brown, Sheldrick, The lesson of ivermectin: metaanalyses based on summary data alone are inherently unreliable, Nat Med,
doi:10.1038/s41591-021-01535-yLier, Tuan, Davis, Case report: disseminated strongyloidiasis in a patient with COVID-19, Am J Trop Med Hyg,
doi:10.4269/ajtmh.20-0699Mahmud, Rahman, Alam, Ivermectin in combination with doxycycline for treating COVID-19 symptoms: a randomized trial, J Int Med Res,
doi:10.1177/03000605211013550Marchese, Crosato, Gulletta, Strongyloides infection manifested during immunosuppressive therapy for SARS-CoV-2 pneumonia, Infection,
doi:10.1007/s15010-020-01522-4Marcos, Terashima, Dupont, Gotuzzo, Strongyloides hyperinfection syndrome: an emerging global infectious disease, Trans R Soc Trop Med Hyg,
doi:10.1016/j.trstmh.2008.01.020Mills, Early treatment of COVID-19 with repurposed therapies: the TOGETHER adaptive platform trial
Myint, Chapman, Suarez, Mehta, Strongyloides hyperinfection syndrome in an immunocompetent host resulting in bandemia and death, BMJ Case Rep,
doi:10.1136/bcr-2016-217911Okumuş, Demirtürk, Çetinkaya, Evaluation of the effectiveness and safety of adding ivermectin to treatment in severe COVID-19 patients, BMC Infect Dis,
doi:10.1186/s12879-021-06104-9Ontario, Science Advisory Table, the Drugs, and Biologics Clinical Practice Guidelines Working Group. Ivermectin treatment for Strongyloides infection in patients with COVID-19, Can Commun Dis Rep,
doi:10.14745/ccdr.v47i78a04Ravikirti, Roy, Pattadar, Evaluation of ivermectin as a potential treatment for mild to moderate COVID-19: a double-blind randomized placebo controlled trial in Eastern India, J Pharm Pharm Sci,
doi:10.18433/jpps32105Shahbaznejad, Davoudi, Eslami, Effects of ivermectin in patients with COVID-19: a multicenter, double-blind, randomized, controlled clinical trial, Clin Ther,
doi:10.1016/j.clinthera.2021.04.007Soni, Evaluation of eosinopenia as a diagnostic and prognostic indicator in COVID-19 infection, Int J Lab Hematol,
doi:10.1111/ijlh.13425Statacorp, None
Statacorp, None, STATA Statistical Software
Vallejos, Zoni, Bangher, Ivermectin to prevent hospitalizations in patients with COVID-19 (IVERCOR-COVID19) a randomized, double-blind, placebo-controlled trial, BMC Infect Dis,
doi:10.1186/s12879-021-06348-5Vasquez-Rios, Pineda-Reyes, Pineda-Reyes, Marin, Ruiz et al., Strongyloides stercoralis hyperinfection syndrome: a deeper understanding of a neglected disease, J Parasit Dis,
doi:10.1007/s12639-019-01090-xWickham, ggplot2: elegant graphics for data analysis
Wolday, Gebrecherkos, Arefaine, Effect of co-infection with intestinal parasites on COVID-19 severity: a prospective observational cohort study, EClinicalMedicine,
doi:10.1016/j.eclinm.2021.101054DOI record:
{
"DOI": "10.1001/jamanetworkopen.2022.3079",
"ISSN": [
"2574-3805"
],
"URL": "http://dx.doi.org/10.1001/jamanetworkopen.2022.3079",
"author": [
{
"affiliation": [
{
"name": "Department of Dermatology, Icahn School of Medicine at Mount Sinai, New York, New York"
}
],
"family": "Bitterman",
"given": "Avi",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Albert Einstein College of Medicine, Bronx, New York"
}
],
"family": "Martins",
"given": "Caitlin Pestana",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Dermatology, Icahn School of Medicine at Mount Sinai, New York, New York"
}
],
"family": "Cices",
"given": "Ahuva",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "University of Denver, Denver, Colorado"
}
],
"family": "Nadendla",
"given": "Makarand Prasad",
"sequence": "additional"
}
],
"container-title": [
"JAMA Network Open"
],
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2022,
3,
21
]
],
"date-time": "2022-03-21T15:36:49Z",
"timestamp": 1647877009000
},
"deposited": {
"date-parts": [
[
2022,
3,
21
]
],
"date-time": "2022-03-21T15:37:05Z",
"timestamp": 1647877025000
},
"indexed": {
"date-parts": [
[
2022,
3,
21
]
],
"date-time": "2022-03-21T16:12:21Z",
"timestamp": 1647879141595
},
"is-referenced-by-count": 0,
"issn-type": [
{
"type": "electronic",
"value": "2574-3805"
}
],
"issue": "3",
"issued": {
"date-parts": [
[
2022,
3,
21
]
]
},
"journal-issue": {
"issue": "3",
"published-print": {
"date-parts": [
[
2022,
3,
1
]
]
}
},
"language": "en",
"link": [
{
"URL": "https://jamanetwork.com/journals/jamanetworkopen/articlepdf/2790173/bitterman_2022_oi_220123_1646862772.92008.pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "10",
"original-title": [],
"page": "e223079",
"prefix": "10.1001",
"published": {
"date-parts": [
[
2022,
3,
21
]
]
},
"published-online": {
"date-parts": [
[
2022,
3,
21
]
]
},
"publisher": "American Medical Association (AMA)",
"reference": [
{
"DOI": "10.1017/S003118201100120X",
"article-title": "Epidemiological aspects of strongyloidiasis in Brazil.",
"author": "Paula",
"doi-asserted-by": "publisher",
"first-page": "1331",
"issue": "11",
"journal-title": "Parasitology",
"key": "zoi220123r1",
"volume": "138",
"year": "2011"
},
{
"DOI": "10.1371/journal.pntd.0003018",
"article-title": "Strongyloidiasis—an insight into its global prevalence and management.",
"author": "Puthiyakunnon",
"doi-asserted-by": "crossref",
"issue": "8",
"journal-title": "PLoS Negl Trop Dis",
"key": "zoi220123r2",
"volume": "8",
"year": "2014"
},
{
"DOI": "10.1007/s12639-019-01090-x",
"article-title": "Strongyloides stercoralis hyperinfection syndrome: a deeper understanding of a neglected disease.",
"author": "Vasquez-Rios",
"doi-asserted-by": "publisher",
"first-page": "167",
"issue": "2",
"journal-title": "J Parasit Dis",
"key": "zoi220123r3",
"volume": "43",
"year": "2019"
},
{
"DOI": "10.3390/pathogens9060468",
"article-title": "The global prevalence of Strongyloides stercoralis infection.",
"author": "Buonfrate",
"doi-asserted-by": "crossref",
"issue": "6",
"journal-title": "Pathogens",
"key": "zoi220123r4",
"volume": "9",
"year": "2020"
},
{
"article-title": "Strongyloides stercoralis hyperinfection syndrome and disseminated disease.",
"author": "Kassalik",
"first-page": "766",
"issue": "11",
"journal-title": "Gastroenterol Hepatol (N Y)",
"key": "zoi220123r5",
"volume": "7",
"year": "2011"
},
{
"DOI": "10.1016/j.trstmh.2008.01.020",
"article-title": "Strongyloides hyperinfection syndrome: an emerging global infectious disease.",
"author": "Marcos",
"doi-asserted-by": "publisher",
"first-page": "314",
"issue": "4",
"journal-title": "Trans R Soc Trop Med Hyg",
"key": "zoi220123r6",
"volume": "102",
"year": "2008"
},
{
"DOI": "10.1111/imj.2018.48.issue-7",
"article-title": "Fatal Strongyloides hyperinfection syndrome in an immunocompetent adult with review of the literature.",
"author": "Chan",
"doi-asserted-by": "publisher",
"first-page": "872",
"issue": "7",
"journal-title": "Intern Med J",
"key": "zoi220123r7",
"volume": "48",
"year": "2018"
},
{
"DOI": "10.1080/20009666.2020.1824333",
"article-title": "Parasitic necrotizing pneumonia in an immunocompetent patient in United States.",
"author": "Gopalakrishna",
"doi-asserted-by": "publisher",
"first-page": "69",
"issue": "1",
"journal-title": "J Community Hosp Intern Med Perspect",
"key": "zoi220123r8",
"volume": "11",
"year": "2021"
},
{
"article-title": "Strongyloides hyperinfection syndrome in an immunocompetent host resulting in bandemia and death.",
"author": "Myint",
"journal-title": "BMJ Case Rep",
"key": "zoi220123r9",
"volume": "2017",
"year": "2017"
},
{
"DOI": "10.1007/s15010-015-0799-1",
"article-title": "Strongyloides stercoralis hyperinfection syndrome: a case series and a review of the literature.",
"author": "Geri",
"doi-asserted-by": "publisher",
"first-page": "691",
"issue": "6",
"journal-title": "Infection",
"key": "zoi220123r10",
"volume": "43",
"year": "2015"
},
{
"DOI": "10.4269/ajtmh.20-0699",
"article-title": "Case report: disseminated strongyloidiasis in a patient with COVID-19.",
"author": "Lier",
"doi-asserted-by": "publisher",
"first-page": "1590",
"issue": "4",
"journal-title": "Am J Trop Med Hyg",
"key": "zoi220123r11",
"volume": "103",
"year": "2020"
},
{
"DOI": "10.1007/s15010-020-01522-4",
"article-title": "Strongyloides infection manifested during immunosuppressive therapy for SARS-CoV-2 pneumonia.",
"author": "Marchese",
"doi-asserted-by": "publisher",
"first-page": "539",
"issue": "3",
"journal-title": "Infection",
"key": "zoi220123r12",
"volume": "49",
"year": "2021"
},
{
"DOI": "10.1111/ijlh.v43.s1",
"article-title": "Evaluation of eosinopenia as a diagnostic and prognostic indicator in COVID-19 infection.",
"author": "Soni",
"doi-asserted-by": "publisher",
"first-page": "137",
"issue": "S1",
"journal-title": "Int J Lab Hematol",
"key": "zoi220123r13",
"volume": "43",
"year": "2021"
},
{
"article-title": "Strongyloidiasis: a multifaceted disease.",
"author": "Ganesh",
"first-page": "194",
"issue": "3",
"journal-title": "Gastroenterol Hepatol (N Y)",
"key": "zoi220123r14",
"volume": "7",
"year": "2011"
},
{
"DOI": "10.1001/jama.2020.13170",
"article-title": "COVID-19 and dexamethasone: a potential strategy to avoid steroid-related Strongyloides hyperinfection.",
"author": "Stauffer",
"doi-asserted-by": "publisher",
"first-page": "623",
"issue": "7",
"journal-title": "JAMA",
"key": "zoi220123r15",
"volume": "324",
"year": "2020"
},
{
"DOI": "10.1097/MJT.0000000000001402",
"article-title": "Ivermectin for prevention and treatment of COVID-19 infection: a systematic review, meta-analysis, and trial sequential analysis to inform clinical guidelines.",
"author": "Bryant",
"doi-asserted-by": "publisher",
"first-page": "e434",
"issue": "4",
"journal-title": "Am J Ther",
"key": "zoi220123r16",
"volume": "28",
"year": "2021"
},
{
"DOI": "10.1186/s12879-021-06348-5",
"article-title": "Ivermectin to prevent hospitalizations in patients with COVID-19 (IVERCOR-COVID19) a randomized, double-blind, placebo-controlled trial.",
"author": "Vallejos",
"doi-asserted-by": "publisher",
"first-page": "635",
"issue": "1",
"journal-title": "BMC Infect Dis",
"key": "zoi220123r19",
"volume": "21",
"year": "2021"
},
{
"DOI": "10.1038/s41591-021-01535-y",
"article-title": "The lesson of ivermectin: meta-analyses based on summary data alone are inherently unreliable.",
"author": "Lawrence",
"doi-asserted-by": "publisher",
"first-page": "1853",
"issue": "11",
"journal-title": "Nat Med",
"key": "zoi220123r20",
"volume": "27",
"year": "2021"
},
{
"DOI": "10.1136/ebmental-2019-300117",
"article-title": "How to perform a meta-analysis with R: a practical tutorial.",
"author": "Balduzzi",
"doi-asserted-by": "publisher",
"first-page": "153",
"issue": "4",
"journal-title": "Evid Based Ment Health",
"key": "zoi220123r22",
"volume": "22",
"year": "2019"
},
{
"DOI": "10.18637/jss.v036.i03",
"article-title": "Conducting meta-analyses in R with the metafor package.",
"author": "Viechtbauer",
"doi-asserted-by": "publisher",
"first-page": "1",
"issue": "3",
"journal-title": "J Stat Software",
"key": "zoi220123r23",
"volume": "36",
"year": "2010"
},
{
"DOI": "10.21105/joss",
"article-title": "Welcome to the tidyverse.",
"author": "Wickham",
"doi-asserted-by": "publisher",
"first-page": "1686",
"issue": "43",
"journal-title": "J Open Source Software",
"key": "zoi220123r24",
"volume": "4",
"year": "2019"
},
{
"DOI": "10.1002/jmv.v93.10",
"article-title": "Clinical study evaluating the efficacy of ivermectin in COVID-19 treatment: a randomized controlled study.",
"author": "Abd-Elsalam",
"doi-asserted-by": "publisher",
"first-page": "5833",
"issue": "10",
"journal-title": "J Med Virol",
"key": "zoi220123r29",
"volume": "93",
"year": "2021"
},
{
"DOI": "10.1016/j.tmaid.2020.101906",
"article-title": "Risk of hospitalization for COVID-19 outpatients treated with various drug regimens in Brazil: comparative analysis.",
"author": "Szente Fonseca",
"doi-asserted-by": "crossref",
"journal-title": "Travel Med Infect Dis",
"key": "zoi220123r30",
"volume": "38",
"year": "2020"
},
{
"article-title": "Efficacy and safety of ivermectin and hydroxychloroquine in patients with severe COVID-19: a randomized controlled trial.",
"author": "Gonzalez",
"journal-title": "medRxiv",
"key": "zoi220123r31"
},
{
"article-title": "Controlled randomized clinical trial on using ivermectin with doxycycline for treating COVID-19 patients in Baghdad, Iraq.",
"author": "Hashim",
"journal-title": "medRxiv",
"key": "zoi220123r32"
},
{
"DOI": "10.1001/jama.2021.3071",
"article-title": "Effect of ivermectin on time to resolution of symptoms among adults with mild COVID-19: a randomized clinical trial.",
"author": "López-Medina",
"doi-asserted-by": "publisher",
"first-page": "1426",
"issue": "14",
"journal-title": "JAMA",
"key": "zoi220123r33",
"volume": "325",
"year": "2021"
},
{
"DOI": "10.1177/03000605211013550",
"article-title": "Ivermectin in combination with doxycycline for treating COVID-19 symptoms: a randomized trial.",
"author": "Mahmud",
"doi-asserted-by": "crossref",
"issue": "5",
"journal-title": "J Int Med Res",
"key": "zoi220123r34",
"volume": "49",
"year": "2021"
},
{
"DOI": "10.1186/s12879-021-06104-9",
"article-title": "Evaluation of the effectiveness and safety of adding ivermectin to treatment in severe COVID-19 patients.",
"author": "Okumus",
"doi-asserted-by": "publisher",
"first-page": "411",
"issue": "1",
"journal-title": "BMC Infect Dis",
"key": "zoi220123r35",
"volume": "21",
"year": "2021"
},
{
"DOI": "10.18433/jpps32105",
"article-title": "Evaluation of ivermectin as a potential treatment for mild to moderate COVID-19: a double-blind randomized placebo controlled trial in Eastern India.",
"author": "Ravikirti",
"doi-asserted-by": "publisher",
"first-page": "343",
"journal-title": "J Pharm Pharm Sci",
"key": "zoi220123r36",
"volume": "24",
"year": "2021"
},
{
"DOI": "10.1016/j.clinthera.2021.04.007",
"article-title": "Effects of ivermectin in patients with COVID-19: a multicenter, double-blind, randomized, controlled clinical trial.",
"author": "Shahbaznejad",
"doi-asserted-by": "publisher",
"first-page": "1007",
"issue": "6",
"journal-title": "Clin Ther",
"key": "zoi220123r37",
"volume": "43",
"year": "2021"
},
{
"DOI": "10.1016/j.eclinm.2021.101054",
"article-title": "Effect of co-infection with intestinal parasites on COVID-19 severity: a prospective observational cohort study.",
"author": "Wolday",
"doi-asserted-by": "crossref",
"journal-title": "EClinicalMedicine",
"key": "zoi220123r38",
"volume": "39",
"year": "2021"
},
{
"article-title": "Ivermectin treatment for Strongyloides infection in patients with COVID-19.",
"author": "Biologics Clinical Practice Guidelines Working Group",
"first-page": "316",
"issue": "7-8",
"journal-title": "Can Commun Dis Rep",
"key": "zoi220123r39",
"volume": "47",
"year": "2021"
},
{
"article-title": "Ivermectin versus albendazole or thiabendazole for Strongyloides stercoralis infection.",
"author": "Henriquez-Camacho",
"issue": "1",
"journal-title": "Cochrane Database Syst Rev",
"key": "zoi220123r40",
"year": "2016"
},
{
"key": "zoi220123r21",
"volume-title": "R: A Language and Environment for Statistical Computing",
"year": "2021"
},
{
"author": "Wickham",
"key": "zoi220123r25",
"volume-title": "Use R! Series",
"year": "2016"
},
{
"DOI": "10.1201/9781003107347",
"author": "Harrer",
"doi-asserted-by": "crossref",
"key": "zoi220123r26",
"volume-title": "Doing Meta-Analysis With R: A Hands-On Guide",
"year": "2021"
},
{
"key": "zoi220123r27",
"volume-title": "STATA Statistical Software: Release 17",
"year": "2021"
},
{
"DOI": "10.1002/9781119536604",
"author": "Higgins",
"doi-asserted-by": "crossref",
"key": "zoi220123r28",
"volume-title": "Cochrane Handbook for Systematic Reviews of Interventions",
"year": "2019"
},
{
"key": "zoi220123r17",
"unstructured": "Director General of Health Malaysia. Kenyataan Akhbar KPK 3 November 2021—Hasil Dapatan Kajian Keberkesanan Rawatan Ivermectin Untuk Pesakit COVID-19 Berisiko Tinggi (I-TECH Study) [in Malaysian]. November 3, 2021. Accessed November 6, 2021. https://kpkesihatan.com/2021/11/03/kenyataan-akhbar-kpk-3-november-2021-hasil-dapatan-kajian-keberkesanan-rawatan-ivermectin-untuk-pesakit-covid-19-berisiko-tinggi-i-tech-study/"
},
{
"key": "zoi220123r18",
"unstructured": "Mills? E. Early treatment of COVID-19 with repurposed therapies: the TOGETHER adaptive platform trial. August 21, 2021. Accessed November 3, 2021. https://rethinkingclinicaltrials.org/news/august-6-2021-early-treatment-of-covid-19-with-repurposed-therapies-the-together-adaptive-platform-trial-edward-mills-phd-frcp/"
}
],
"reference-count": 40,
"references-count": 40,
"relation": {},
"resource": {
"primary": {
"URL": "https://jamanetwork.com/journals/jamanetworkopen/fullarticle/2790173"
}
},
"score": 1,
"short-container-title": [
"JAMA Netw Open"
],
"short-title": [],
"source": "Crossref",
"subject": [
"General Medicine"
],
"subtitle": [
"A Meta-analysis"
],
"title": [
"Comparison of Trials Using Ivermectin for COVID-19 Between Regions With High and Low Prevalence of Strongyloidiasis"
],
"type": "journal-article",
"volume": "5"
}