An Updated Systematic Review and Meta-Analysis of Mortality, Need for ICU admission, Use of Mechanical Ventilation, Adverse effects and other Clinical Outcomes of Ivermectin Treatment in COVID-19 Patients
MBBS Smruti Karale, MBBS, MPH Vikas Bansal, MBBS Janaki Makadia, Muhammad Tayyeb, Hira Khan, Shree Spandana Ghanta, MBBS Romil Singh, MD Aysun Tekin, Abhishek Bhurwal, MD Hemant Mutneja, MBB Ishita Mehra, MBBS, MBA Rahul Kashyap
doi:10.1101/2021.04.30.21256415
Highlights What We Already Know about This Topic
1. COVID-19 is an ongoing global pandemic, for which Ivermectin has been tried on a therapeutic and prophylactic basis.
2. Results from several clinical trials and observational studies suggest that Ivermectin may improve survival and clinical outcomes with a good safety profile when compared with other treatments; however, the current evidence is limited. .
What This Article Tells Us That Is New 1. This systematic review and meta-analysis provide a summary of the latest literature on the efficacy and safety of Ivermectin use for COVID-19.
2. Based on our analysis of the latest evidence, we found that Ivermectin's benefit in reducing mortality cannot be concluded with confidence . However, as an adjuvant
References
Abate, Ali, Mantfardo, Basu, Rate of Intensive Care Unit admission and outcomes among patients with coronavirus: A systematic review and Meta-analysis, PLoS One
Abd-Elsalam, Noor, Badawi, Clinical study evaluating the efficacy of ivermectin in COVID-19 treatment: A randomized controlled study, J Med Virol
Afsar, Ghauri, Abbas, Mukarram, Peracha et al., Ivermectin Use Associated with Reduced Duration of COVID-19 Febrile Illness in a Community Setting
Ahmed, Karim, Ross, A five-day course of ivermectin for the treatment of COVID-19 may reduce the duration of illness, Int J Infect Dis
Ahsan, Rani, Siddiqui, Clinical Variants, Characteristics, and Outcomes Among COVID-19 Patients: A Case Series Analysis at a Tertiary Care Hospital in Karachi, Pakistan, Cureus
Aref, Bazeed, Hassan, Clinical, Biochemical and Molecular Evaluations of Ivermectin Mucoadhesive Nanosuspension Nasal Spray in Reducing Upper Respiratory Symptoms of Mild COVID-19, Int J Nanomedicine
Babalola, Bode, Ajayi, Ivermectin shows clinical benefits in mild to moderate COVID19: A randomised controlled double-blind, dose-response study in Lagos, QJM
Balk, Bonis, Moskowitz, Correlation of quality measures with estimates of treatment effect in meta-analyses of randomized controlled trials, Jama
Bansal, Mahapure, Bhurwal, Mortality Benefit of Remdesivir in COVID-19: A Systematic Review and Meta-Analysis, Front Med
Bansal, Mahapure, Mehra, Mortality Benefit of Convalescent Plasma in COVID-19: A Systematic Review and Meta-Analysis, Front Med
Bansal, Singh, Bhurwal, Rathore, Kashyap, Obesity Is a Risk Factor for Increased COVID-19 Severity: A Systemic Review and Meta-Regression, Critical Care Medicine
Bauer, Kapoor, Rath, Thomas, What is the role of supplementation with ascorbic acid, zinc, vitamin D, or N-acetylcysteine for prevention or treatment of COVID-19?, Cleve Clin J Med
Bayram, Ozsaygili, Sav, Susceptibility of severe COVID-19 patients to rhinoorbital mucormycosis fungal infection in different clinical manifestations, Jpn J Ophthalmol
Beigel, Tomashek, Dodd, Remdesivir for the Treatment of Covid-19 -Final Report, N Engl J Med
Bhalala, Gist, Tripathi, Pediatric COVID-19: A Report From Viral Infection and Respiratory Illness Universal Study (VIRUS), Critical Care Medicine
Bhattacharya, Ray, Mukherjee, Chowdhury, Kulasreshtha et al., Observational Study on Clinical Features, Treatment and Outcome of Covid 19 in a Tertiary Care Centre in India -a Retrospective Case Series, International Journal of Scientific Research
Biber, Mandelboim, Harmelin, randomized placebo-controlled trial
Borenstein, Hedges, Higgins, Rothstein, Comprehensive Meta-Analysis Version 3
Boscolo-Rizzo, Guida, Polesel, Sequelae in adults at 12 months after mild-tomoderate coronavirus disease 2019 (COVID-19), Int Forum Allergy Rhinol
Bryant, Lawrie, Dowswell, Ivermectin for Prevention and Treatment of COVID-19 Infection: A Systematic Review, Meta-analysis, and Trial Sequential Analysis to Inform Clinical Guidelines, Am J Ther
Budhiraja, Soni, Jha, Clinical Profile of First
Cadegiani, Goren, Wambier, Mccoy, Early COVID-19 Therapy with Azithromycin Plus Nitazoxanide, Ivermectin or Hydroxychloroquine in Outpatient Settings Significantly Reduced Symptoms Compared to Known Outcomes in Untreated Patients
Caly, Druce, Catton, Jans, Wagstaff, The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro, Antiviral Res
Camprubi, Almuedo-Riera, Marti-Soler, Lack of efficacy of standard doses of ivermectin in severe COVID-19 patients, PLoS One
Carvallo, Ivermectin, Aspirin, Dexamethasone and Enoxaparin as Treatment of Covid 19 -Study Results -ClinicalTrials
Castañeda-Sabogal, Chambergo-Michilot, Toro-Huamanchumo, Silva-Rengifo, Gonzales-Zamora et al., Outcomes of Ivermectin in the treatment of COVID-19: a systematic review and meta-analysis
Cdc, Interim clinical guidance for management of patients with confirmed coronavirus disease (COVID-19)
Chaccour, Casellas, Blanco-Di Matteo, The effect of early treatment with ivermectin on viral load, symptoms and humoral response in patients with non-severe COVID-19: A pilot, double-blind, placebo-controlled, randomized clinical trial, EClinicalMedicine
Chahla, Ruiz, Mena, Cluster Randomised Trials-Ivermectin Repurposing For COVID-19 Treatment Of Outpatients With Mild Disease In Primary Health Care Centers
Chamie-Quintero, Hibberd, Scheim, Ivermectin for COVID-19 in Peru: 14-fold reduction in nationwide excess deaths
Chamie-Quintero, Hibberd, Scheim, Sharp reductions in COVID-19 case fatalities and excess deaths in Peru in close time conjunction, state-by-state, with ivermectin treatments, State-By-State, with Ivermectin Treatments
Chowdhury, Shahbaz, Karim, Islam, Dan et al., A Comparative Study on Ivermectin-Doxycycline and Hydroxychloroquine-Azithromycin Therapy on COVID-19 Patients, Eurasian Journal of Medicine and Oncology
Crump, Ivermectin: enigmatic multifaceted 'wonder' drug continues to surprise and exceed expectations, J Antibiot
Cucinotta, Vanelli, WHO Declares COVID-19 a Pandemic, Acta Biomed
Datta, Talwar, Lee, A Proposed Framework and Timeline of the Spectrum of Disease Due to SARS-CoV-2 Infection: Illness Beyond Acute Infection and Public Health Implications, Jama
Derbyshire, Delange, COVID-19: is there a role for immunonutrition, particularly in the over 65s?, BMJ Nutr Prev Health
Dinicolantonio, Barroso, Mccarty, Ivermectin may be a clinically useful antiinflammatory agent for late-stage COVID-19, Open Heart
Domecq, Lal, Sheldrick, Receiving Organ Support Therapies: The International Viral Infection and Respiratory Illness Universal Study Registry, Critical Care Medicine
Dong, Du, Gardner, An interactive web-based dashboard to track COVID-19 in real time, Lancet Infect Dis
Elalfy, Besheer, El-Mesery, Effect of a combination of nitazoxanide, ribavirin, and ivermectin plus zinc supplement (MANS.NRIZ study) on the clearance of mild COVID-19, J Med Virol
Elgazzar, Eltaweel, Youssef, Hany, Hafez et al., Efficacy and Safety of Ivermectin for Treatment and prophylaxis of COVID-19 Pandemic
Elsayed, Hassaballa, Ahmed, Gumaa, Sharkawy, for patients with severe COVID-19: a systematic review and meta-analysis
Espitia-Hernandez, Munguia, Diaz-Chiguer, López-Elizalde, Jimenez-Ponce, Effects of Ivermectin-azithromycin-cholecalciferol combined therapy on COVID-19 infected patients: A proof of concept study, Biomedical Research
Fda, FDA experts discuss COVID-19 therapeutic clinical trials | American Medical Association
Fda, Why You Should Not Use Ivermectin to Treat or Prevent COVID-19
Festic, Kor, Gajic, Prevention of acute respiratory distress syndrome, Curr Opin Crit Care
Gajic, Dabbagh, Park, Early identification of patients at risk of acute lung injury: evaluation of lung injury prediction score in a multicenter cohort study, Am J Respir Crit Care Med
Galan, Santos, Asato, Phase 2 randomized study on chloroquine, hydroxychloroquine or ivermectin in hospitalized patients with severe manifestations of SARS-CoV-2 infection, Pathog Glob Health
Garibaldi, Wang, Robinson, Comparison of Time to Clinical Improvement With vs Without Remdesivir Treatment in Hospitalized Patients With COVID-19, JAMA Netw Open
Gilzad-Kohan, Jamali, Anti-Inflammatory Properties of Drugs Used to Control COVID-19 and their Effects on the Renin-Angiotensin System and Angiotensin-Converting Enzyme-2, J Pharm Pharm Sci
Gonzalez, Gámez, Enciso, Efficacy and safety of Ivermectin and Hydroxychloroquine in patients with severe COVID-19. A randomized controlled trial
Gorial, Mashhadani, Sayaly, Effectiveness of Ivermectin as add-on Therapy in COVID-19 Management
Group, Convalescent plasma in patients admitted to hospital with COVID-19 (RECOVERY): a randomised controlled, open-label, platform trial, Lancet
Gunster, Busse, Spoden, 6-month mortality and readmissions of hospitalized COVID-19 patients: A nationwide cohort study of 8,679 patients in Germany, PLoS One
Guzmán, Castillo-Gonzalez, Gonzalez, Factors associated with increased mortality in critically ill COVID-19 patients in a Mexican public hospital: the other faces of health system oversaturation
Hariyanto, Halim, Gunawan, Kurniawan, Ivermectin and outcomes from Covid-19 pneumonia: A systematic review and meta-analysis of randomized clinical trial studies, Reviews in Medical Virology
Hashim, Maulood, Rasheed, Fatak, Kabah et al., Controlled randomized clinical trial on using Ivermectin with Doxycycline for treating COVID-19 patients in Baghdad, Iraq. medRxiv
Hazan, Gunaratne, Effectiveness of Ivermectin-Based Multidrug Therapy in Severe Hypoxic Ambulatory COVID-19 Patients
Higgins, Altman, Gotzsche, The Cochrane Collaboration's tool for assessing risk of bias in randomised trials, BMJ
Higgins, Thomas, Chandler, Cochrane handbook for systematic reviews of interventions
Hill, Abdulamir, Ahmed, Meta-analysis of randomized trials of ivermectin to treat SARS-CoV-2 infection
Hill, Garratt, Levi, Erratum: Expression of Concern: "Meta-analysis of Randomized Trials of Ivermectin to Treat SARS-CoV-2 Infection, Open Forum Infect Dis
Hussain, Shuayb, Rahman, Outcome of ivermectin and doxycycline in cancer patients with COVID-19: A positive experience in Bangladesh, International Journal of Molecular & Immuno Oncology
Janiaud, Axfors, Schmitt, Association of Convalescent Plasma Treatment With Clinical Outcomes in Patients With COVID-19: A Systematic Review and Metaanalysis, Jama
Jaspersen, Drug-induced oesophageal disorders: pathogenesis, incidence, prevention and management, Drug Saf
Khan Chachar, Khan, Asif, Tanveer, Khaqan et al., Effectiveness of Ivermectin in SARS-CoV-2/COVID-19 Patients, International Journal of Sciences
Khan, Khan, Debnath, Ivermectin Treatment May Improve the Prognosis of Patients With COVID-19, Arch Bronconeumol (Engl Ed)
Khan, Sabzposh, Deshpande, Kashyap, Pregnancy during COVID-19 pandemic -Maternal and neonatal outcomes: A concise review, International Journal of Academic Medicine
Kim, An, Kim, Hwang, Comparative efficacy and safety of pharmacological interventions for the treatment of COVID-19: A systematic review and network meta-analysis, PLoS Med
Kishoria, Mathur, Parmar, IVERMECTIN AS ADJUVANT TO HYDROXYCHOLOROQUINE IN PATIENTS RESISTANT TO STANDARD TREATMENT FOR SARS-CoV-2: RESULTS OF AN OPEN-LABEL RANDOMIZED CLINICAL STUDY, Paripex Indian Journal of Research
Kory, Meduri, Varon, Iglesias, Marik, Review of the Emerging Evidence Demonstrating the Efficacy of Ivermectin in the Prophylaxis and Treatment of COVID-19, American Journal of Therapeutics
Kow, Merchant, Mustafa, Hasan, The association between the use of ivermectin and mortality in patients with COVID-19: a meta-analysis, Pharmacol Rep
Krolewiecki, Lifschitz, Moragas, Antiviral effect of high-dose ivermectin in adults with COVID-19: A proof-of-concept randomized trial, EClinicalMedicine
Lat, Coopersmith, Backer, The Surviving Sepsis Campaign: Fluid Resuscitation and Vasopressor Therapy Research Priorities in Adult Patients, Crit Care Med
Lawrie, Ivermectin reduces the risk of death from COVID-19 -a rapid review and metaanalysis in support of the recommendation of the Front Line COVID-19 Critical Care Alliance
Lima-Morales, Mendez-Hernandez, Flores, Effectiveness of a multidrug therapy consisting of Ivermectin, Azithromycin, Montelukast, and Acetylsalicylic acid to prevent hospitalization and death among ambulatory COVID-19 cases in Tlaxcala, Mexico, Int J Infect Dis
Lopez-Medina, Lopez, Hurtado, Effect of Ivermectin on Time to Resolution of Symptoms Among Adults With Mild COVID-19: A Randomized Clinical Trial, Jama
Lundberg, Pinkham, Baer, Nuclear import and export inhibitors alter capsid protein distribution in mammalian cells and reduce Venezuelan Equine Encephalitis Virus replication, Antiviral Res
Mahmud, Nagraj, Karia, Efficacy and Safety of Tocilizumab in Hospitalized COVID-19 Patients: A Systematic Review, Critical Care Medicine
Mahmud, Rahman, Alam, Ivermectin in combination with doxycycline for treating COVID-19 symptoms: a randomized trial, J Int Med Res
Md, Islam, Current Drugs with Potential for Treatment of COVID-19: A Literature Review, J Pharm Pharm Sci
Mega, Latin America's embrace of an unproven COVID treatment is hindering drug trials, Nature
Menon, Gandhi, Tariq, Impact of Chronic Kidney Disease on Severity and Mortality in COVID-19 Patients: A Systematic Review and Meta-analysis, Cureus
Menon, Sharma, Earthineni, Association of Gastrointestinal System With Severity and Mortality of COVID-19: A Systematic Review and Meta-Analysis, Cureus
Menon, Sharma, Kataria, The Association of Acute Kidney Injury With Disease Severity and Mortality in COVID-19: A Systematic Review and Meta-Analysis, Cureus
Mohan, Tiwari, Suri, ): a randomized, placebo-controlled trial
Morgenstern, Redondo, De León, The use of compassionate Ivermectin in the management of symptomatic outpatients and hospitalized patients with clinical diagnosis of COVID-19 at the Medical Center Bournigal and the Medical Center Punta Cana, Rescue Group, Dominican Republic, from may 1 to august 10, 2020, J Clin Trials
Mourya, Thakur, Hada, Kulshreshtha, Sharma, Comparative Analytical Study of Two Different Drug Regimens in Treatment of Covid 19 Positive Patients in Index Medical College Hospital and Research Center, Indore, India, International Journal of Health and Clinical Research
Nalbandian, Sehgal, Gupta, Post-acute COVID-19 syndrome, Nat Med
Nardelli, Zangrillo, Sanchini, Crying wolf in time of Corona: the strange case of ivermectin and hydroxychloroquine. Is the fear of failure withholding potential lifesaving treatment from clinical use?, Signa Vitae
Nayar, Khanna, Anand, Ivermectin in Covid-19: Review of the Current Evidence, The Indian Practitioner
Niaee, Gheibi, Namdar, Ivermectin as an adjunct treatment for hospitalized adult COVID-19 patients: A randomized multi-center clinical trial
Núñez, Yuca, Cervantes, Murcia, Juárez et al., Therapeutic Efficacy of Ivermectin as an Adjuvant in the Treatment of Patients with COVID-19 Study conducted at the Social Security Institute for Workers of the State of Chiapas, ISSTECH, Mexico, International Journal of Innovative Science and Research Technology
Okumus, Demirturk, Cetinkaya, Evaluation of the effectiveness and safety of adding ivermectin to treatment in severe COVID-19 patients, BMC Infect Dis
Padhy, Mohanty, Das, Meher, Therapeutic potential of ivermectin as add on treatment in COVID 19: A systematic review and meta-analysis, J Pharm Pharm Sci
Pierre, Ivermectin and COVID-19 in Care Home: Case Report, Journal of Infectious Diseases and Epidemiology
Podder, Chowdhury, Sina, Haque, Outcome of ivermectin treated mild to moderate COVID-19 cases: a single-centre, open-label, randomised controlled study, IMC Journal of Medical Science
Popp, Stegemann, Metzendorf, Ivermectin for preventing and treating COVID-19, Cochrane Database Syst Rev
Pott-Junior, Paoliello, Miguel, Use of ivermectin in the treatment of Covid-19: A pilot trial, Toxicol Rep
Rahman, Iqbal, Islam, Niaz, Hussain et al., Comparison of viral clearance between ivermectin with doxycycline and hydroxychloroquine with azithromycin in COVID-19 patients, Journal of Bangladesh College of Physicians and Surgeons
Rajter, Sherman, Fatteh, Vogel, Sacks et al., Use of Ivermectin Is Associated With Lower Mortality in Hospitalized Patients With Coronavirus Disease 2019: The Ivermectin in COVID Nineteen Study, Chest
Rathore, Rojas, Sondhi, Myocarditis associated with Covid-19 disease: a systematic review of published Case reports and Case series, Preprints
Ravikirti, Pattadar, Ivermectin as a potential treatment for mild to moderate COVID-19 -A double blind randomized placebo-controlled trial
Ravindra, Chitra, Madhur, Retrospective Assessment of Treatments of Hospitalized Covid-19 Patients
Razonable, Pennington, Meehan, A Collaborative Multidisciplinary Approach to the Management of Coronavirus Disease 2019 in the Hospital Setting, Mayo Clinic Proceedings
Release, Kovid-19 -Huvemek® Phase 2 clinical trial
Richardson, Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area
Robin, Alam, Saber, Bhiuyan, Murshed et al., A Case Series of 100 COVID-19 Positive Patients Treated with Combination of Ivermectin and Doxycycline, Journal of Bangladesh College of Physicians and Surgeons
Roman, Burela, Pasupuleti, Piscoya, Vidal et al., Ivermectin for the treatment of COVID-19: A systematic review and meta-analysis of randomized controlled trials
Samaha, Mouawia, Fawaz, Effects of a Single Dose of Ivermectin on Viral and Clinical Outcomes in Asymptomatic SARS-CoV-2 Infected Subjects: A Pilot Clinical Trial in Lebanon, Viruses
Schünemann, Brożek, Guyatt, Oxman, Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach
Shah Bukhari, Asghar, Perveen, Efficacy of Ivermectin in COVID-19 Patients with Mild to Moderate Disease
Shah, Mann, Singh, Bangar, Kulkarni, Impact of COVID-19 on the Mental Health of Children and Adolescents, Cureus
Shahbaznejad, Davoudi, Eslami, Effects of Ivermectin in Patients With COVID-19: A Multicenter, Double-Blind, Randomized, Controlled Clinical Trial, Clin Ther
Sharun, Dhama, Patel, Ivermectin, a new candidate therapeutic against SARS-CoV-2/COVID-19, Ann Clin Microbiol Antimicrob
Sheraton, Deo, Dutt, Surani, Hall-Flavin et al., Psychological effects of the COVID 19 pandemic on healthcare workers globally: A systematic review, Psychiatry Res
Sheraton, Deo, Kashyap, Surani, A Review of Neurological Complications of COVID-19, Cureus
Singh, Kashyap, Hutton, Sharma, Surani, A Review of Cardiac Complications in Coronavirus Disease, Cureus
Singh, Rathore, Khan, Mortality and Severity in COVID-19 Patients on ACEIs & ARBs-A Meta-Regression Analysis
Singh, Shiza, Saadat, Dawe, Rehman, Association of Guillain-Barre Syndrome With COVID-19: A Case Report and Literature Review, Cureus
Soto-Becerra, Culquichicón, Hurtado-Roca, Araujo-Castillo, Real-world effectiveness of hydroxychloroquine, azithromycin, and ivermectin among hospitalized COVID-19 patients: results of a target trial emulation using observational data from a nationwide healthcare system in Peru
Spoorthi, Sasank, Utility of ivermectin and doxycycline combination for the treatment of SARS-CoV2, IAIM
Tay, Fraser, Chan, Nuclear localization of dengue virus (DENV) 1-4 non-structural protein 5; protection against all 4 DENV serotypes by the inhibitor Ivermectin, Antiviral Res
Tripathi, Gist, Chiotos, Risk Factors for Severe COVID-19 Illness in Children: Analysis of the VIRUS: COVID-19 Registry, Critical Care Medicine
Vallejos, Zoni, Bangher, Ivermectin to prevent hospitalizations in patients with COVID-19 (IVERCOR-COVID19) a randomized, double-blind, placebo-controlled trial, BMC Infect Dis
Wagstaff, Sivakumaran, Heaton, Harrich, Jans, Ivermectin is a specific inhibitor of importin alpha/beta-mediated nuclear import able to inhibit replication of HIV-1 and dengue virus, Biochem J
Walkey, Kumar, Harhay, The Viral Infection and Respiratory Illness Universal Study (VIRUS): An International Registry of Coronavirus 2019-Related Critical Illness, Crit Care Explor
Walkey, Sheldrick, Kashyap, Guiding Principles for the Conduct of Observational Critical Care Research for Coronavirus Disease 2019 Pandemics and Beyond: The Society of Critical Care Medicine Discovery Viral Infection and Respiratory Illness Universal Study Registry, Crit Care Med
Whoreafc, Sterne, Murthy, Association Between Administration of Systemic Corticosteroids and Mortality Among Critically Ill Patients With COVID-19: A Meta-analysis, Jama
Wu, Mcgoogan, Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72314 Cases From the Chinese Center for Disease Control and Prevention, Jama
Yagisawa, Foster, Hanaki, Ōmura, Global trends in clinical studies of ivermectin in COVID-19, THE JAPANESE JOURNAL OF ANTIBIOTICS
Youkee, Hulme, Roberts, Daniels, Nutbeam et al., Time Matters: Antibiotic Timing in Sepsis and Septic Shock, Crit Care Med
Yousaf, Sd, Al-Soub, Mohamed, COVID-19-associated SIADH: a clue in the times of pandemic!, Am J Physiol Endocrinol Metab
Zhang, Song, Ci, Ivermectin inhibits LPS-induced production of inflammatory cytokines and improves LPS-induced survival in mice, Inflamm Res
DOI record:
{
"DOI": "10.1101/2021.04.30.21256415",
"URL": "http://dx.doi.org/10.1101/2021.04.30.21256415",
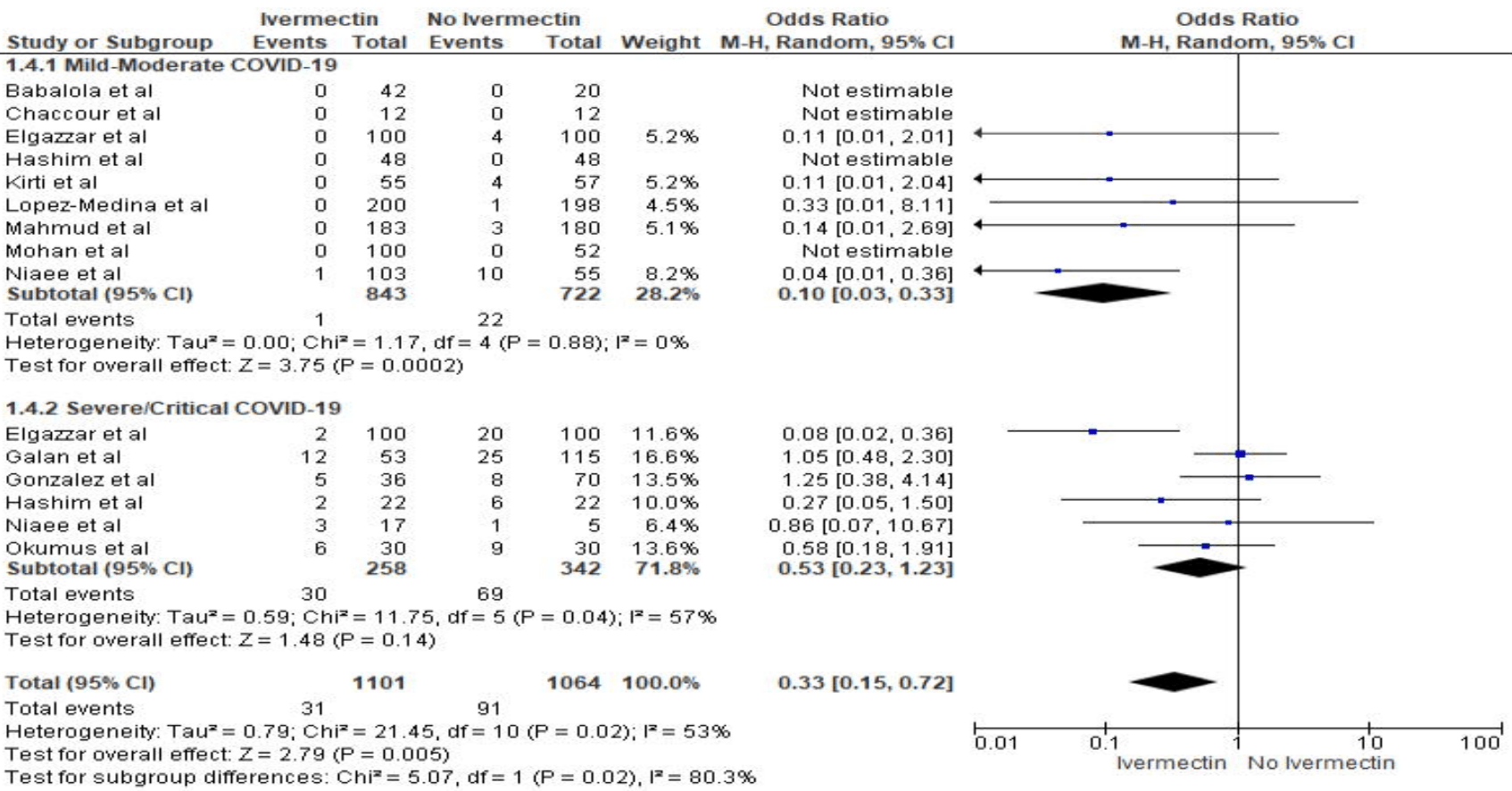
"abstract": "<jats:title>Abstract</jats:title><jats:sec><jats:title>Importance</jats:title><jats:p>Repurposing Ivermectin, a known anti-parasitic agent, for treating COVID-19 has demonstrated positive results in several studies. We aim to evaluate the benefit and risk of Ivermectin in COVID-19.</jats:p></jats:sec><jats:sec><jats:title>Methods</jats:title><jats:p>We conducted a systematic search for full-text manuscripts published from February 1, 2020, to August 15th, 2021 focusing on Ivermectin therapy against COVID-19. The primary outcomes were mortality, need for intensive care unit (ICU) admission; secondary outcomes were - adverse effects, need for mechanical ventilation, viral clearance, time to viral clearance, need for hospitalization, and length of hospital stay. Random-effects models were used for all analyses.</jats:p></jats:sec><jats:sec><jats:title>Results</jats:title><jats:p>We included a total of 52 studies (n=17561) in the qualitative analysis, out of these, 44 studies (n=14019) were included in the meta-analysis. In the mortality meta-analysis (N=29), odds of death were lower in the Ivermectin-arm compared to control (OR 0.54, p=0.009). Although lower odds of mortality were observed in various subgroup analyses of RCTs, they did not reach statistical significance: therapeutic RCTs: mild-moderate COVID-19 (OR 0.31, p=0.06), therapeutic RCTs: severe/critical COVID-19 (OR 0.86, p=0.56), inpatient RCTs: mild-moderate COVID-19 (OR 0.18, p=0.08), inpatient RCTs: severe/critical COVID-19 (OR 0.86, p=0.56). Ivermectin, mostly as adjuvant therapy, was associated with higher odds of viral clearance (N=22) (OR 3.52, p=0.0002), shorter duration to achieve viral clearance (N=8) (MD - 4.12, p=0.02), reduced need for hospitalization (N=6) (OR 0.34, p=008).</jats:p></jats:sec><jats:sec><jats:title>Conclusion</jats:title><jats:p>Our meta-analysis suggests that the mortality benefit of Ivermectin in COVID-19 is uncertain. But as adjuvant therapy, Ivermectin may improve viral clearance and reduce the need for hospitalization.</jats:p></jats:sec><jats:sec><jats:title>Highlights</jats:title><jats:sec><jats:title>What We Already Know about This Topic</jats:title><jats:list list-type=\"order\"><jats:list-item><jats:p>COVID-19 is an ongoing global pandemic, for which Ivermectin has been tried on a therapeutic and prophylactic basis.</jats:p></jats:list-item><jats:list-item><jats:p>Results from several clinical trials and observational studies suggest that Ivermectin may improve survival and clinical outcomes with a good safety profile when compared with other treatments; however, the current evidence is limited..</jats:p></jats:list-item></jats:list></jats:sec><jats:sec><jats:title>What This Article Tells Us That Is New</jats:title><jats:list list-type=\"order\"><jats:list-item><jats:p>This systematic review and meta-analysis provide a summary of the latest literature on the efficacy and safety of Ivermectin use for COVID-19.</jats:p></jats:list-item><jats:list-item><jats:p>Based on our analysis of the latest evidence, we found that Ivermectin’s benefit in reducing mortality cannot be concluded with confidence. However, as an adjuvant therapy it may help reduce the need for hospitalization, duration for viral clearance while increasing the likelihood of achieving viral clearance.</jats:p></jats:list-item><jats:list-item><jats:p>We need more high-quality data for conclusive evidence regarding the benefit of Ivermectin in reducing the need for ICU admissions, mechanical ventilation and duration of hospital stay in COVID-19 patients.</jats:p></jats:list-item></jats:list></jats:sec></jats:sec>",
"accepted": {
"date-parts": [
[
2021,
9,
17
]
]
},
"author": [
{
"affiliation": [],
"family": "Karale",
"given": "Smruti",
"sequence": "first"
},
{
"ORCID": "http://orcid.org/0000-0001-6047-5559",
"affiliation": [],
"authenticated-orcid": false,
"family": "Bansal",
"given": "Vikas",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Makadia",
"given": "Janaki",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tayyeb",
"given": "Muhammad",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Khan",
"given": "Hira",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ghanta",
"given": "Shree Spandana",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-3777-5670",
"affiliation": [],
"authenticated-orcid": false,
"family": "Singh",
"given": "Romil",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-1891-2118",
"affiliation": [],
"authenticated-orcid": false,
"family": "Tekin",
"given": "Aysun",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bhurwal",
"given": "Abhishek",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mutneja",
"given": "Hemant",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mehra",
"given": "Ishita",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-4383-3411",
"affiliation": [],
"authenticated-orcid": false,
"family": "Kashyap",
"given": "Rahul",
"sequence": "additional"
}
],
"container-title": [],
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2021,
5,
6
]
],
"date-time": "2021-05-06T16:08:53Z",
"timestamp": 1620317333000
},
"deposited": {
"date-parts": [
[
2021,
9,
20
]
],
"date-time": "2021-09-20T08:59:03Z",
"timestamp": 1632128343000
},
"group-title": "Infectious Diseases (except HIV/AIDS)",
"indexed": {
"date-parts": [
[
2024,
5,
1
]
],
"date-time": "2024-05-01T16:17:29Z",
"timestamp": 1714580249537
},
"institution": [
{
"name": "medRxiv"
}
],
"is-referenced-by-count": 10,
"issued": {
"date-parts": [
[
2021,
5,
4
]
]
},
"link": [
{
"URL": "https://syndication.highwire.org/content/doi/10.1101/2021.04.30.21256415",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "246",
"original-title": [],
"posted": {
"date-parts": [
[
2021,
5,
4
]
]
},
"prefix": "10.1101",
"published": {
"date-parts": [
[
2021,
5,
4
]
]
},
"publisher": "Cold Spring Harbor Laboratory",
"reference": [
{
"DOI": "10.23750/abm.v91i1.9397",
"doi-asserted-by": "publisher",
"key": "2021092001550866000_2021.04.30.21256415v2.1"
},
{
"DOI": "10.1016/S1473-3099(20)30120-1",
"doi-asserted-by": "publisher",
"key": "2021092001550866000_2021.04.30.21256415v2.2"
},
{
"key": "2021092001550866000_2021.04.30.21256415v2.3",
"unstructured": "Domecq JP , Lal A , Sheldrick CR , et al. Outcomes of Patients With Coronavirus Disease 2019 Receiving Organ Support Therapies: The International Viral Infection and Respiratory Illness Universal Study Registry. Critical Care Medicine. 9000;Online First."
},
{
"DOI": "10.1097/01.ccm.0000726356.00193.e7",
"doi-asserted-by": "crossref",
"key": "2021092001550866000_2021.04.30.21256415v2.4",
"unstructured": "Bansal V , Singh R , Bhurwal A , Rathore S , Kashyap R. 117: Obesity Is a Risk Factor for Increased COVID-19 Severity: A Systemic Review and Meta-Regression. Critical Care Medicine.49(1):43."
},
{
"article-title": "Impact of Chronic Kidney Disease on Severity and Mortality in COVID-19 Patients: A Systematic Review and Meta-analysis",
"first-page": "e14279",
"issue": "4",
"journal-title": "Cureus",
"key": "2021092001550866000_2021.04.30.21256415v2.5",
"volume": "13",
"year": "2021"
},
{
"article-title": "The Association of Acute Kidney Injury With Disease Severity and Mortality in COVID-19: A Systematic Review and Meta-Analysis",
"first-page": "e13894",
"issue": "3",
"journal-title": "Cureus",
"key": "2021092001550866000_2021.04.30.21256415v2.6",
"volume": "13",
"year": "2021"
},
{
"article-title": "Association of Guillain-Barre Syndrome With COVID-19: A Case Report and Literature Review",
"first-page": "e13828",
"issue": "3",
"journal-title": "Cureus",
"key": "2021092001550866000_2021.04.30.21256415v2.7",
"volume": "13",
"year": "2021"
},
{
"article-title": "Association of Gastrointestinal System With Severity and Mortality of COVID-19: A Systematic Review and Meta-Analysis",
"first-page": "e13317",
"issue": "2",
"journal-title": "Cureus",
"key": "2021092001550866000_2021.04.30.21256415v2.8",
"volume": "13",
"year": "2021"
},
{
"DOI": "10.1016/j.mayocp.2020.05.010",
"article-title": "A Collaborative Multidisciplinary Approach to the Management of Coronavirus Disease 2019 in the Hospital Setting",
"doi-asserted-by": "crossref",
"first-page": "1467",
"issue": "7",
"journal-title": "Mayo Clinic Proceedings",
"key": "2021092001550866000_2021.04.30.21256415v2.9",
"volume": "95",
"year": "2020"
},
{
"DOI": "10.22541/au.161219538.89676033/v1",
"doi-asserted-by": "crossref",
"key": "2021092001550866000_2021.04.30.21256415v2.10",
"unstructured": "Rathore SS , Rojas GA , Sondhi M , et al. Myocarditis associated with Covid-19 disease: a systematic review of published Case reports and Case series. Preprints; 2021/02/01/ 2021."
},
{
"article-title": "A Review of Neurological Complications of COVID-19",
"first-page": "e8192",
"issue": "5",
"journal-title": "Cureus",
"key": "2021092001550866000_2021.04.30.21256415v2.11",
"volume": "12",
"year": "2020"
},
{
"DOI": "10.4103/IJAM.IJAM_94_20",
"article-title": "Pregnancy during COVID-19 pandemic - Maternal and neonatal outcomes: A concise review",
"doi-asserted-by": "crossref",
"first-page": "287",
"issue": "4",
"journal-title": "International Journal of Academic Medicine",
"key": "2021092001550866000_2021.04.30.21256415v2.12",
"volume": "6",
"year": "2020"
},
{
"article-title": "Impact of COVID-19 on the Mental Health of Children and Adolescents",
"first-page": "e10051",
"issue": "8",
"journal-title": "Cureus",
"key": "2021092001550866000_2021.04.30.21256415v2.13",
"volume": "12",
"year": "2020"
},
{
"DOI": "10.1016/j.psychres.2020.113360",
"article-title": "Psychological effects of the COVID 19 pandemic on healthcare workers globally: A systematic review",
"doi-asserted-by": "crossref",
"first-page": "113360",
"journal-title": "Psychiatry Res",
"key": "2021092001550866000_2021.04.30.21256415v2.14",
"volume": "292",
"year": "2020"
},
{
"article-title": "A Review of Cardiac Complications in Coronavirus Disease 2019",
"first-page": "e8034",
"issue": "5",
"journal-title": "Cureus",
"key": "2021092001550866000_2021.04.30.21256415v2.15",
"volume": "12",
"year": "2020"
},
{
"DOI": "10.1097/01.ccm.0000726468.36252.01",
"doi-asserted-by": "crossref",
"key": "2021092001550866000_2021.04.30.21256415v2.16",
"unstructured": "Bhalala U , Gist K , Tripathi S , et al. 145: Pediatric COVID-19: A Report From Viral Infection and Respiratory Illness Universal Study (VIRUS). Critical Care Medicine.49(1):58."
},
{
"DOI": "10.1097/01.ccm.0000726272.88301.cd",
"doi-asserted-by": "crossref",
"key": "2021092001550866000_2021.04.30.21256415v2.17",
"unstructured": "Tripathi S , Gist K , Chiotos K , et al. 61: Risk Factors for Severe COVID-19 Illness in Children: Analysis of the VIRUS: COVID-19 Registry. Critical Care Medicine.49(1):32."
},
{
"DOI": "10.18433/jpps31002",
"article-title": "Current Drugs with Potential for Treatment of COVID-19: A Literature Review",
"doi-asserted-by": "crossref",
"first-page": "58",
"issue": "1",
"journal-title": "J Pharm Pharm Sci",
"key": "2021092001550866000_2021.04.30.21256415v2.18",
"volume": "23",
"year": "2020"
},
{
"DOI": "10.18433/jpps31346",
"article-title": "Anti-Inflammatory Properties of Drugs Used to Control COVID-19 and their Effects on the Renin-Angiotensin System and Angiotensin-Converting Enzyme-2",
"doi-asserted-by": "crossref",
"first-page": "259",
"journal-title": "J Pharm Pharm Sci",
"key": "2021092001550866000_2021.04.30.21256415v2.19",
"volume": "23",
"year": "2020"
},
{
"article-title": "Mortality Benefit of Convalescent Plasma in COVID-19: A Systematic Review and Meta-Analysis",
"first-page": "624924",
"journal-title": "Front Med (Lausanne)",
"key": "2021092001550866000_2021.04.30.21256415v2.20",
"volume": "8",
"year": "2021"
},
{
"DOI": "10.1101/2021.03.14.21253557",
"doi-asserted-by": "crossref",
"key": "2021092001550866000_2021.04.30.21256415v2.21",
"unstructured": "Singh R , Rathore SS , Khan H , et al. Mortality and Severity in COVID-19 Patients on ACEIs & ARBs-A Meta-Regression Analysis. medRxiv. 2021."
},
{
"DOI": "10.1097/01.ccm.0000726448.40803.c6",
"article-title": "140: Efficacy and Safety of Tocilizumab in Hospitalized COVID-19 Patients: A Systematic Review",
"doi-asserted-by": "crossref",
"first-page": "55",
"issue": "1",
"journal-title": "Critical Care Medicine",
"key": "2021092001550866000_2021.04.30.21256415v2.22",
"volume": "49",
"year": "2021"
},
{
"article-title": "Mortality Benefit of Remdesivir in COVID-19: A Systematic Review and Meta-Analysis",
"first-page": "606429",
"journal-title": "Front Med (Lausanne)",
"key": "2021092001550866000_2021.04.30.21256415v2.23",
"volume": "7",
"year": "2020"
},
{
"DOI": "10.1038/ja.2017.11",
"doi-asserted-by": "publisher",
"key": "2021092001550866000_2021.04.30.21256415v2.24"
},
{
"DOI": "10.1186/s12941-020-00368-w",
"doi-asserted-by": "publisher",
"key": "2021092001550866000_2021.04.30.21256415v2.25"
},
{
"DOI": "10.1016/j.antiviral.2013.10.004",
"doi-asserted-by": "publisher",
"key": "2021092001550866000_2021.04.30.21256415v2.26"
},
{
"DOI": "10.1016/j.antiviral.2013.06.002",
"doi-asserted-by": "publisher",
"key": "2021092001550866000_2021.04.30.21256415v2.27"
},
{
"DOI": "10.1042/BJ20120150",
"doi-asserted-by": "publisher",
"key": "2021092001550866000_2021.04.30.21256415v2.28"
},
{
"DOI": "10.1136/openhrt-2020-001350",
"doi-asserted-by": "crossref",
"key": "2021092001550866000_2021.04.30.21256415v2.29",
"unstructured": "DiNicolantonio JJ , Barroso J , McCarty M. Ivermectin may be a clinically useful anti-inflammatory agent for late-stage COVID-19. Open Heart. 2020;7(2)."
},
{
"DOI": "10.1007/s00011-008-8007-8",
"doi-asserted-by": "publisher",
"key": "2021092001550866000_2021.04.30.21256415v2.30"
},
{
"DOI": "10.1016/j.antiviral.2020.104787",
"article-title": "The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro",
"doi-asserted-by": "crossref",
"first-page": "104787",
"journal-title": "Antiviral Res",
"key": "2021092001550866000_2021.04.30.21256415v2.31",
"volume": "178",
"year": "2020"
},
{
"DOI": "10.1101/2020.07.07.20145979",
"doi-asserted-by": "crossref",
"key": "2021092001550866000_2021.04.30.21256415v2.32",
"unstructured": "Gorial FI , Mashhadani S , Sayaly HM , et al. Effectiveness of Ivermectin as add-on Therapy in COVID-19 Management (Pilot Trial). medRxiv. 2020."
},
{
"DOI": "10.1016/jchest.2020.10.009",
"doi-asserted-by": "publisher",
"key": "2021092001550866000_2021.04.30.21256415v2.33"
},
{
"article-title": "Observational Study on Clinical Features, Treatment and Outcome of Covid 19 in a Tertiary Care Centre in India - a Retrospective Case Series",
"first-page": "1",
"issue": "10",
"journal-title": "International Journal of Scientific Research",
"key": "2021092001550866000_2021.04.30.21256415v2.34",
"volume": "9",
"year": "2020"
},
{
"article-title": "A Comparative Study on Ivermectin-Doxycycline and Hydroxychloroquine-Azithromycin Therapy on COVID-19 Patients",
"first-page": "63",
"issue": "1",
"journal-title": "Eurasian Journal of Medicine and Oncology",
"key": "2021092001550866000_2021.04.30.21256415v2.35",
"volume": "5",
"year": "2021"
},
{
"DOI": "10.1038/d41586-020-02958-2",
"article-title": "Latin America’s embrace of an unproven COVID treatment is hindering drug trials",
"doi-asserted-by": "crossref",
"first-page": "481",
"issue": "7830",
"journal-title": "Nature",
"key": "2021092001550866000_2021.04.30.21256415v2.36",
"volume": "586",
"year": "2020"
},
{
"key": "2021092001550866000_2021.04.30.21256415v2.37",
"unstructured": "Trial Site Staff. Beyond The Roundup | First EU Nation To Approve Ivermectin For Covid-19. https://trialsitenews.com/beyond-the-roundup-first-eu-nation-to-approve-ivermectin-for-covid-19/. Published January 30, 2021. Accessed 02.14.2021."
},
{
"article-title": "Ivermectin in Covid-19: Review of the Current Evidence",
"first-page": "27",
"issue": "3",
"journal-title": "The Indian Practitioner",
"key": "2021092001550866000_2021.04.30.21256415v2.38",
"volume": "74",
"year": "2021"
},
{
"key": "2021092001550866000_2021.04.30.21256415v2.39",
"unstructured": "AIIMS/ ICMR-COVID-19 National Task Force/Joint Monitoring Group (Dte.GHS). CLINICAL GUIDANCE FOR MANAGEMENT OF ADULT COVID-19 PATIENTS. Ministry of Health & Family Welfare, Government of India. https://www.mohfw.gov.in/pdf/COVID19ManagementAlgorithm22042021v1.pdf. Published 2021. Updated 04/22/2021. Accessed 04/25/2021."
},
{
"DOI": "10.2139/ssrn.3765018",
"doi-asserted-by": "crossref",
"key": "2021092001550866000_2021.04.30.21256415v2.40",
"unstructured": "Chamie-Quintero JJ , Hibberd J , Scheim D. Sharp reductions in COVID-19 case fatalities and excess deaths in Peru in close time conjunction, state-by-state, with ivermectin treatments. State-By-State, with Ivermectin Treatments (January 12, 2021). 2021."
},
{
"article-title": "Global trends in clinical studies of ivermectin in COVID-19",
"first-page": "1",
"journal-title": "THE JAPANESE JOURNAL OF ANTIBIOTICS",
"key": "2021092001550866000_2021.04.30.21256415v2.41",
"volume": "74",
"year": "2021"
},
{
"DOI": "10.21203/rs.3.rs-100994/v1",
"doi-asserted-by": "crossref",
"key": "2021092001550866000_2021.04.30.21256415v2.42",
"unstructured": "Cadegiani FA , Goren A , Wambier CG , McCoy J. Early COVID-19 Therapy with Azithromycin Plus Nitazoxanide, Ivermectin or Hydroxychloroquine in Outpatient Settings Significantly Reduced Symptoms Compared to Known Outcomes in Untreated Patients. medRxiv. 2020."
},
{
"DOI": "10.1016/j.ijid.2021.02.014",
"article-title": "Effectiveness of a multidrug therapy consisting of Ivermectin, Azithromycin, Montelukast, and Acetylsalicylic acid to prevent hospitalization and death among ambulatory COVID-19 cases in Tlaxcala, Mexico",
"doi-asserted-by": "crossref",
"first-page": "598",
"journal-title": "Int J Infect Dis",
"key": "2021092001550866000_2021.04.30.21256415v2.43",
"volume": "105",
"year": "2021"
},
{
"DOI": "10.21203/rs.3.rs-109670/v1",
"doi-asserted-by": "crossref",
"key": "2021092001550866000_2021.04.30.21256415v2.44",
"unstructured": "Niaee MS , Gheibi N , Namdar P , et al. Ivermectin as an adjunct treatment for hospitalized adult COVID-19 patients: A randomized multi-center clinical trial. Research Square. 2020."
},
{
"DOI": "10.1177/03000605211013550",
"doi-asserted-by": "crossref",
"key": "2021092001550866000_2021.04.30.21256415v2.45",
"unstructured": "Mahmud R , Rahman MM , Alam I , et al. Ivermectin in combination with doxycycline for treating COVID-19 symptoms: a randomized trial. J Int Med Res. 2021;49(5):3000605211013550."
},
{
"DOI": "10.1101/2021.04.20.21255792",
"doi-asserted-by": "crossref",
"key": "2021092001550866000_2021.04.30.21256415v2.46",
"unstructured": "Ravindra G , Chitra L , Madhur M , et al. Retrospective Assessment of Treatments of Hospitalized Covid-19 Patients. medRxiv. 2021."
},
{
"DOI": "10.3390/v13060989",
"doi-asserted-by": "crossref",
"key": "2021092001550866000_2021.04.30.21256415v2.47",
"unstructured": "Samaha AA , Mouawia H , Fawaz M , et al. Effects of a Single Dose of Ivermectin on Viral and Clinical Outcomes in Asymptomatic SARS-CoV-2 Infected Subjects: A Pilot Clinical Trial in Lebanon. Viruses. 2021;13(6)."
},
{
"DOI": "10.21203/rs.3.rs-100956/v3",
"doi-asserted-by": "crossref",
"key": "2021092001550866000_2021.04.30.21256415v2.48",
"unstructured": "Elgazzar A , Eltaweel A , Youssef SA , Hany B , Hafez M , Moussa H. Efficacy and Safety of Ivermectin for Treatment and prophylaxis of COVID-19 Pandemic. Research Square. 2020."
},
{
"key": "2021092001550866000_2021.04.30.21256415v2.49",
"unstructured": "The Cochrane Collaboration. Review Manager (RevMan) [Computer program]. Version 5.4 for Windows. Oxford, England: The Cochrane Collaboration. https://training.cochrane.org/online-learning/core-software-cochrane-reviews/revman/revman-5-download. Published 2020. Accessed 01/31/2021."
},
{
"key": "2021092001550866000_2021.04.30.21256415v2.50",
"unstructured": "Borenstein M , Hedges L , Higgins J , Rothstein H. Comprehensive Meta-Analysis Version Biostat, Englewood, NJ 2013. https://www.meta-analysis.com/index.php?cart=BBFA4702757. Published 2013. Accessed 01/31/2021."
},
{
"DOI": "10.1002/9781119536604",
"doi-asserted-by": "crossref",
"key": "2021092001550866000_2021.04.30.21256415v2.51",
"unstructured": "Higgins JPT , Thomas J , Chandler J , et al. Cochrane handbook for systematic reviews of interventions. John Wiley & Sons; 2019."
},
{
"DOI": "10.1136/bmj.d5928",
"doi-asserted-by": "publisher",
"key": "2021092001550866000_2021.04.30.21256415v2.52"
},
{
"DOI": "10.1001/jama.287.22.2973",
"doi-asserted-by": "publisher",
"key": "2021092001550866000_2021.04.30.21256415v2.53"
},
{
"key": "2021092001550866000_2021.04.30.21256415v2.54",
"unstructured": "National Heart L, and Blood Institute,. Development and Use of Study Quality Assessment Tools. https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools. Updated 04/14/2021. Accessed 04/14/2021, 2020."
},
{
"key": "2021092001550866000_2021.04.30.21256415v2.55",
"unstructured": "Schünemann H , Brożek J , Guyatt G , Oxman A. Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach. Updated October. 2013;2013."
},
{
"DOI": "10.2139/ssrn.3734478",
"doi-asserted-by": "crossref",
"key": "2021092001550866000_2021.04.30.21256415v2.56",
"unstructured": "Afsar N , Ghauri MI , Abbas M , Mukarram MS , Peracha MY , Ishaq K. Ivermectin Use Associated with Reduced Duration of COVID-19 Febrile Illness in a Community Setting. Available at SSRN 3734478. 2020."
},
{
"DOI": "10.1016/j.ijid.2020.11.191",
"doi-asserted-by": "publisher",
"key": "2021092001550866000_2021.04.30.21256415v2.57"
},
{
"article-title": "Clinical Variants, Characteristics, and Outcomes Among COVID-19 Patients: A Case Series Analysis at a Tertiary Care Hospital in Karachi, Pakistan",
"first-page": "e14761",
"issue": "4",
"journal-title": "Cureus",
"key": "2021092001550866000_2021.04.30.21256415v2.58",
"volume": "13",
"year": "2021"
},
{
"article-title": "A Case Series of 100 COVID-19 Positive Patients Treated with Combination of Ivermectin and Doxycycline",
"first-page": "10",
"issue": "0",
"journal-title": "Journal of Bangladesh College of Physicians and Surgeons",
"key": "2021092001550866000_2021.04.30.21256415v2.59",
"volume": "38",
"year": "2020"
},
{
"DOI": "10.1093/qjmed/hcab035",
"doi-asserted-by": "crossref",
"key": "2021092001550866000_2021.04.30.21256415v2.60",
"unstructured": "Babalola OE , Bode CO , Ajayi AA , et al. Ivermectin shows clinical benefits in mild to moderate COVID19: A randomised controlled double-blind, dose-response study in Lagos. QJM. 2021."
},
{
"DOI": "10.1101/2021.05.31.21258081",
"doi-asserted-by": "crossref",
"key": "2021092001550866000_2021.04.30.21256415v2.61",
"unstructured": "Biber A , Mandelboim M , Harmelin G , et al. Favorable outcome on viral load and culture viability using Ivermectin in early treatment of non-hospitalized patients with mild COVID-19, A double-blind, randomized placebo-controlled trial. medRxiv. 2021."
},
{
"DOI": "10.1101/2020.11.16.20232223",
"doi-asserted-by": "crossref",
"key": "2021092001550866000_2021.04.30.21256415v2.62",
"unstructured": "Budhiraja S , Soni A , Jha V , et al. Clinical Profile of First 1000 COVID-19 Cases Admitted at Tertiary Care Hospitals and the Correlates of their Mortality: An Indian Experience. medRxiv. 2020."
},
{
"DOI": "10.1101/2021.02.02.21250840",
"doi-asserted-by": "crossref",
"key": "2021092001550866000_2021.04.30.21256415v2.63",
"unstructured": "Shah Bukhari KH , Asghar A , Perveen N , et al. Efficacy of Ivermectin in COVID-19 Patients with Mild to Moderate Disease. medRxiv. 2021."
},
{
"DOI": "10.1371/journal.pone.0242184",
"doi-asserted-by": "publisher",
"key": "2021092001550866000_2021.04.30.21256415v2.64"
},
{
"key": "2021092001550866000_2021.04.30.21256415v2.65",
"unstructured": "Carvallo H. Ivermectin, Aspirin, Dexamethasone and Enoxaparin as Treatment of Covid 19 - Study Results - ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/results/NCT04425863?view=results. Published 2020. Updated 10/19/2020. Accessed 02/12/2021."
},
{
"article-title": "The effect of early treatment with ivermectin on viral load, symptoms and humoral response in patients with non-severe COVID-19: A pilot, double-blind, placebo-controlled, randomized clinical trial",
"first-page": "100720",
"issue": "100720",
"journal-title": "EClinicalMedicine",
"key": "2021092001550866000_2021.04.30.21256415v2.66",
"volume": "32",
"year": "2021"
},
{
"DOI": "10.18483/ijSci.2378",
"doi-asserted-by": "publisher",
"key": "2021092001550866000_2021.04.30.21256415v2.67"
},
{
"DOI": "10.21203/rs.3.rs-495945/v1",
"doi-asserted-by": "crossref",
"key": "2021092001550866000_2021.04.30.21256415v2.68",
"unstructured": "Chahla RE , Ruiz LM , Mena T , et al. Cluster Randomised Trials-Ivermectin Repurposing For COVID-19 Treatment Of Outpatients With Mild Disease In Primary Health Care Centers. Research Square. 2021."
},
{
"DOI": "10.1002/jmv.26880",
"article-title": "Effect of a combination of nitazoxanide, ribavirin, and ivermectin plus zinc supplement (MANS.NRIZ study) on the clearance of mild COVID-19",
"doi-asserted-by": "crossref",
"first-page": "3176",
"issue": "5",
"journal-title": "J Med Virol",
"key": "2021092001550866000_2021.04.30.21256415v2.69",
"volume": "93",
"year": "2021"
},
{
"article-title": "Effects of Ivermectin-azithromycin-cholecalciferol combined therapy on COVID-19 infected patients: A proof of concept study",
"first-page": "129",
"issue": "5",
"journal-title": "Biomedical Research",
"key": "2021092001550866000_2021.04.30.21256415v2.70",
"volume": "31",
"year": "2020"
},
{
"DOI": "10.1080/20477724.2021.1890887",
"article-title": "Phase 2 randomized study on chloroquine, hydroxychloroquine or ivermectin in hospitalized patients with severe manifestations of SARS-CoV-2 infection",
"doi-asserted-by": "crossref",
"first-page": "235",
"issue": "4",
"journal-title": "Pathog Glob Health",
"key": "2021092001550866000_2021.04.30.21256415v2.71",
"volume": "115",
"year": "2021"
},
{
"DOI": "10.1101/2021.02.18.21252037",
"doi-asserted-by": "crossref",
"key": "2021092001550866000_2021.04.30.21256415v2.72",
"unstructured": "Gonzalez JLB , González Gámez M , Enciso EAM , et al. Efficacy and safety of Ivermectin and Hydroxychloroquine in patients with severe COVID-19. A randomized controlled trial. medRxiv. 2021."
},
{
"DOI": "10.1101/2021.03.04.21252084",
"doi-asserted-by": "crossref",
"key": "2021092001550866000_2021.04.30.21256415v2.73",
"unstructured": "Guzmán MJM , Castillo-Gonzalez A , Gonzalez JLB , et al. Factors associated with increased mortality in critically ill COVID-19 patients in a Mexican public hospital: the other faces of health system oversaturation. medRxiv. 2021."
},
{
"DOI": "10.1101/2020.10.26.20219345",
"doi-asserted-by": "crossref",
"key": "2021092001550866000_2021.04.30.21256415v2.74",
"unstructured": "Hashim HA , Maulood MF , Rasheed AM , Fatak DF , Kabah KK , Abdulamir AS . Controlled randomized clinical trial on using Ivermectin with Doxycycline for treating COVID-19 patients in Baghdad, Iraq. medRxiv. 2020:2020.2010.2026.20219345."
},
{
"article-title": "Outcome of ivermectin and doxycycline in cancer patients with COVID-19: A positive experience in Bangladesh",
"first-page": "27",
"issue": "1",
"journal-title": "International Journal of Molecular & Immuno Oncology",
"key": "2021092001550866000_2021.04.30.21256415v2.75",
"volume": "6",
"year": "2021"
},
{
"key": "2021092001550866000_2021.04.30.21256415v2.76",
"unstructured": "Release HP . Kovid-19 - Huvemek® Phase 2 clinical trial -. Huvepharma https://huvemec.bg/covid-19-huvemec-klinichno-izpitanie/za-isledvaneto/. Updated 03/25/2021. Accessed 05/22/2021."
},
{
"DOI": "10.1016/j.arbr.2020.08.011",
"article-title": "Ivermectin Treatment May Improve the Prognosis of Patients With COVID-19",
"doi-asserted-by": "crossref",
"first-page": "828",
"issue": "12",
"journal-title": "Arch Bronconeumol (Engl Ed)",
"key": "2021092001550866000_2021.04.30.21256415v2.77",
"volume": "56",
"year": "2020"
},
{
"DOI": "10.1101/2021.01.05.21249310",
"doi-asserted-by": "crossref",
"key": "2021092001550866000_2021.04.30.21256415v2.78",
"unstructured": "Ravikirti, Roy R , Pattadar C , et al. Ivermectin as a potential treatment for mild to moderate COVID-19 – A double blind randomized placebo-controlled trial. medRxiv. 2021."
},
{
"article-title": "IVERMECTIN AS ADJUVANT TO HYDROXYCHOLOROQUINE IN PATIENTS RESISTANT TO STANDARD TREATMENT FOR SARS-CoV-2: RESULTS OF AN OPEN-LABEL RANDOMIZED CLINICAL STUDY",
"first-page": "1",
"issue": "8",
"journal-title": "Paripex Indian Journal of Research",
"key": "2021092001550866000_2021.04.30.21256415v2.79",
"volume": "9",
"year": "2020"
},
{
"DOI": "10.1016/j.eclinm.2021.100959",
"doi-asserted-by": "crossref",
"key": "2021092001550866000_2021.04.30.21256415v2.80",
"unstructured": "Krolewiecki A , Lifschitz A , Moragas M , et al. Antiviral effect of high-dose ivermectin in adults with COVID-19: A proof-of-concept randomized trial. EClinicalMedicine. 2021;37."
},
{
"DOI": "10.1001/jama.2021.3071",
"doi-asserted-by": "publisher",
"key": "2021092001550866000_2021.04.30.21256415v2.81"
},
{
"article-title": "Ivermectin and COVID-19 in Care Home: Case Report",
"first-page": "1",
"issue": "4",
"journal-title": "Journal of Infectious Diseases and Epidemiology",
"key": "2021092001550866000_2021.04.30.21256415v2.82",
"volume": "7",
"year": "2021"
},
{
"DOI": "10.21203/rs.3.rs-191648/v1",
"doi-asserted-by": "crossref",
"key": "2021092001550866000_2021.04.30.21256415v2.83",
"unstructured": "Mohan A , Tiwari P , Suri T , et al. Ivermectin in mild and moderate COVID-19 (RIVET-COV): a randomized, placebo-controlled trial. Research Square. 2021."
},
{
"article-title": "The use of compassionate Ivermectin in the management of symptomatic outpatients and hospitalized patients with clinical diagnosis of COVID-19 at the Medical Center Bournigal and the Medical Center Punta Cana, Rescue Group, Dominican Republic, from may 1 to august 10, 2020",
"first-page": "1",
"issue": "S9:1000002",
"journal-title": "J Clin Trials",
"key": "2021092001550866000_2021.04.30.21256415v2.84",
"volume": "11",
"year": "2020"
},
{
"article-title": "Comparative Analytical Study of Two Different Drug Regimens in Treatment of Covid 19 Positive Patients in Index Medical College Hospital and Research Center, Indore, India",
"first-page": "265",
"issue": "6",
"journal-title": "International Journal of Health and Clinical Research",
"key": "2021092001550866000_2021.04.30.21256415v2.85",
"volume": "4",
"year": "2021"
},
{
"DOI": "10.38124/IJISRT20JUL252",
"article-title": "Therapeutic Efficacy of Ivermectin as an Adjuvant in the Treatment of Patients with COVID-19 Study conducted at the Social Security Institute for Workers of the State of Chiapas, ISSTECH, Mexico",
"doi-asserted-by": "crossref",
"first-page": "211",
"issue": "7",
"journal-title": "International Journal of Innovative Science and Research Technology",
"key": "2021092001550866000_2021.04.30.21256415v2.86",
"volume": "5",
"year": "2020"
},
{
"article-title": "Outcome of ivermectin treated mild to moderate COVID-19 cases: a single-centre, open-label, randomised controlled study",
"first-page": "11",
"issue": "2:002",
"journal-title": "IMC Journal of Medical Science",
"key": "2021092001550866000_2021.04.30.21256415v2.87",
"volume": "14",
"year": "2020"
},
{
"DOI": "10.1186/s12879-021-06104-9",
"article-title": "Evaluation of the effectiveness and safety of adding ivermectin to treatment in severe COVID-19 patients",
"doi-asserted-by": "crossref",
"first-page": "411",
"issue": "1",
"journal-title": "BMC Infect Dis",
"key": "2021092001550866000_2021.04.30.21256415v2.88",
"volume": "21",
"year": "2021"
},
{
"article-title": "Use of ivermectin in the treatment of Covid-19: A pilot trial",
"first-page": "505",
"issue": "0",
"journal-title": "Toxicol Rep",
"key": "2021092001550866000_2021.04.30.21256415v2.89",
"volume": "8",
"year": "2021"
},
{
"article-title": "Comparison of viral clearance between ivermectin with doxycycline and hydroxychloroquine with azithromycin in COVID-19 patients",
"first-page": "5",
"journal-title": "Journal of Bangladesh College of Physicians and Surgeons",
"key": "2021092001550866000_2021.04.30.21256415v2.90",
"volume": "38",
"year": "2020"
},
{
"DOI": "10.1016/j.clinthera.2021.07.006",
"doi-asserted-by": "crossref",
"key": "2021092001550866000_2021.04.30.21256415v2.91",
"unstructured": "Shahbaznejad L , Davoudi A , Eslami G , et al. Effects of Ivermectin in Patients With COVID-19: A Multicenter, Double-Blind, Randomized, Controlled Clinical Trial. Clin Ther. 2021."
},
{
"DOI": "10.2139/ssrn.3710623",
"doi-asserted-by": "crossref",
"key": "2021092001550866000_2021.04.30.21256415v2.92",
"unstructured": "Soto-Becerra P , Culquichicón C , Hurtado-Roca Y , Araujo-Castillo RV . Real-world effectiveness of hydroxychloroquine, azithromycin, and ivermectin among hospitalized COVID-19 patients: results of a target trial emulation using observational data from a nationwide healthcare system in Peru. medRxiv. 2020."
},
{
"article-title": "Utility of ivermectin and doxycycline combination for the treatment of SARS-CoV2",
"first-page": "177",
"issue": "10",
"journal-title": "IAIM",
"key": "2021092001550866000_2021.04.30.21256415v2.93",
"volume": "7",
"year": "2020"
},
{
"DOI": "10.1002/jmv.27122",
"article-title": "Clinical study evaluating the efficacy of ivermectin in COVID-19 treatment: A randomized controlled study",
"doi-asserted-by": "crossref",
"first-page": "5833",
"issue": "10",
"journal-title": "J Med Virol",
"key": "2021092001550866000_2021.04.30.21256415v2.94",
"volume": "93",
"year": "2021"
},
{
"DOI": "10.2147/IJN.S313093",
"article-title": "Clinical, Biochemical and Molecular Evaluations of Ivermectin Mucoadhesive Nanosuspension Nasal Spray in Reducing Upper Respiratory Symptoms of Mild COVID-19",
"doi-asserted-by": "crossref",
"first-page": "4063",
"journal-title": "Int J Nanomedicine",
"key": "2021092001550866000_2021.04.30.21256415v2.95",
"volume": "16",
"year": "2021"
},
{
"DOI": "10.1186/s12879-021-06348-5",
"article-title": "Ivermectin to prevent hospitalizations in patients with COVID-19 (IVERCOR-COVID19) a randomized, double-blind, placebo-controlled trial",
"doi-asserted-by": "crossref",
"first-page": "635",
"issue": "1",
"journal-title": "BMC Infect Dis",
"key": "2021092001550866000_2021.04.30.21256415v2.96",
"volume": "21",
"year": "2021"
},
{
"DOI": "10.1101/2021.07.06.21259924",
"doi-asserted-by": "crossref",
"key": "2021092001550866000_2021.04.30.21256415v2.97",
"unstructured": "Hazan S , Dave S , Gunaratne AW , et al. Effectiveness of Ivermectin-Based Multidrug Therapy in Severe Hypoxic Ambulatory COVID-19 Patients. medRxiv. 2021."
},
{
"DOI": "10.18433/jpps31457",
"doi-asserted-by": "publisher",
"key": "2021092001550866000_2021.04.30.21256415v2.98"
},
{
"DOI": "10.1093/ofid/ofab358",
"doi-asserted-by": "crossref",
"key": "2021092001550866000_2021.04.30.21256415v2.99",
"unstructured": "Hill A , Abdulamir A , Ahmed S , et al. Meta-analysis of randomized trials of ivermectin to treat SARS-CoV-2 infection. Research Square. 2021."
},
{
"DOI": "10.1093/ofid/ofab394",
"article-title": "Erratum: Expression of Concern: “Meta-analysis of Randomized Trials of Ivermectin to Treat SARS-CoV-2 Infection”",
"doi-asserted-by": "crossref",
"first-page": "ofab394",
"issue": "8",
"journal-title": "Open Forum Infect Dis",
"key": "2021092001550866000_2021.04.30.21256415v2.100",
"volume": "8",
"year": "2021"
},
{
"key": "2021092001550866000_2021.04.30.21256415v2.101",
"unstructured": "Lawrie T. Ivermectin reduces the risk of death from COVID-19 -a rapid review and meta-analysis in support of the recommendation of the Front Line COVID-19 Critical Care Alliance. (Latest version v1.2 - 6 Jan 2021). 2021."
},
{
"article-title": "Crying wolf in time of Corona: the strange case of ivermectin and hydroxychloroquine",
"first-page": "2",
"journal-title": "Is the fear of failure withholding potential life-saving treatment from clinical use? Signa Vitae",
"key": "2021092001550866000_2021.04.30.21256415v2.102",
"volume": "1",
"year": "2021"
},
{
"DOI": "10.1007/s43440-021-00245-z",
"doi-asserted-by": "crossref",
"key": "2021092001550866000_2021.04.30.21256415v2.103",
"unstructured": "Kow CS , Merchant HA , Mustafa ZU , Hasan SS . The association between the use of ivermectin and mortality in patients with COVID-19: a meta-analysis. Pharmacol Rep. 2021:1–7."
},
{
"DOI": "10.31219/osf.io/dzs2v",
"doi-asserted-by": "crossref",
"key": "2021092001550866000_2021.04.30.21256415v2.104",
"unstructured": "Bryant A , Lawrie TA , Dowswell T , et al. Ivermectin for Prevention and Treatment of COVID-19 Infection: A Systematic Review, Meta-analysis, and Trial Sequential Analysis to Inform Clinical Guidelines. Am J Ther. 2021."
},
{
"DOI": "10.1097/MJT.0000000000001377",
"article-title": "Review of the Emerging Evidence Demonstrating the Efficacy of Ivermectin in the Prophylaxis and Treatment of COVID-19",
"doi-asserted-by": "crossref",
"first-page": "e299",
"issue": "3",
"journal-title": "American Journal of Therapeutics",
"key": "2021092001550866000_2021.04.30.21256415v2.105",
"volume": "28",
"year": "2021"
},
{
"article-title": "Ivermectin and outcomes from Covid-19 pneumonia: A systematic review and meta-analysis of randomized clinical trial studies",
"first-page": "1",
"journal-title": "Reviews in Medical Virology",
"key": "2021092001550866000_2021.04.30.21256415v2.106",
"volume": "e2265",
"year": "2021"
},
{
"key": "2021092001550866000_2021.04.30.21256415v2.107",
"unstructured": "British Ivermectin Recommendation Development. The BIRD Recommendation on the Use of Ivermectin for Covid-19. https://www.francesoir.fr/sites/francesoir/files/media-icons/bird-proceedings-02-03-2021-v151.pdf. Published 2021. Accessed 04.01.2021."
},
{
"DOI": "10.1371/journal.pmed.1003501",
"doi-asserted-by": "publisher",
"key": "2021092001550866000_2021.04.30.21256415v2.108"
},
{
"DOI": "10.1101/2021.01.26.21250420",
"doi-asserted-by": "crossref",
"key": "2021092001550866000_2021.04.30.21256415v2.109",
"unstructured": "Castañeda-Sabogal A , Chambergo-Michilot D , Toro-Huamanchumo CJ , Silva-Rengifo C , Gonzales-Zamora J , Barboza JJ . Outcomes of Ivermectin in the treatment of COVID-19: a systematic review and meta-analysis. medRxiv. 2021:2021.2001.2026.21250420."
},
{
"DOI": "10.1101/2021.05.21.21257595",
"doi-asserted-by": "crossref",
"key": "2021092001550866000_2021.04.30.21256415v2.110",
"unstructured": "Roman YM , Burela PA , Pasupuleti V , Piscoya A , Vidal JE , Hernandez AV . Ivermectin for the treatment of COVID-19: A systematic review and meta-analysis of randomized controlled trials. medRxiv. 2021."
},
{
"article-title": "Ivermectin for preventing and treating COVID-19",
"first-page": "CD015017",
"issue": "7",
"journal-title": "Cochrane Database Syst Rev",
"key": "2021092001550866000_2021.04.30.21256415v2.111",
"volume": "7",
"year": "2021"
},
{
"key": "2021092001550866000_2021.04.30.21256415v2.112",
"unstructured": "NIH. Corticosteroids | COVID-19 Treatment Guidelines. https://www.covid19treatmentguidelines.nih.gov/therapies/immunomodulators/corticosteroids/. Published 2021. Updated 08/04/2021. Accessed 08/25/2021."
},
{
"DOI": "10.1136/bmjnph-2020-000071",
"article-title": "COVID-19: is there a role for immunonutrition, particularly in the over 65s?",
"doi-asserted-by": "crossref",
"first-page": "100",
"issue": "1",
"journal-title": "BMJ Nutr Prev Health",
"key": "2021092001550866000_2021.04.30.21256415v2.113",
"volume": "3",
"year": "2020"
},
{
"DOI": "10.3949/ccjm.87a.ccc046",
"doi-asserted-by": "crossref",
"key": "2021092001550866000_2021.04.30.21256415v2.114",
"unstructured": "Bauer SR , Kapoor A , Rath M , Thomas SA . What is the role of supplementation with ascorbic acid, zinc, vitamin D, or N-acetylcysteine for prevention or treatment of COVID-19? Cleve Clin J Med. 2020."
},
{
"key": "2021092001550866000_2021.04.30.21256415v2.115",
"unstructured": "World Health Organization. WHO Coronavirus Disease (COVID-19) Dashboard. WHO. https://covid19.who.int/. Updated 02.14.2021. Accessed 02.14.2021."
},
{
"DOI": "10.31219/osf.io/9egh4",
"doi-asserted-by": "crossref",
"key": "2021092001550866000_2021.04.30.21256415v2.116",
"unstructured": "Chamie-Quintero J , Hibberd JA , Scheim D. Ivermectin for COVID-19 in Peru: 14-fold reduction in nationwide excess deaths, p=. 002 for effect by state, then 13-fold increase after ivermectin use restricted. 2021."
},
{
"DOI": "10.1371/journal.pone.0235653",
"doi-asserted-by": "publisher",
"key": "2021092001550866000_2021.04.30.21256415v2.117"
},
{
"DOI": "10.21203/rs.3.rs-52224/v1",
"doi-asserted-by": "crossref",
"key": "2021092001550866000_2021.04.30.21256415v2.118",
"unstructured": "Elsayed HH , Hassaballa AS , Ahmed TA , Gumaa M , Sharkawy HY . Variation in outcome of invasive mechanical ventilation between different countries for patients with severe COVID-19: a systematic review and meta-analysis. 2020."
},
{
"key": "2021092001550866000_2021.04.30.21256415v2.119",
"unstructured": "Richardson S. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area (Published online ahead of print, 2020 Apr 22). Jama."
},
{
"DOI": "10.2165/00002018-200022030-00007",
"doi-asserted-by": "publisher",
"key": "2021092001550866000_2021.04.30.21256415v2.120"
},
{
"DOI": "10.1152/ajpendo.00178.2020",
"article-title": "COVID-19-associated SIADH: a clue in the times of pandemic!",
"doi-asserted-by": "crossref",
"first-page": "E882",
"issue": "6",
"journal-title": "Am J Physiol Endocrinol Metab",
"key": "2021092001550866000_2021.04.30.21256415v2.121",
"volume": "318",
"year": "2020"
},
{
"key": "2021092001550866000_2021.04.30.21256415v2.122",
"unstructured": "Mahmud R. Clinical Trial of Ivermectin Plus Doxycycline for the Treatment of Confirmed Covid-19 Infection. Dhaka Medical College Bangladesh. https://clinicaltrials.gov/ct2/show/study/NCT04523831. Published 2020. Updated October 9, 2020. Accessed 02.14.2021."
},
{
"DOI": "10.1056/NEJMoa2007764",
"doi-asserted-by": "publisher",
"key": "2021092001550866000_2021.04.30.21256415v2.123"
},
{
"DOI": "10.1001/jama.2020.17023",
"doi-asserted-by": "publisher",
"key": "2021092001550866000_2021.04.30.21256415v2.124"
},
{
"DOI": "10.1001/jama.2020.22717",
"doi-asserted-by": "publisher",
"key": "2021092001550866000_2021.04.30.21256415v2.125"
},
{
"DOI": "10.1002/alr.22832",
"doi-asserted-by": "crossref",
"key": "2021092001550866000_2021.04.30.21256415v2.126",
"unstructured": "Boscolo-Rizzo P , Guida F , Polesel J , et al. Sequelae in adults at 12 months after mild-to-moderate coronavirus disease 2019 (COVID-19). Int Forum Allergy Rhinol. 2021."
},
{
"DOI": "10.1038/s41591-021-01283-z",
"doi-asserted-by": "publisher",
"key": "2021092001550866000_2021.04.30.21256415v2.127"
},
{
"DOI": "10.1371/journal.pone.0255427",
"article-title": "6-month mortality and readmissions of hospitalized COVID-19 patients: A nationwide cohort study of 8,679 patients in Germany",
"doi-asserted-by": "crossref",
"first-page": "e0255427",
"issue": "8",
"journal-title": "PLoS One",
"key": "2021092001550866000_2021.04.30.21256415v2.128",
"volume": "16",
"year": "2021"
},
{
"DOI": "10.1001/jama.2020.2648",
"doi-asserted-by": "publisher",
"key": "2021092001550866000_2021.04.30.21256415v2.129"
},
{
"key": "2021092001550866000_2021.04.30.21256415v2.130",
"unstructured": "CDC. Interim clinical guidance for management of patients with confirmed coronavirus disease (COVID-19). Centers for Disease Control Prevention. https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-guidance-management-patients.html. Published 2020. Updated 02/12/2021. Accessed 06/19/2021."
},
{
"DOI": "10.1016/s0140-6736(21)00897-7",
"doi-asserted-by": "publisher",
"key": "2021092001550866000_2021.04.30.21256415v2.131"
},
{
"DOI": "10.1001/jama.2021.2747",
"article-title": "Association of Convalescent Plasma Treatment With Clinical Outcomes in Patients With COVID-19: A Systematic Review and Meta-analysis",
"doi-asserted-by": "crossref",
"first-page": "1185",
"issue": "12",
"journal-title": "Jama",
"key": "2021092001550866000_2021.04.30.21256415v2.132",
"volume": "325",
"year": "2021"
},
{
"DOI": "10.1001/jamanetworkopen.2021.3071",
"article-title": "Comparison of Time to Clinical Improvement With vs Without Remdesivir Treatment in Hospitalized Patients With COVID-19",
"doi-asserted-by": "crossref",
"first-page": "e213071",
"issue": "3",
"journal-title": "JAMA Netw Open",
"key": "2021092001550866000_2021.04.30.21256415v2.133",
"volume": "4",
"year": "2021"
},
{
"DOI": "10.1007/s10384-021-00845-5",
"article-title": "Susceptibility of severe COVID-19 patients to rhino-orbital mucormycosis fungal infection in different clinical manifestations",
"doi-asserted-by": "crossref",
"first-page": "515",
"issue": "4",
"journal-title": "Jpn J Ophthalmol",
"key": "2021092001550866000_2021.04.30.21256415v2.134",
"volume": "65",
"year": "2021"
},
{
"DOI": "10.1097/MCC.0000000000000174",
"article-title": "Prevention of acute respiratory distress syndrome",
"doi-asserted-by": "crossref",
"first-page": "82",
"issue": "1",
"journal-title": "Curr Opin Crit Care",
"key": "2021092001550866000_2021.04.30.21256415v2.135",
"volume": "21",
"year": "2015"
},
{
"DOI": "10.1164/rccm.201004-0549OC",
"doi-asserted-by": "publisher",
"key": "2021092001550866000_2021.04.30.21256415v2.136"
},
{
"DOI": "10.1097/CCM.0000000000004864",
"article-title": "The Surviving Sepsis Campaign: Fluid Resuscitation and Vasopressor Therapy Research Priorities in Adult Patients",
"doi-asserted-by": "crossref",
"first-page": "623",
"issue": "4",
"journal-title": "Crit Care Med",
"key": "2021092001550866000_2021.04.30.21256415v2.137",
"volume": "49",
"year": "2021"
},
{
"DOI": "10.1097/CCM.0000000000001968",
"article-title": "Time Matters: Antibiotic Timing in Sepsis and Septic Shock",
"doi-asserted-by": "crossref",
"first-page": "e1016",
"issue": "10",
"journal-title": "Crit Care Med",
"key": "2021092001550866000_2021.04.30.21256415v2.138",
"volume": "44",
"year": "2016"
},
{
"key": "2021092001550866000_2021.04.30.21256415v2.139",
"unstructured": "National Institutes of Health. Ivermectin | COVID-19 Treatment Guidelines. National Institutes of Health. https://www.covid19treatmentguidelines.nih.gov/antiviral-therapy/ivermectin/. Published 2020. Updated February 11, 2021. Accessed 02.14.2021."
},
{
"key": "2021092001550866000_2021.04.30.21256415v2.140",
"unstructured": "FDA. FAQ: COVID-19 and Ivermectin Intended for Animals | FDA. FDA. https://www.fda.gov/animal-veterinary/product-safety-information/faq-covid-19-and-ivermectin-intended-animals. Published 2020. Updated 12/16/2020. Accessed 02.14.2021."
},
{
"key": "2021092001550866000_2021.04.30.21256415v2.141",
"unstructured": "FDA. Why You Should Not Use Ivermectin to Treat or Prevent COVID-19 | FDA. https://www.fda.gov/consumers/consumer-updates/why-you-should-not-use-ivermectin-treat-or-prevent-covid-19. Published 2021. Updated 03.05.2021. Accessed 04.10.2021."
},
{
"key": "2021092001550866000_2021.04.30.21256415v2.142",
"unstructured": "European Medicines Agency. EMA advises against use of ivermectin for the prevention or treatment of COVID-19 outside randomised clinical trials | European Medicines Agency. https://www.ema.europa.eu/en/news/ema-advises-against-use-ivermectin-prevention-treatment-covid-19-outside-randomised-clinical-trials. Published 2021. Updated 03.22.2021. Accessed 04.10.2021."
},
{
"key": "2021092001550866000_2021.04.30.21256415v2.143",
"unstructured": "World Health Organization. WHO advises that ivermectin only be used to treat COVID-19 within clinical trials. https://www.who.int/news-room/feature-stories/detail/who-advises-that-ivermectin-only-be-used-to-treat-covid-19-within-clinical-trials. Published 2021. Updated 03/31/2021. Accessed 08/25/2021."
},
{
"key": "2021092001550866000_2021.04.30.21256415v2.144",
"unstructured": "ACTIV-6: COVID-19 Study of Repurposed Medications. https://clinicaltrials.gov/ct2/show/NCT04885530. Published 2021. Updated 08/30/2021. Accessed 09/02/2021."
},
{
"key": "2021092001550866000_2021.04.30.21256415v2.145",
"unstructured": "National Institutes of Health. NIH to launch public-private partnership to speed COVID-19 vaccine and treatment options. https://www.nih.gov/news-events/news-releases/nih-launch-public-private-partnership-speed-covid-19-vaccine-treatment-options. Updated 04/17/2020. Accessed 08/25/2021."
},
{
"key": "2021092001550866000_2021.04.30.21256415v2.146",
"unstructured": "CDC Health Alert Network. CDCHAN-00449: Rapid Increase in Ivermectin Prescriptions and Reports of Severe Illness Associated with Use of Products Containing Ivermectin to Prevent or Treat COVID-19. https://emergency.cdc.gov/han/2021/han00449.asp?ACSTrackingID=USCDC_511-DM64535&ACSTrackingLabel=HAN%20449%20-%20General%20Public&deliveryName=USCDC_511-DM64535. Published 2021. Updated 08/26/2021. Accessed 09/03/2021."
},
{
"DOI": "10.1097/CCM.0000000000004572",
"article-title": "Guiding Principles for the Conduct of Observational Critical Care Research for Coronavirus Disease 2019 Pandemics and Beyond: The Society of Critical Care Medicine Discovery Viral Infection and Respiratory Illness Universal Study Registry",
"doi-asserted-by": "crossref",
"first-page": "e1038",
"issue": "11",
"journal-title": "Crit Care Med",
"key": "2021092001550866000_2021.04.30.21256415v2.147",
"volume": "48",
"year": "2020"
},
{
"DOI": "10.1097/CCE.0000000000000113",
"article-title": "The Viral Infection and Respiratory Illness Universal Study (VIRUS): An International Registry of Coronavirus 2019-Related Critical Illness",
"doi-asserted-by": "crossref",
"first-page": "e0113",
"issue": "4",
"journal-title": "Crit Care Explor",
"key": "2021092001550866000_2021.04.30.21256415v2.148",
"volume": "2",
"year": "2020"
},
{
"key": "2021092001550866000_2021.04.30.21256415v2.149",
"unstructured": "FDA. FDA experts discuss COVID-19 therapeutic clinical trials | American Medical Association. AMA Webinar Series. https://www.ama-assn.org/delivering-care/public-health/fda-experts-discuss-covid-19-therapeutic-clinical-trials. Updated 03/17/2021. Accessed 04/15/2021."
}
],
"reference-count": 149,
"references-count": 149,
"relation": {},
"resource": {
"primary": {
"URL": "http://medrxiv.org/lookup/doi/10.1101/2021.04.30.21256415"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"subtype": "preprint",
"title": "An Updated Systematic Review and Meta-Analysis of Mortality, Need for ICU admission, Use of Mechanical Ventilation, Adverse effects and other Clinical Outcomes of Ivermectin Treatment in COVID-19 Patients",
"type": "posted-content"
}