Quality of clinical evidence and political justifications of ivermectin mass distribution of COVID-19 kits in eight Latin American countries
José Antonio Requejo Domínguez, Dolores Mino-León, Dr Veronika J Wirtz
BMJ Global Health, doi:10.1136/bmjgh-2022-010962
Background Several countries in Latin America conducted mass distribution of COVID-19 kits intended to treat mild COVID-19, thereby preventing excess hospitalisations. Many of the kits contained ivermectin, an antiparasitic medicine that was not approved at the time for the treatment of COVID-19. The study objective was to compare the timing of the publication of scientific evidence about the efficacy of ivermectin for COVID-19 with the timeline of distribution of COVID-19 kits in eight Latin American countries and to analyse whether evidence was used to justify ivermectin distribution. Methods We conducted a systematic review of randomised controlled trials (RCTs) published on the efficacy of ivermectin or ivermectin as adjuvant therapy on mortality from, or as prevention for, COVID-19. Each RCT was assessed using the Cochrane Grading of Recommendations, Assessment, Development and Evaluations (GRADE). Information on the timing and justification of government decisions was collected through a systematic search of leading newspapers and government press releases. Results After removing the duplicates and abstracts without full text, 33 RCTs met our inclusion criteria. According to GRADE, the majority had a substantial risk of bias. Many government officials made claims that ivermectin was effective and safe in the prevention or treatment of COVID-19, despite the lack of published evidence. Conclusion All eight governments distributed COVID-19 kits to their populations despite the absence of high-quality evidence on the efficacy of ivermectin for prevention, hospitalisation and mortality in COVID-19 patients. Lessons learnt from this situation could be used to strengthen government institutions' capacities to implement evidenceinformed public health policies.
BACKGROUND In 2020, at least eight countries in Latin America
Competing interests None declared. Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research. Patient consent for publication Not applicable. Provenance and peer review Not commissioned; externally peer reviewed. Data availability statement Data are available in a public, open access repository. No applicable. Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Supplement 2 Nexis Uni search strategy
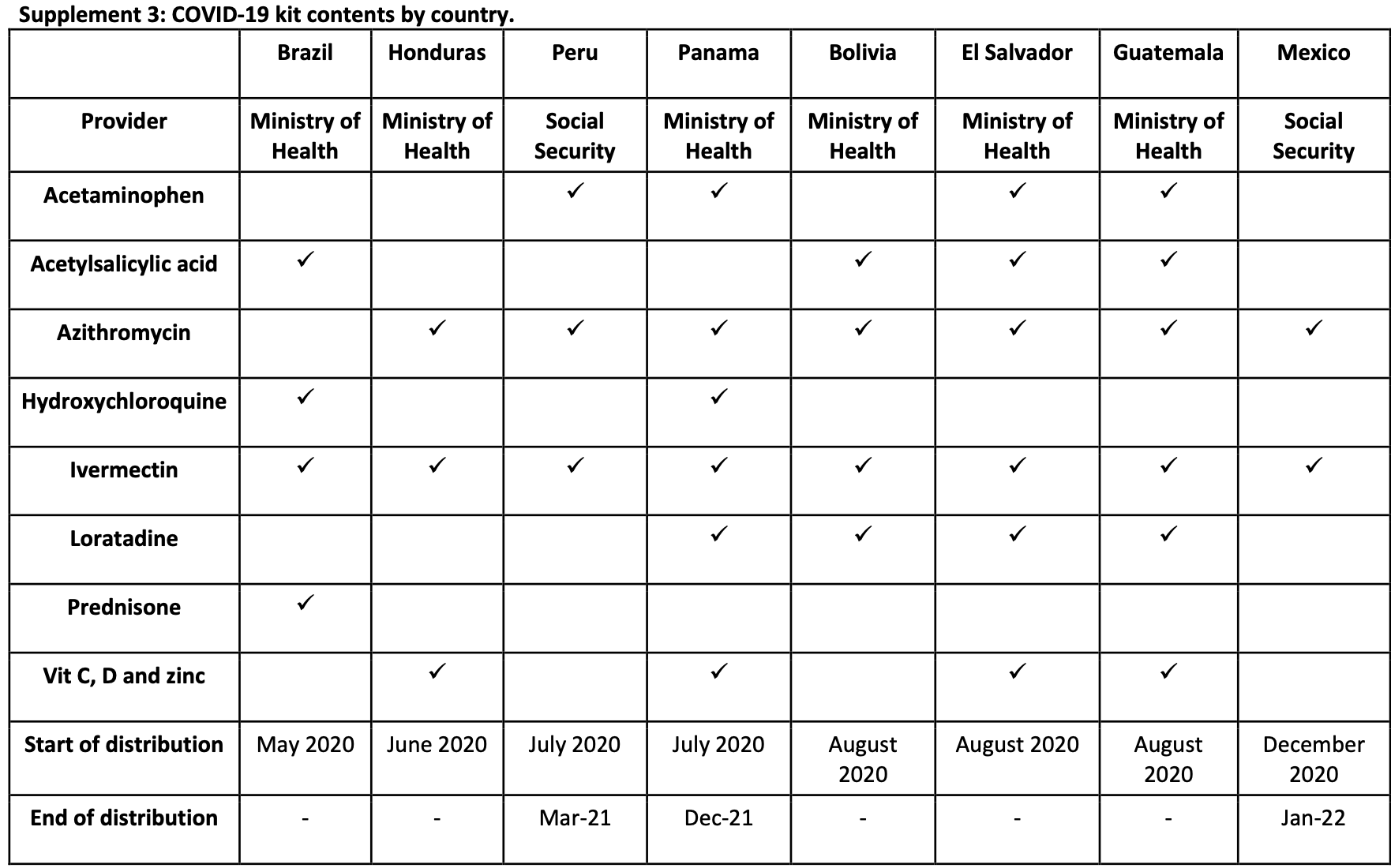
Ministry of Health Social Security
Ministry of Health
Ministry of Health
Ministry of Health
Ministry of Health Social Security
Acetaminophen
References
Bryant, Lawrie, Dowswell, Ivermectin for prevention and treatment of COVID-19 infection: a systematic review, meta-analysis, and trial sequential analysis to inform clinical guidelines, Am J Ther,
doi:10.1097/MJT.0000000000001402Camhaji, Gortázar, Santaeulalia, El accidentado viaje de la ivermectina POR América. El País
Campillo, Boussinesq, Bertout, Serious adverse reactions associated with ivermectin: a systematic pharmacovigilance study in sub-Saharan Africa and in the rest of the world, PLOS Negl Trop Dis,
doi:10.1371/journal.pntd.0009354Chacón, Ivermectin is no longer in COVID-19 treatment kits in Mexico, AP News
Crosby, Crosby, Applying the precautionary principle to personal protective equipment (PPE) guidance during the COVID-19 pandemic: did we learn the lessons of SARS?, Can J Anaesth,
doi:10.1007/s12630-020-01760-yDe, Gobierno envía Los primeros 22.000 tratamientos maíz Y catracho para atender a pacientes de covid-19
De, Paulo, Ministério da Saúde pressiona Manaus e diz ser 'inadmissível' não usar cloroquina contra Covid-19
De, Tomás-López, Álvarez-Medina, A multimodal strategy to reduce the risk of hospitalization/death in ambulatory patients with COVID-19, Arch Med Res,
doi:10.1016/j.arcmed.2022.01.002Dyer, Domínguez, Covid-19: Mexico City gave ivermectin kits to people with covid in "unethical'' experiment, BMJ Global,
doi:10.1136/bmj.o453Freelon, Hanbury, Brazil's main COVID strategy is a cocktail of unproven drugs: goats and soda, NPR
Health,
Hellmann, Homedes, An unethical trial and the politicization of the COVID-19 pandemic in Brazil: the case of prevent senior, Dev World Bioeth,
doi:10.1111/dewb.12363Henrique, COVID-19 vaccination in Brazil is a success, despite the failure of the federal government
Hill, Garratt, Levi, Meta-Analysis of randomized trials of ivermectin to treat SARS-cov-2 infection, Open Forum Infect Dis,
doi:10.1093/ofid/ofab358Hill, Mirchandani, Pilkington, Ivermectin for COVID-19: addressing potential bias and medical fraud, Open Forum Infect Dis,
doi:10.1093/ofid/ofab645Kim, Sparks, Liew, A rush to judgment? rapid reporting and dissemination of results and its consequences regarding the use of hydroxychloroquine for covid-19, Ann Intern Med,
doi:10.7326/M20-1223Kow, Merchant, Mustafa, The association between the use of ivermectin and mortality in patients with COVID-19: a meta-analysis, Pharmacol Rep,
doi:10.1007/s43440-021-00245-zLee, Morgan, Chaiyachati, Pulse oximetry for monitoring patients with COVID-19 at home-a pragmatic, randomized trial, N Engl J Med,
doi:10.1056/NEJMc2201541Lowe, Voo, Lee, Uncertainty, scarcity and transparency: public health ethics and risk communication in a pandemic, Lancet Reg Health Am,
doi:10.1016/j.lana.2022.100374Machiaco, Vargas, Bolivian city gives out free doses of de-worming drug in Bid to combat coronavirus
Merino, Borja, Lopez, Ivermectin and the odds of hospitalization due to covid-19: evidence from a quasi, SocArXiv
Mt, Prefeitura em MT CRIA kit para tratamento de COVID-19 contendo cloroquina
Nacional, RESOLUÇÃO de DIRETORIA COLEGIADA-RDC no 420, de 1o de SETEMBRO de 2020. DIáRIO OFICIAL dA UNIãO
Nación, Perú dejará de utilizar antiparasitario ivermectina Contra El COVID-19
News, Some Alaska legislators urge easier ivermectin access
Panamericana De La Salud, Ongoing living update of potential COVID-19 therapeutics: summary of rapid systematic reviews
Panamericana De La Salud, Ongoing living update of potential COVID-19 therapeutics: summary of rapid systematic reviews
Peruano, Modifican El documento Técnico: prevencióN, diagnóstico Y tratamiento de personas afectadas POR COVID-19 en El perú-REsolucion Ministerial-N° 270-2020-MINSA-PODER EJECUTIVO-SALUD
República, Coronavirus: ivermectina, antiparasitario animal Y usado Contra rosácea, ES aprobado POR Bolivia para tratar COVID-19
Roman, Burela, Pasupuleti, Ivermectin for the treatment of coronavirus disease 2019: a systematic review and meta-analysis of randomized controlled trials, Clin Infect Dis,
doi:10.1093/cid/ciab591Saavedra, Cañás, Tratamiento farmacológico para covid-19 en protocolos latinoamericanos: Una revisión narrativa de la eficacia Y seguridad, Visa Em Debate,
doi:10.22239/2317-269x.01741Shalders, Tratamento precoce': governo bolsonaro gasta quase R $ 90 milhões em remédios ineficazes, MAS ainda não pagou butantan POR vacinas
Tello, Ivermectina e hidroxicloroquina no son recomendables para tratamiento contra la COVID-19
Trigo, Kurmanaev, Cabrera, With officials' backing, dubious virus remedies surge in Latin America. the New York times
Zaidi, Dehgani-Mobaraki, Retracted article: the mechanisms of action of ivermectin against SARS-cov-2: an evidence-based clinical review article, J Antibiot (Tokyo),
doi:10.1038/s41429-021-00430-5DOI record:
{
"DOI": "10.1136/bmjgh-2022-010962",
"ISSN": [
"2059-7908"
],
"URL": "http://dx.doi.org/10.1136/bmjgh-2022-010962",
"abstract": "<jats:sec><jats:title>Background</jats:title><jats:p>Several countries in Latin America conducted mass distribution of COVID-19 kits intended to treat mild COVID-19, thereby preventing excess hospitalisations. Many of the kits contained ivermectin, an antiparasitic medicine that was not approved at the time for the treatment of COVID-19. The study objective was to compare the timing of the publication of scientific evidence about the efficacy of ivermectin for COVID-19 with the timeline of distribution of COVID-19 kits in eight Latin American countries and to analyse whether evidence was used to justify ivermectin distribution.</jats:p></jats:sec><jats:sec><jats:title>Methods</jats:title><jats:p>We conducted a systematic review of randomised controlled trials (RCTs) published on the efficacy of ivermectin or ivermectin as adjuvant therapy on mortality from, or as prevention for, COVID-19. Each RCT was assessed using the Cochrane Grading of Recommendations, Assessment, Development and Evaluations (GRADE). Information on the timing and justification of government decisions was collected through a systematic search of leading newspapers and government press releases.</jats:p></jats:sec><jats:sec><jats:title>Results</jats:title><jats:p>After removing the duplicates and abstracts without full text, 33 RCTs met our inclusion criteria. According to GRADE, the majority had a substantial risk of bias. Many government officials made claims that ivermectin was effective and safe in the prevention or treatment of COVID-19, despite the lack of published evidence.</jats:p></jats:sec><jats:sec><jats:title>Conclusion</jats:title><jats:p>All eight governments distributed COVID-19 kits to their populations despite the absence of high-quality evidence on the efficacy of ivermectin for prevention, hospitalisation and mortality in COVID-19 patients. Lessons learnt from this situation could be used to strengthen government institutions’ capacities to implement evidence-informed public health policies.</jats:p></jats:sec>",
"alternative-id": [
"10.1136/bmjgh-2022-010962"
],
"author": [
{
"ORCID": "http://orcid.org/0000-0002-2352-4232",
"affiliation": [],
"authenticated-orcid": false,
"family": "Requejo Domínguez",
"given": "José Antonio",
"sequence": "first"
},
{
"ORCID": "http://orcid.org/0000-0002-5144-2728",
"affiliation": [],
"authenticated-orcid": false,
"family": "Mino-León",
"given": "Dolores",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-0863-8768",
"affiliation": [],
"authenticated-orcid": false,
"family": "Wirtz",
"given": "Veronika J",
"sequence": "additional"
}
],
"container-title": "BMJ Global Health",
"container-title-short": "BMJ Glob Health",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"bmj.com"
]
},
"created": {
"date-parts": [
[
2023,
5,
24
]
],
"date-time": "2023-05-24T15:00:19Z",
"timestamp": 1684940419000
},
"deposited": {
"date-parts": [
[
2023,
5,
24
]
],
"date-time": "2023-05-24T15:00:37Z",
"timestamp": 1684940437000
},
"indexed": {
"date-parts": [
[
2023,
5,
25
]
],
"date-time": "2023-05-25T04:35:50Z",
"timestamp": 1684989350894
},
"is-referenced-by-count": 0,
"issue": "5",
"issued": {
"date-parts": [
[
2023,
5
]
]
},
"journal-issue": {
"issue": "5",
"published-online": {
"date-parts": [
[
2023,
5,
24
]
]
},
"published-print": {
"date-parts": [
[
2023,
5
]
]
}
},
"language": "en",
"license": [
{
"URL": "http://creativecommons.org/licenses/by-nc/4.0/",
"content-version": "unspecified",
"delay-in-days": 22,
"start": {
"date-parts": [
[
2023,
5,
23
]
],
"date-time": "2023-05-23T00:00:00Z",
"timestamp": 1684800000000
}
}
],
"link": [
{
"URL": "https://syndication.highwire.org/content/doi/10.1136/bmjgh-2022-010962",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "239",
"original-title": [],
"page": "e010962",
"prefix": "10.1136",
"published": {
"date-parts": [
[
2023,
5
]
]
},
"published-online": {
"date-parts": [
[
2023,
5,
24
]
]
},
"published-print": {
"date-parts": [
[
2023,
5
]
]
},
"publisher": "BMJ",
"reference": [
{
"key": "2023052408000907000_8.5.e010962.1",
"unstructured": "Camhaji E , Galarraga Gortázar N , Santaeulalia I , et al . El accidentado viaje de la ivermectina POR América. El País. 2022. Available: https://elpais.com/internacional/2022-02-15/el-accidentado-viaje-de-la-ivermectina-por-america.html [Accessed 7 Aug 2022]."
},
{
"DOI": "10.1038/d41586-020-02958-2",
"article-title": "Latin America’s embrace of an unproven COVID treatment is hindering drug trials",
"author": "Mega",
"doi-asserted-by": "crossref",
"first-page": "481",
"journal-title": "Nature",
"key": "2023052408000907000_8.5.e010962.2",
"volume": "586",
"year": "2020"
},
{
"DOI": "10.1038/d41586-020-03289-y",
"doi-asserted-by": "crossref",
"key": "2023052408000907000_8.5.e010962.3",
"unstructured": "Torres-Atencio I , Goodridge A , Vega S . COVID-19: Panama stockpiles unproven drugs. Nature 2020;587:548. doi:10.1038/d41586-020-03289-y"
},
{
"DOI": "10.1136/bmj.o453",
"article-title": "Covid-19: Mexico City gave ivermectin kits to people with covid in \"unethical'' experiment",
"author": "Dyer",
"doi-asserted-by": "crossref",
"first-page": "453",
"journal-title": "BMJ",
"key": "2023052408000907000_8.5.e010962.4",
"volume": "376",
"year": "2022"
},
{
"DOI": "10.1016/j.lana.2021.100089",
"doi-asserted-by": "crossref",
"key": "2023052408000907000_8.5.e010962.5",
"unstructured": "Furlan L , Caramelli B . The regrettable story of the \"covid k\" and the \"early treatment of covid-19\" in Brazil. Lancet Reg Health Am 2021;4:100089. doi:10.1016/j.lana.2021.100089"
},
{
"key": "2023052408000907000_8.5.e010962.6",
"unstructured": "Salud con lupa . El Salvador Guatemala Y Bolivia ofrecen kits de medicinas para COVID-19 Sin prever reacciones adversas. 2020. Available: https://saludconlupa.com/noticias/el-salvador-guatemala-y-bolivia-ofrecen-kits-de-medicinas-para-covid-19-sin-prever-reacciones-adversas/ [Accessed 11 Aug 2022]."
},
{
"key": "2023052408000907000_8.5.e010962.7",
"unstructured": "Redacción Animal Politico . Dan en CDMX ivermectina a pacientes COVID, pese a dudas sobre SU uso. 2021. Available: https://www.animalpolitico.com/2021/01/cdmx-usan-ivermectina-azitromicina-tratar-pacientes-covid/"
},
{
"key": "2023052408000907000_8.5.e010962.8",
"unstructured": "Secretaría de Salud . Gobierno envía Los primeros 22.000 tratamientos maíz Y catracho para atender a pacientes de covid-19. 2020. Available: https://www.salud.gob.hn/site/index.php/component/k2/item/1708-gobierno-envia-los-primeros-22-000-tratamientos-maiz-y-catracho-para-atender-a-pacientes-de-covid-19 [Accessed 23 Aug 2022]."
},
{
"key": "2023052408000907000_8.5.e010962.9",
"unstructured": "Trigo MS , Kurmanaev A , León Cabrera JM . With officials’ backing, dubious virus remedies surge in Latin America. the New York times. 2020. Available: https://www.nytimes.com/2020/07/23/world/americas/chlorine-coronavirus-bolivia-latin-america.html [Accessed 11 2022]."
},
{
"DOI": "10.1038/s41429-021-00430-5",
"doi-asserted-by": "crossref",
"key": "2023052408000907000_8.5.e010962.10",
"unstructured": "Zaidi AK , Dehgani-Mobaraki P . Retracted article: the mechanisms of action of ivermectin against SARS-cov-2: an evidence-based clinical review article. J Antibiot (Tokyo) 2022;75:122. doi:10.1038/s41429-021-00430-5"
},
{
"DOI": "10.1016/j.pt.2017.02.004",
"doi-asserted-by": "publisher",
"key": "2023052408000907000_8.5.e010962.11"
},
{
"DOI": "10.1371/journal.pntd.0009354",
"doi-asserted-by": "crossref",
"key": "2023052408000907000_8.5.e010962.12",
"unstructured": "Campillo JT , Boussinesq M , Bertout S , et al . Serious adverse reactions associated with ivermectin: a systematic pharmacovigilance study in sub-Saharan Africa and in the rest of the world. PLOS Negl Trop Dis 2021;15:e0009354. doi:10.1371/journal.pntd.0009354"
},
{
"DOI": "10.1016/j.antiviral.2020.104787",
"article-title": "The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro",
"author": "Caly",
"doi-asserted-by": "crossref",
"first-page": "104787",
"journal-title": "Antiviral Res",
"key": "2023052408000907000_8.5.e010962.13",
"volume": "178",
"year": "2020"
},
{
"DOI": "10.1016/j.jclinepi.2010.07.015",
"doi-asserted-by": "publisher",
"key": "2023052408000907000_8.5.e010962.14"
},
{
"DOI": "10.1002/14651858.CD015017",
"doi-asserted-by": "crossref",
"key": "2023052408000907000_8.5.e010962.15",
"unstructured": "Popp M , Stegemann M , Metzendorf M-I , et al . Ivermectin for preventing and treating COVID-19. Cochrane Database Syst Rev 2021;7:CD015017. doi:10.1002/14651858.CD015017.pub2"
},
{
"DOI": "10.1007/s43440-021-00245-z",
"article-title": "The association between the use of ivermectin and mortality in patients with COVID-19: a meta-analysis",
"author": "Kow",
"doi-asserted-by": "crossref",
"first-page": "1473",
"journal-title": "Pharmacol Rep",
"key": "2023052408000907000_8.5.e010962.16",
"volume": "73",
"year": "2021"
},
{
"DOI": "10.1093/cid/ciab591",
"article-title": "Ivermectin for the treatment of coronavirus disease 2019: a systematic review and meta-analysis of randomized controlled trials",
"author": "Roman",
"doi-asserted-by": "crossref",
"first-page": "1022",
"journal-title": "Clin Infect Dis",
"key": "2023052408000907000_8.5.e010962.17",
"volume": "74",
"year": "2022"
},
{
"DOI": "10.1093/ofid/ofab358",
"doi-asserted-by": "crossref",
"key": "2023052408000907000_8.5.e010962.18",
"unstructured": "Hill A , Garratt A , Levi J , et al . Meta-Analysis of randomized trials of ivermectin to treat SARS-cov-2 infection. Open Forum Infect Dis 2021;8:ofab358. doi:10.1093/ofid/ofab358"
},
{
"DOI": "10.1093/ofid/ofab645",
"doi-asserted-by": "crossref",
"key": "2023052408000907000_8.5.e010962.19",
"unstructured": "Hill A , Mirchandani M , Pilkington V . Ivermectin for COVID-19: addressing potential bias and medical fraud. Open Forum Infect Dis 2022;9:ofab645. doi:10.1093/ofid/ofab645"
},
{
"DOI": "10.1097/MJT.0000000000001402",
"doi-asserted-by": "publisher",
"key": "2023052408000907000_8.5.e010962.20"
},
{
"key": "2023052408000907000_8.5.e010962.21",
"unstructured": "El Peruano . Modifican El documento Técnico: prevencióN, diagnóstico Y tratamiento de personas afectadas POR COVID-19 en El perú-REsolucion Ministerial-N° 270-2020-MINSA-PODER EJECUTIVO-SALUD. 2020. Available: https://busquedas.elperuano.pe/normaslegales/modifican-el-documento-tecnico-prevencion-diagnostico-y-tr-RESOLUCION-MINISTERIAL-N-270-2020-MINSA-1866159-4/ [Accessed 7 Aug 2022]."
},
{
"key": "2023052408000907000_8.5.e010962.22",
"unstructured": "La República . Coronavirus: ivermectina, antiparasitario animal Y usado Contra rosácea, ES aprobado POR Bolivia para tratar COVID-19. 2020. Available: https://larepublica.pe/mundo/2020/05/12/coronavirus-ivermectina-antiparasitario-animal-y-usado-contra-rosacea-es-aprobado-por-bolivia-para-tratar-covid-19/ [Accessed 7 Aug 2022]."
},
{
"key": "2023052408000907000_8.5.e010962.23",
"unstructured": "Machiaco M , Vargas C . Bolivian city gives out free doses of de-worming drug in Bid to combat coronavirus. 2020. Available: https://www.reuters.com/article/us-health-coronavirus-bolivia-drug-idUSKBN22V2U7 [Accessed 7 Aug 2022]."
},
{
"key": "2023052408000907000_8.5.e010962.24",
"unstructured": "G1 MT . Prefeitura em MT CRIA kit para tratamento de COVID-19 contendo cloroquina. 2020. Available: https://g1.globo.com/mt/mato-grosso/noticia/2020/05/22/prefeitura-em-mt-cria-kit-para-tratamento-de-covid-19-contendo-cloroquina.ghtml [Accessed 11 Aug 2022]."
},
{
"key": "2023052408000907000_8.5.e010962.25",
"unstructured": "Gobierno del Perú . EsSalud entregó al gobierno regional kits COVID-19 Y fórmula para preparación de ivermectina. 2020. Available: https://www.gob.pe/institucion/regionucayali/noticias/211206-essalud-entrego-al-gobierno-regional-kits-covid-19-y-formula-para-preparacion-de-ivermectina [Accessed 11 Aug 2022]."
},
{
"key": "2023052408000907000_8.5.e010962.26",
"unstructured": "Agencia Peruana de Noticias Andina . EsSalud Y FFAA preparan 100,000 kits para atender La pandemia en regiones. 2020. Available: https://andina.pe/agencia/noticia-coronavirus-essalud-y-ffaa-preparan-100000-kits-para-atender-pandemia-regiones-807594.aspx [Accessed 11 Aug 2022]."
},
{
"key": "2023052408000907000_8.5.e010962.27",
"unstructured": "Redacción de la Prensa . Minsa entrega kits para pacientes Con covid-19. La prensa. 2020. Available: https://www.prensa.com/sociedad/minsa-entrega-kits-para-pacientes-con-covid-19/ [Accessed 10 Aug 2022]."
},
{
"key": "2023052408000907000_8.5.e010962.28",
"unstructured": "Larios B . Gobierno entregará un millón de kits de medicamentos para pacientes Con COVID-19. Agencia Guatemalteca de Noticias, 2020. Available: https://agn.gt/archivo/para-corregir-gobierno-entregara-un-millon-de-kits-de-medicamentos-para-pacientes-con-covid-19/"
},
{
"key": "2023052408000907000_8.5.e010962.29",
"unstructured": "Imprensa Nacional . RESOLUÇÃO de DIRETORIA COLEGIADA-RDC no 420, de 1o de SETEMBRO de 2020. DIáRIO OFICIAL dA UNIãO. 2020. Available: https://www.in.gov.br/en/web/dou/-/resolucao-de-diretoria-colegiada-rdc-n-420-de-1-de-setembro-de-2020-275243243 [Accessed 11 2022]."
},
{
"key": "2023052408000907000_8.5.e010962.30",
"unstructured": "Reuters Fact . Fact check-Mexico no longer including ivermectin in home COVID-19 care kits, contrary to claims on social media. Reuters, 2022. Available: https://www.reuters.com/article/factcheck-imssmexico-ivermectin-idUSL1N2U626I"
},
{
"key": "2023052408000907000_8.5.e010962.31",
"unstructured": "Folha de Sao Paulo . Ministério da Saúde pressiona Manaus e diz ser ‘inadmissível’ não usar cloroquina contra Covid-19. Folha de Sao Paulo, 2021. Available: https://www1.folha.uol.com.br/colunas/painel/2021/01/ministerio-da-saude-pressiona-manaus-e-diz-ser-inadmissivel-nao-usar-cloroquina-contra-covid-19.shtml"
},
{
"key": "2023052408000907000_8.5.e010962.32",
"unstructured": "Freelon K , Hanbury S . Brazil’s main COVID strategy is a cocktail of unproven drugs: goats and soda. NPR, 2021. Available: https://www.npr.org/sections/goatsandsoda/2021/06/15/1006198151/covid-pseudoscience-is-choking-brazil"
},
{
"key": "2023052408000907000_8.5.e010962.33",
"unstructured": "Ministerio de Salud y Deportes de Bolivia . Brigadas de salud distribuirán 150.000 kits de medicamentos para tratar síntomas de coronavirus. 2020. Available: https://www.minsalud.gob.bo/es/4483-brigadas-de-salud-distribuiran-150-000-kits-de-medicamentos-para-tratar-sintomas-de-coronavirus [Accessed 11 Aug 2022]."
},
{
"key": "2023052408000907000_8.5.e010962.34",
"unstructured": "La Nación . Perú dejará de utilizar antiparasitario ivermectina Contra El COVID-19. 2021. Available: https://www.lanacion.com.py/mundo/2021/03/28/peru-dejara-de-utilizar-antiparasitario-ivermectina-contra-el-covid-19/ [Accessed 16 Aug 2022]."
},
{
"key": "2023052408000907000_8.5.e010962.35",
"unstructured": "Cruz Walkiria Tello N . Ivermectina e hidroxicloroquina no son recomendables para tratamiento contra la COVID-19. Caja de Seguro Social, 2021. Available: https://prensa.css.gob.pa/2021/12/30/ivermectina-e-hidroxicloroquina-no-son-recomendables-para-tratamiento-contra-la-covid-19/"
},
{
"key": "2023052408000907000_8.5.e010962.36",
"unstructured": "Martínez Chacón M . Ivermectin is no longer in COVID-19 treatment kits in Mexico. AP News, 2022. Available: https://apnews.com/article/fact-checking-867018157676"
},
{
"DOI": "10.1016/j.lana.2022.100374",
"doi-asserted-by": "crossref",
"key": "2023052408000907000_8.5.e010962.37",
"unstructured": "Lowe AE , Voo TC , Lee LM , et al . Uncertainty, scarcity and transparency: public health ethics and risk communication in a pandemic. Lancet Reg Health Am 2022;16:100374. doi:10.1016/j.lana.2022.100374"
},
{
"DOI": "10.1007/s12630-020-01760-y",
"article-title": "Applying the precautionary principle to personal protective equipment (PPE) guidance during the COVID-19 pandemic: did we learn the lessons of SARS?",
"author": "Crosby",
"doi-asserted-by": "crossref",
"first-page": "1327",
"journal-title": "Can J Anaesth",
"key": "2023052408000907000_8.5.e010962.38",
"volume": "67",
"year": "2020"
},
{
"key": "2023052408000907000_8.5.e010962.39",
"unstructured": "Organización Panamericana de la Salud . Ongoing living update of potential COVID-19 therapeutics: summary of rapid systematic reviews. 2020."
},
{
"key": "2023052408000907000_8.5.e010962.40",
"unstructured": "Organización Panamericana de la Salud . Ongoing living update of potential COVID-19 therapeutics: summary of rapid systematic reviews (May). 2020."
},
{
"DOI": "10.22239/2317-269x.01741",
"article-title": "Tratamiento farmacológico para covid-19 en protocolos latinoamericanos: Una revisión narrativa de la eficacia Y seguridad",
"author": "Saavedra",
"doi-asserted-by": "crossref",
"first-page": "150",
"journal-title": "Visa Em Debate",
"key": "2023052408000907000_8.5.e010962.41",
"volume": "8",
"year": "2020"
},
{
"DOI": "10.1111/dewb.12363",
"doi-asserted-by": "crossref",
"key": "2023052408000907000_8.5.e010962.42",
"unstructured": "Hellmann F , Homedes N . An unethical trial and the politicization of the COVID-19 pandemic in Brazil: the case of prevent senior. Dev World Bioeth 2022. doi:10.1111/dewb.12363 [Epub ahead of print 28 Jun 2022]."
},
{
"DOI": "10.31235/osf.io/r93g4",
"doi-asserted-by": "crossref",
"key": "2023052408000907000_8.5.e010962.43",
"unstructured": "Merino J , Borja VH , Lopez O , et al . Ivermectin and the odds of hospitalization due to covid-19: evidence from a quasi-experimental analysis based on a public intervention in Mexico City. SocArXiv [Preprint]. doi:10.31235/osf.io/r93g4"
},
{
"key": "2023052408000907000_8.5.e010962.44",
"unstructured": "Sarabia D . CDMX gastó 29 MDP en tratamiento Con ivermectina no autorizado Contra COVID. 2022. Available: https://www.animalpolitico.com/2022/02/gobierno-cdmx-gasto-tratamiento-covid-ivermectina/ [Accessed 8 Aug 2022]."
},
{
"key": "2023052408000907000_8.5.e010962.45",
"unstructured": "Bertin Henrique AL . COVID-19 vaccination in Brazil is a success, despite the failure of the federal government. O’Neill Institute. 2022. Available: https://oneill.law.georgetown.edu/covid-19-vaccination-in-brazil-is-a-success-despite-the-failure-of-the-federal-government/ [Accessed 16 Aug 2022]."
},
{
"key": "2023052408000907000_8.5.e010962.46",
"unstructured": "Instituto Mexicano del Seguro Social . Algoritmos interinos para La atención del COVID-19. 2021. Available: http://educacionensalud.imss.gob.mx/es/system/files/Algoritmos_interinos_COVID19_CTEC.pdf"
},
{
"DOI": "10.1016/j.arcmed.2022.01.002",
"article-title": "A multimodal strategy to reduce the risk of hospitalization/death in ambulatory patients with COVID-19",
"author": "Ascencio-Montiel",
"doi-asserted-by": "crossref",
"first-page": "323",
"journal-title": "Arch Med Res",
"key": "2023052408000907000_8.5.e010962.47",
"volume": "53",
"year": "2022"
},
{
"key": "2023052408000907000_8.5.e010962.48",
"unstructured": "Shalders A . Tratamento precoce’: governo bolsonaro gasta quase R $ 90 milhões em remédios ineficazes, MAS ainda não pagou butantan POR vacinas. BBC News Brasil. 2021. Available: https://www.bbc.com/portuguese/brasil-55747043 [Accessed 8 Aug 2022]."
},
{
"DOI": "10.1056/NEJMc2201541",
"article-title": "Pulse oximetry for monitoring patients with COVID-19 at home-a pragmatic, randomized trial",
"author": "Lee",
"doi-asserted-by": "crossref",
"first-page": "1857",
"journal-title": "N Engl J Med",
"key": "2023052408000907000_8.5.e010962.49",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.1001/jama.2021.24352",
"article-title": "Us insurer spending on ivermectin prescriptions for COVID-19",
"author": "Chua",
"doi-asserted-by": "crossref",
"first-page": "584",
"journal-title": "JAMA",
"key": "2023052408000907000_8.5.e010962.50",
"volume": "327",
"year": "2022"
},
{
"key": "2023052408000907000_8.5.e010962.51",
"unstructured": "AP News . Some Alaska legislators urge easier ivermectin access. 2021. Available: https://apnews.com/article/coronavirus-pandemic-us-food-and-drug-administration-anchorage-alaska-pandemics-53b41f35cdf640cd3e743482aca565f4 [Accessed 16 Aug 2022]."
},
{
"key": "2023052408000907000_8.5.e010962.52",
"unstructured": "WHO . Who advises that ivermectin only be used to treat COVID-19 within clinical trials. 2021. Available: https://www.who.int/news-room/feature-stories/detail/who-advises-that-ivermectin-only-be-used-to-treat-covid-19-within-clinical-trials [Accessed 8 Aug 2022]."
},
{
"key": "2023052408000907000_8.5.e010962.53",
"unstructured": "The Japan Times . Japan to give two masks each to 50 million households to fight virus. 2022. Available: https://www.japantimes.co.jp/news/2020/04/02/national/japanese-government-distribute-two-masks-per-household-abe/ [Accessed 16 Aug 2022]."
},
{
"key": "2023052408000907000_8.5.e010962.54",
"unstructured": "FDA . Fda advises patients on use of non-steroidal anti-inflammatory drugs (NSAIDs) for COVID-19. Available: https://www.fda.gov/drugs/drug-safety-and-availability/fda-advises-patients-use-non-steroidal-anti-inflammatory-drugs-nsaids-covid-19 [Accessed 6 Mar 2023]."
},
{
"DOI": "10.7326/M20-1223",
"article-title": "A rush to judgment? rapid reporting and dissemination of results and its consequences regarding the use of hydroxychloroquine for covid-19",
"author": "Kim",
"doi-asserted-by": "crossref",
"first-page": "819",
"journal-title": "Ann Intern Med",
"key": "2023052408000907000_8.5.e010962.55",
"volume": "172",
"year": "2020"
},
{
"key": "2023052408000907000_8.5.e010962.56",
"unstructured": "Pan American Health Organization . COVID-19: chloroquine and hydroxychloroquine research. 2020. Available: https://iris.paho.org/handle/10665.2/52094 [Accessed 5 Sep 2022]."
},
{
"key": "2023052408000907000_8.5.e010962.57",
"unstructured": "Agencia Guatemalteca de Noticias . Gobierno entregará un millón de kits de medicamentos para pacientes Con COVID-19. 2020. Available: https://agn.gt/archivo/para-corregir-gobierno-entregara-un-millon-de-kits-de-medicamentos-para-pacientes-con-covid-19/ [Accessed Aug 2022]."
},
{
"key": "2023052408000907000_8.5.e010962.58",
"unstructured": "González Pinilla J . No hay POR qué temerle a la hidroxicloroquina’, dice ministro sucre, pese a advertencia de la OPS. La Prensa. 2020. Available: https://www.prensa.com/sociedad/no-hay-porque-temerle-a-la-hidroxicloroquina-ministro-sucre/ [Accessed 11 Aug 2022]."
}
],
"reference-count": 58,
"references-count": 58,
"relation": {},
"resource": {
"primary": {
"URL": "https://gh.bmj.com/lookup/doi/10.1136/bmjgh-2022-010962"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Public Health, Environmental and Occupational Health",
"Health Policy"
],
"subtitle": [],
"title": "Quality of clinical evidence and political justifications of ivermectin mass distribution of COVID-19 kits in eight Latin American countries",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1136/crossmarkpolicy",
"volume": "8"
}