Ivermectin as a Potential Addition to the Limited Anti-COVID-19 Arsenal: A Double-Blinded Clinical Trial
Mehran Varnaseri, Fatemeh Amini, Ramin Jamshididan, Mehrdad Dargahi, Nematollah Gheibi, Sara Abolghasemi, Mohammadreza Dayer, Negar Varnasseri, Khojasteh Hoseinynejad, Sahar Kheradhoosh, Pedram Nazari, Ebrahim Babadi, Seyedeh Maryam Mousavinezhad, Pouya Ebrahimi
Jundishapur Journal of Health Sciences, doi:10.5812/jjhs-146703
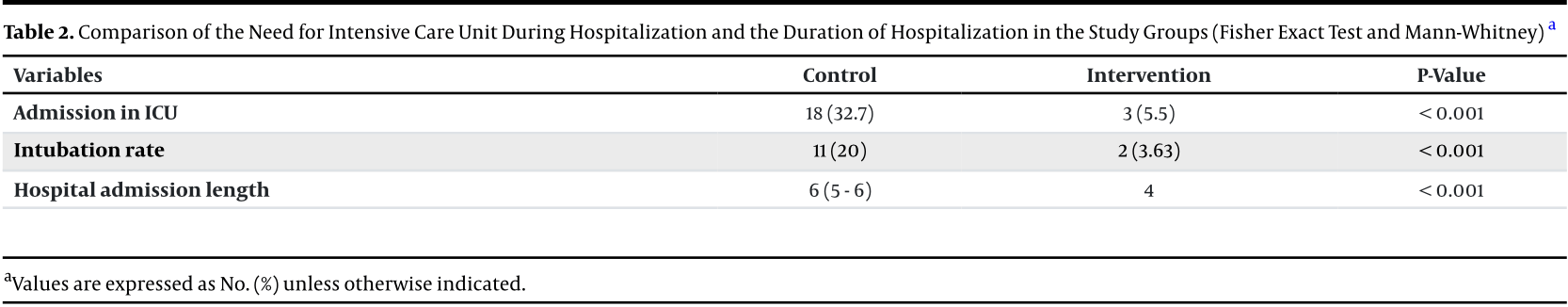
Background: Addressing the Coronavirus disease 2019 (COVID-19) pandemic remains a significant challenge for healthcare systems globally. Despite the absence of a proven cure, ivermectin has been proposed as a potentially effective agent against it. Objectives: This study aimed to evaluate the therapeutic effects of ivermectin compared to a placebo group in non-critically ill confirmed COVID-19 patients. Methods: A double-blind, randomized clinical trial was conducted on 110 patients with moderate-to-severe (non-critical) confirmed COVID-19 infection. The patients were equally divided into two groups, with one group receiving ivermectin tablets (14 mg every 12 hours for three days) and the other group receiving a placebo. The efficacy and safety of ivermectin were assessed in both groups. Results: A total of 110 patients, including 62 (56.4%) men and 48 (43.6%) women, with an average age of 53.36 ± 15.10 years, were enrolled in our double-blind, randomized clinical trial. The baseline characteristics of the two groups were similar. The findings demonstrated that ivermectin significantly reduced the need for Intensive Care Unit admission (32.7% vs. 5.5%; P < 0.001), hospitalization duration (six vs. four days; P < 0.001), and median time to symptom resolution period (P < 0.05) in COVID-19 patients compared to the placebo group, without any serious side effects (P > 0.05). Conclusions: Ivermectin appears to be a potentially effective and safe medication for COVID-19 patients with moderate disease.
Authors' Contribution: Study concept and design: M.V., M.D., P.N., K.H., F.A., R.J.; acquisition of data: N.G., S.A., M.D.; analysis and interpretation of data: N.V., K.H., S.K.; drafting of the manuscript: P.N., E.B., S.M., P.E.; critical revision of the manuscript for important intellectual content: M.V., M.D.; statistical analysis: S.A., M.D.; administrative, technical, and material support: M.V., M.D., P.N., K.H., F.A., R.J.; study supervision: N.G., S.A., M.D.
Clinical
References
Abd-Elsalam, Noor, Badawi, Khalaf, Esmail et al., Clinical study evaluating the efficacy of ivermectin in COVID-19 treatment: A randomized controlled study, J Med Virol,
doi:10.1002/jmv.27122Ahmed, Karim, Ross, Hossain, Clemens et al., A five-day course of ivermectin for the treatment of COVID-19 may reduce the duration of illness, Int J Infect Dis,
doi:10.1016/j.ijid.2020.11.191Bhowmick, Dang, Vallish, Dang, Safety and Efficacy of Ivermectin and Doxycycline Monotherapy and in Combination in the Treatment of COVID-19: A Scoping Review, Drug Saf,
doi:10.1007/s40264-021-01066-yChaccour, Casellas, Blanco-Di Matteo, Pineda, Fernandez-Montero et al., The effect of early treatment with ivermectin on viral load, symptoms and humoral response in patients with non-severe COVID-19: A pilot, double-blind, placebocontrolled, randomized clinical trial, EClin Med,
doi:10.1016/j.eclinm.2020.100720Chahla, Ruiz, Mena, Brepe, Terranova et al., Randomized trials -Ivermectin repurposing for COVID-19 treatment of outpatients with mild disease in primary health care centers, Health Sci,
doi:10.21203/rs.3.rs-495945/v1Dror, Eisenbach, Taiber, Morozov, Mizrachi et al., Vaccine hesitancy: the next challenge in the fight against COVID-19, Eur J Epidemiol,
doi:10.1007/s10654-020-00671-yEksombatchai, Wongsinin, Phongnarudech, Thammavaranucupt, Amornputtisathaporn et al., Pulmonary function and six-minute-walk test in patients after recovery from COVID-19: A prospective cohort study, PLoS One,
doi:10.1371/journal.pone.0257040Fattahi, Mohseni, Beheshtian, Jafarpour, Jalalvand et al., Disease Waves of SARS-CoV-2 in Iran Closely Mirror Global Pandemic Trends, Arch Iran Med,
doi:10.34172/aim.2022.83Gonzalez Canga, Prieto, Liebana, Martinez, Vega et al., The pharmacokinetics and interactions of ivermectin in humans--a mini-review, AAPS J,
doi:10.1208/s12248-007-9000-9Gorial, Mashhadani, Sayaly, Dakhil, Almashhadani et al., Effectiveness of Ivermectin as add-on Therapy in COVID-19 Management (Pilot Trial), medRxiv,
doi:10.1101/2020.07.07.20145979Karale, Bansal, Makadia, Tayyeb, Khan et al., An Updated Systematic Review and Meta-Analysis of Mortality, Need for ICU admission, Use of Mechanical Ventilation, Adverse effects and other Clinical Outcomes of Ivermectin Treatment in COVID-19 Patients, medRxiv,
doi:10.1101/2021.04.30.21256415Kaur, Shekhar, Sharma, Sarma, Prakash et al., Ivermectin as a potential drug for treatment of COVID-19: an in-sync review with clinical and computational attributes, Pharmacol Rep,
doi:10.1007/s43440-020-00195-yKhan, Khan, Debnath, Nath, Mahtab et al., Ivermectin Treatment May Improve the Prognosis of Patients With COVID-19, Arch Bronconeumol (Engl Ed),
doi:10.1016/j.arbres.2020.08.007Kim, An, Kim, Hwang, Comparative efficacy and safety of pharmacological interventions for the treatment of COVID-19: A systematic review and network meta-analysis, PLoS Med,
doi:10.1371/journal.pmed.1003501Lopez-Medina, Lopez, Hurtado, Davalos, Ramirez et al., Effect of Ivermectin on Time to Resolution of Symptoms Among Adults With Mild COVID-19: A Randomized Clinical Trial, JAMA,
doi:10.1001/jama.2021.3071Rahmanzade, Rahmanzadeh, Hashemian, Tabarsi, Iran's Approach to COVID-19: Evolving Treatment Protocols and Ongoing Clinical Trials, Front Public Health,
doi:10.3389/fpubh.2020.551889Robin, Alam, Saber, Bhiuyan, Murshed et al., A Case Series of 100 COVID-19 Positive Patients Treated with Combination of Ivermectin and Doxycycline, Journal of Bangladesh College of Physicians and Surgeons,
doi:10.3329/jbcps.v38i0.47512Saeed, Gaba, Shah, Helali, Raidullah et al., Correlation between Chest CT Severity Scores and the Clinical Parameters of Adult Patients with COVID-19 Pneumonia, Radiol Res Pract,
doi:10.1155/2021/6697677Soto-Becerra, Culquichicón, Araujo-Castillo, Hurtado-Roca, Real-World Effectiveness of Hydroxychloroquine, Azithromycin, and Ivermectin Among Hospitalized COVID-19 Patients: Results of a Target Trial Emulation Using Observational Data from a Nationwide Healthcare System in Peru
Wagstaff, Rawlinson, Hearps, Jans, An AlphaScreen(R)based assay for high-throughput screening for specific inhibitors of nuclear import, J Biomol Screen,
doi:10.1177/1087057110390360DOI record:
{
"DOI": "10.5812/jjhs-146703",
"ISSN": [
"2345-4075"
],
"URL": "http://dx.doi.org/10.5812/jjhs-146703",
"abstract": "<jats:p>Background: Addressing the Coronavirus disease 2019 (COVID-19) pandemic remains a significant challenge for healthcare systems globally. Despite the absence of a proven cure, ivermectin has been proposed as a potentially effective agent against it. Objectives: This study aimed to evaluate the therapeutic effects of ivermectin compared to a placebo group in non-critically ill confirmed COVID-19 patients. Methods: A double-blind, randomized clinical trial was conducted on 110 patients with moderate-to-severe (non-critical) confirmed COVID-19 infection. The patients were equally divided into two groups, with one group receiving ivermectin tablets (14 mg every 12 hours for three days) and the other group receiving a placebo. The efficacy and safety of ivermectin were assessed in both groups. Results: A total of 110 patients, including 62 (56.4%) men and 48 (43.6%) women, with an average age of 53.36 ± 15.10 years, were enrolled in our double-blind, randomized clinical trial. The baseline characteristics of the two groups were similar. The findings demonstrated that ivermectin significantly reduced the need for Intensive Care Unit admission (32.7% vs. 5.5%; P < 0.001), hospitalization duration (six vs. four days; P < 0.001), and median time to symptom resolution period (P < 0.05) in COVID-19 patients compared to the placebo group, without any serious side effects (P > 0.05). Conclusions: Ivermectin appears to be a potentially effective and safe medication for COVID-19 patients with moderate disease.</jats:p>",
"alternative-id": [
"05108263c44b0054f749ca47511bd8a9463c24e2"
],
"author": [
{
"ORCID": "http://orcid.org/0000-0002-5863-487X",
"affiliation": [],
"authenticated-orcid": false,
"family": "Varnaseri",
"given": "Mehran",
"sequence": "first"
},
{
"affiliation": [],
"family": "Amini",
"given": "Fatemeh",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Jamshididan",
"given": "Ramin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Dargahi",
"given": "Mehrdad",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-7503-0894",
"affiliation": [],
"authenticated-orcid": false,
"family": "Gheibi",
"given": "Nematollah",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-7117-6079",
"affiliation": [],
"authenticated-orcid": false,
"family": "Abolghasemi",
"given": "Sara",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Dayer",
"given": "Mohammadreza",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Varnasseri",
"given": "Negar",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hoseinynejad",
"given": "Khojasteh",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kheradhoosh",
"given": "Sahar",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Nazari",
"given": "Pedram",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Babadi",
"given": "Ebrahim",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mousavinezhad",
"given": "Seyedeh Maryam",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0009-0005-3694-6863",
"affiliation": [],
"authenticated-orcid": false,
"family": "Ebrahimi",
"given": "Pouya",
"sequence": "additional"
}
],
"container-title": "Jundishapur Journal of Health Sciences",
"container-title-short": "Jundishapur J Health Sci",
"content-domain": {
"crossmark-restriction": false,
"domain": [
"brieflands.com"
]
},
"created": {
"date-parts": [
[
2024,
5,
1
]
],
"date-time": "2024-05-01T15:02:00Z",
"timestamp": 1714575720000
},
"deposited": {
"date-parts": [
[
2024,
5,
1
]
],
"date-time": "2024-05-01T15:02:06Z",
"timestamp": 1714575726000
},
"indexed": {
"date-parts": [
[
2024,
5,
2
]
],
"date-time": "2024-05-02T00:34:25Z",
"timestamp": 1714610065611
},
"is-referenced-by-count": 0,
"issue": "2",
"issued": {
"date-parts": [
[
2024,
4,
30
]
]
},
"journal-issue": {
"issue": "2",
"published-online": {
"date-parts": [
[
2024,
4,
30
]
]
}
},
"license": [
{
"URL": "https://creativecommons.org/licenses/by-nc/4.0/",
"content-version": "am",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
4,
9
]
],
"date-time": "2024-04-09T00:00:00Z",
"timestamp": 1712620800000
}
}
],
"link": [
{
"URL": "https://brieflands.com/articles/jjhs-146703",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://brieflands.com/articles/jjhs-146703",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "3819",
"original-title": [],
"prefix": "10.5812",
"published": {
"date-parts": [
[
2024,
4,
30
]
]
},
"published-online": {
"date-parts": [
[
2024,
4,
30
]
]
},
"publisher": "Briefland",
"reference": [
{
"DOI": "10.34172/aim.2022.83",
"doi-asserted-by": "publisher",
"key": "key-A146703REF1-1"
},
{
"author": " ",
"journal-title": "Number of COVID-19 cases reported to WHO.",
"key": "key-A146703REF2-2",
"year": "2024"
},
{
"DOI": "10.1007/s10654-020-00671-y",
"doi-asserted-by": "publisher",
"key": "key-A146703REF3-3"
},
{
"DOI": "10.1016/j.antiviral.2020.104787",
"doi-asserted-by": "publisher",
"key": "key-A146703REF4-4"
},
{
"DOI": "10.1056/NEJMe2402224",
"doi-asserted-by": "publisher",
"key": "key-A146703REF5-5"
},
{
"DOI": "10.1208/s12248-007-9000-9",
"doi-asserted-by": "publisher",
"key": "key-A146703REF6-6"
},
{
"DOI": "10.1177/1087057110390360",
"doi-asserted-by": "publisher",
"key": "key-A146703REF7-7"
},
{
"DOI": "10.1371/journal.pmed.1003501",
"doi-asserted-by": "publisher",
"key": "key-A146703REF8-8"
},
{
"DOI": "10.1007/s40264-021-01066-y",
"doi-asserted-by": "publisher",
"key": "key-A146703REF9-9"
},
{
"author": " ",
"journal-title": "Coronavirus Disease 2019 (COVID-19) Treatment Guidelines.",
"key": "key-A146703REF10-10",
"year": "2021"
},
{
"DOI": "10.3389/fpubh.2020.551889",
"doi-asserted-by": "publisher",
"key": "key-A146703REF11-11"
},
{
"DOI": "10.1002/jmv.27122",
"doi-asserted-by": "publisher",
"key": "key-A146703REF12-12"
},
{
"DOI": "10.1155/2021/6697677",
"doi-asserted-by": "publisher",
"key": "key-A146703REF13-13"
},
{
"DOI": "10.1371/journal.pone.0257040",
"doi-asserted-by": "publisher",
"key": "key-A146703REF14-14"
},
{
"DOI": "10.1016/j.ygeno.2020.07.001",
"doi-asserted-by": "publisher",
"key": "key-A146703REF15-15"
},
{
"DOI": "10.3329/jbcps.v38i0.47512",
"doi-asserted-by": "publisher",
"key": "key-A146703REF16-16"
},
{
"DOI": "10.1016/j.eclinm.2020.100720",
"doi-asserted-by": "publisher",
"key": "key-A146703REF17-17"
},
{
"DOI": "10.21203/rs.3.rs-495945/v1",
"doi-asserted-by": "publisher",
"key": "key-A146703REF18-18"
},
{
"DOI": "10.1001/jama.2021.3071",
"doi-asserted-by": "publisher",
"key": "key-A146703REF19-19"
},
{
"DOI": "10.1101/2020.07.07.20145979",
"doi-asserted-by": "publisher",
"key": "key-A146703REF20-20"
},
{
"author": "Soto-Becerra P",
"first-page": "30",
"journal-title": "SSRN.",
"key": "key-A146703REF21-21",
"volume": "Preprint",
"year": "2020"
},
{
"DOI": "10.1016/j.arbres.2020.08.007",
"doi-asserted-by": "publisher",
"key": "key-A146703REF22-22"
},
{
"DOI": "10.1016/j.ijid.2020.11.191",
"doi-asserted-by": "publisher",
"key": "key-A146703REF23-23"
},
{
"DOI": "10.1101/2021.04.30.21256415",
"doi-asserted-by": "publisher",
"key": "key-A146703REF24-24"
},
{
"DOI": "10.1007/s43440-020-00195-y",
"doi-asserted-by": "publisher",
"key": "key-A146703REF25-25"
},
{
"DOI": "10.3390/pathogens12020167",
"doi-asserted-by": "publisher",
"key": "key-A146703REF26-26"
}
],
"reference-count": 26,
"references-count": 26,
"relation": {},
"resource": {
"primary": {
"URL": "https://brieflands.com/articles/jjhs-146703"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Ivermectin as a Potential Addition to the Limited Anti-COVID-19 Arsenal: A Double-Blinded Clinical Trial",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.5812/crossmark_update_policy",
"volume": "16"
}