A Comparative Study on Ivermectin-Doxycycline and Hydroxychloroquine-Azithromycin Therapy on COVID-19 Patients
Abu Taiub, MD. Mohammed Mohiuddin Chowdhury, Mohammad Shahbaz, Md Rezaul Karim, Jahirul Islam, Guo Dan, MD; PhD. Shuixiang He
Eurasian Journal of Medicine and Oncology, doi:10.14744/ejmo.2021.16263
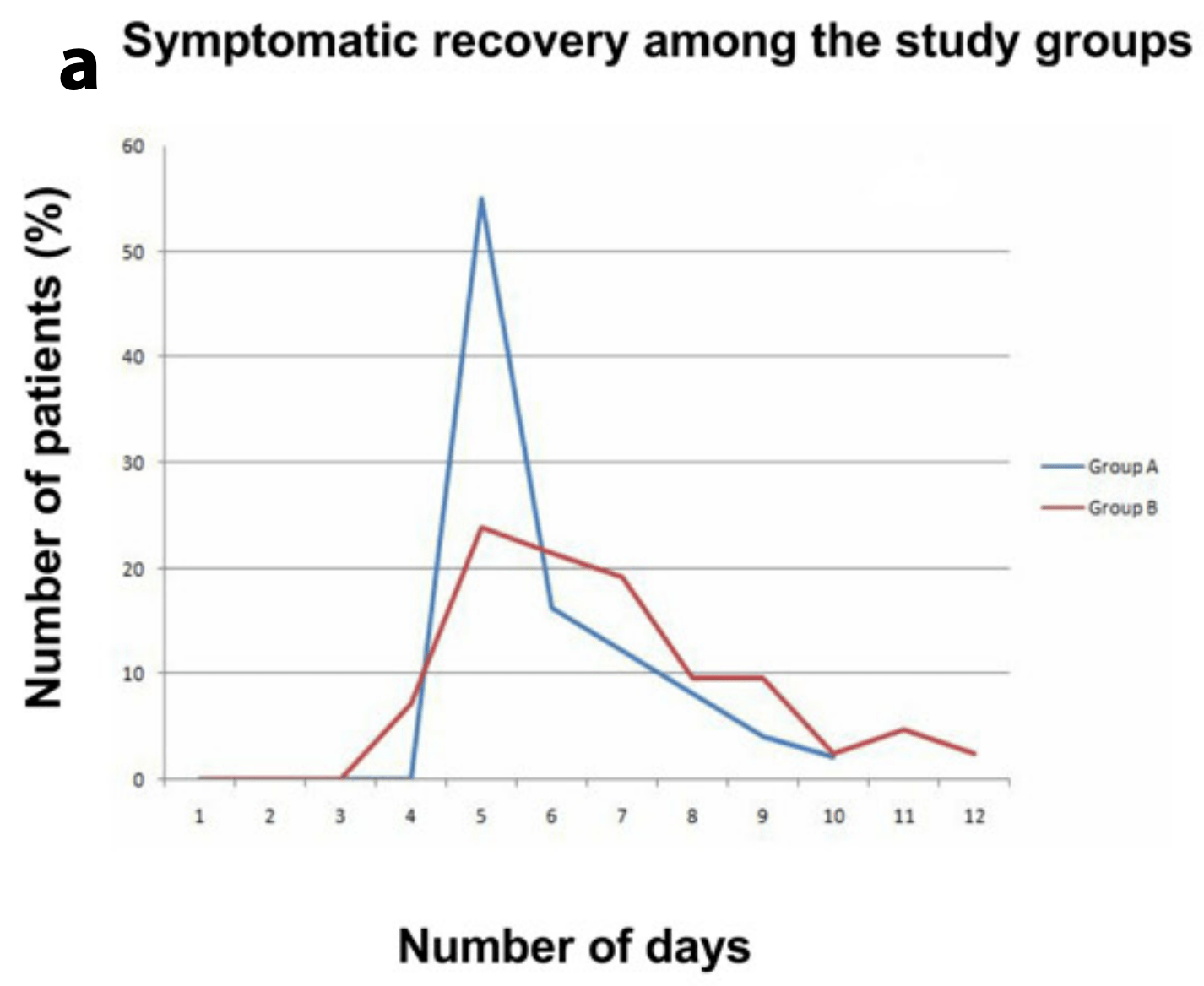
C oronavirus disease 2019 (COVID-19) is a global pan- demic declared by the world health organization (WHO). Over ninety million people have already been infected by severe acute respiratory syndrome-corona-virus-2 (SARS-CoV-2), and billions have been affected by the socioeconomic squeal. As SARS-CoV-2 is a novel virus, there are no proven treatment options yet. Early treatment before the disease becomes severe would be optimal. The Objectives: We investigated the outcomes of Ivermectin-Doxycycline vs. Hydroxychloroquine-Azithromycin combination therapy in mild to moderate COVID19 patients. Methods: Patients were divided randomly into two groups: Ivermectin 200µgm/kg single dose + Doxycycline 100mg BID for ten days in group A, and Hydroxychloroquine 400mg for the first day, then 200mg BID for nine days + Azithromycin 500mg daily for five days in group B (Control group). RT-PCR for SARS-CoV-2 infection was repeated in all symptomatic patients on the second day onward without symptoms. Repeat PCR was done every two days onward if the result found positive. Time to the negative PCR and symptomatic recovery was measured for each group. Results: All subjects in Group A reached a negative PCR, at a mean of 8.93 days, and reached symptomatic recovery, at a mean of 5.93 days, with 55.10% symptom-free by the fifth day. In group B, 96.36% reached a negative PCR at a mean of 9.33 days and were symptoms-free at 6.99 days. In group A 31.67% of patients expressed symptoms caused by medication, this was 46.43% in group B.
Conclusion: The combination therapy of Ivermectin-Doxycycline showed a trend towards superiority to the combination of Hydroxychloroquine-Azithromycin for mild to moderate COVID19 disease.
Disclosures Acknowledgment: Alexis Lieberman, MD; Associate chief for Ambulatory Pediatrics and Director of the Adolescent Program at Albert Einstein Medical Center in Philadelphia, Pennsylvania, for his kind assistance in editing this manuscript.
References
Abegunde, Doxycycline plus ivermectin versus ivermectin alone for treatment of patients with onchocerciasis, Cochrane Database of Systematic Reviews,
doi:10.1002/14651858.CD011146.pub2Accapezzato, Chloroquine enhances human CD8+ T cell responses against soluble antigens in vivo, Journal of Experimental Medicine,
doi:10.1084/jem.20051106Bhiuyan, Saber, A Case Series of 100 COVID-19 Positive Patients Treated with Combination of Ivermectin and Doxycycline Mtalam, R Murshed, Bangladesh Coll Phys Surg,
doi:10.3329/jbcps.v38i0.47512Chan, A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster, The Lancet,
doi:10.1016/S0140-6736(20)30154-9Conforti, Doxycycline, a widely used antibiotic in dermatology with a possible anti-inflammatory action against IL-6 in COVID-19 outbreak, Dermatologic Therapy,
doi:10.1111/dth.13437Gautret, Lagier, Parola, Hoang, Meddeb et al., Clinical and microbiological effect of a combination of Hydroxychloroquine and Azithromycin in 80 COVID-19 patients with at least a six-day follow up: an observational study,
doi:10.1016/j.tmaid.2020.101663Gautret, Lagier, Parola, Hoang, Meddeb et al., Clinical and microbiological effect of a combination of Hydroxychloroquine and Azithromycin in 80 COVID-19 patients with at least a six-day follow up: an observational study, Mediterr-Infect,
doi:10.1016/j.tmaid.2020.101663Gautret, Lagier, Parola, Hydroxychloroquine and Azithromycin as a treatment of COVID-19: results of an openlabel non-randomized clinical trial, Int J Antimicrob Agents
Holmes, Charles, Safety and Efficacy Review of Doxycycline, Clinical Medicine Insights: Therapeutics,
doi:10.4137/CMT.S2035Juurlink, Safety considerations with chloroquine, hydroxychloroquine and azithromycin in the management of SARS-CoV-2 infection, Canadian Medical Association Journal,
doi:10.1503/cmaj.200528Liu, Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro, Cell Discovery,
doi:10.1038/s41421-020-0156-0Meyerowitz, Rethinking the role of hydroxychloroquine in the treatment of COVID-19, The FASEB Journal,
doi:10.1096/fj.202000919Molina, No evidence of rapid antiviral clearance or clinical benefit with the combination of hydroxychloroquine and azithromycin in patients with severe COVID-19 infection, Médecine et Maladies Infectieuses,
doi:10.1016/j.medmal.2020.03.006Momekov, Ivermectin as a potential COVID-19 treatment from the pharmacokinetic point of view: antiviral levels are not likely attainable with known dosing regimens,
doi:10.1101/2020.04.11.20061804Rajter, ICON (Ivermectin in COvid Nineteen) study: Use of Ivermectin is Associated with Lower Mortality in Hospitalized Patients with COVID19,
doi:10.1101/2020.06.06.20124461Savarino, Tarek, Pharmacokinetic bases of the hydroxychloroquine response in COVID-19: implications for therapy and prevention
Sodhi, Therapeutic Potential for Tetracyclines in the Treatment of COVID-19, The Journal of Human Pharmacology and Drug Therapy,
doi:10.1002/phar.2395Wang, Cao, Zhang, Remdesivir and chloroquine effectively inhibit the recently emerged novel Coronavirus (2019-nCoV) in vitro, Cell Res,
doi:10.1038/s41422-020-0282-0Weniger, Organization WH Review of side effects and toxicity of chloroquine
Yao, Ye, Zhang, In vitro antiviral activity and projection of optimized dosing design of Hydroxychloroquine for the treatment of severe acute respiratory syndrome Coronavirus 2 (SARS-CoV-2), Clin Infect Dis,
doi:10.1093/cid/ciaa237Şimşek Yavuz, Serap, Serhat, Antiviral treatment of COVID-19, Turkish Journal of Medical Sciences,
doi:10.3906/sag-2004-145DOI record:
{
"DOI": "10.14744/ejmo.2021.16263",
"ISSN": [
"2587-196X"
],
"URL": "http://dx.doi.org/10.14744/ejmo.2021.16263",
"author": [
{
"affiliation": [],
"family": "chowdhury",
"given": "abu taiub mohammed mohiuddin",
"sequence": "first"
}
],
"container-title": "Eurasian Journal of Medicine and Oncology",
"container-title-short": "EJMO",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2021,
3,
2
]
],
"date-time": "2021-03-02T14:46:16Z",
"timestamp": 1614696376000
},
"deposited": {
"date-parts": [
[
2023,
5,
5
]
],
"date-time": "2023-05-05T17:29:44Z",
"timestamp": 1683307784000
},
"indexed": {
"date-parts": [
[
2024,
3,
29
]
],
"date-time": "2024-03-29T11:54:26Z",
"timestamp": 1711713266156
},
"is-referenced-by-count": 15,
"issued": {
"date-parts": [
[
2021
]
]
},
"link": [
{
"URL": "https://www.ejmo.org/pdf/A%20Comparative%20Study%20on%20IvermectinDoxycycline%20and%20HydroxychloroquineAzithromycin%20Therapy%20on%20COVID19%20Patients-16263.pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "5966",
"original-title": [],
"prefix": "10.14744",
"published": {
"date-parts": [
[
2021
]
]
},
"published-online": {
"date-parts": [
[
2021
]
]
},
"published-print": {
"date-parts": [
[
2021
]
]
},
"publisher": "Kare Publishing",
"reference-count": 0,
"references-count": 0,
"relation": {
"has-preprint": [
{
"asserted-by": "object",
"id": "10.21203/rs.3.rs-38896/v1",
"id-type": "doi"
}
]
},
"resource": {
"primary": {
"URL": "https://ejmo.org/10.14744/ejmo.2021.16263/"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Oncology",
"Internal Medicine"
],
"subtitle": [],
"title": "A Comparative Study on Ivermectin-Doxycycline and Hydroxychloroquine-Azithromycin Therapy on COVID-19 Patients",
"type": "journal-article"
}