Protocol violations in López-Medina et al.: 38 switched ivermectin (IVM) and placebo doses, failure of blinding, widespread IVM sales OTC in Cali, and nearly identical AEs for the IVM and control groups
David E Scheim, Jennifer A Hibberd, Juan J Chamie-Quintero
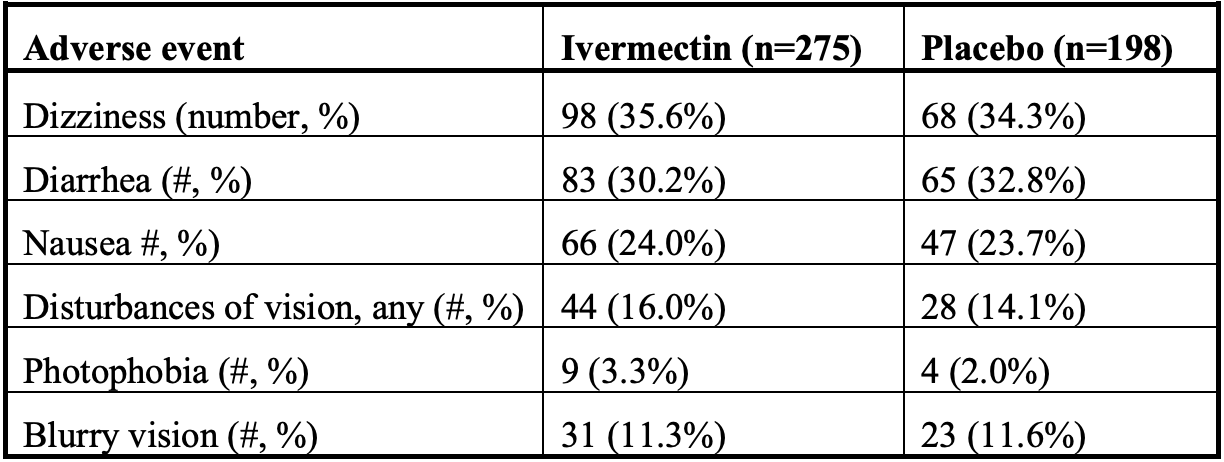
A randomized controlled trial for treatment of mild cases of COVID-19 conducted in Cali, Colombia reported no statistically significant differences in outcomes for its ivermectin (IVM) and placebo arms. A striking anomaly, however, was that certain adverse events (AEs) that are distinctive for the study's high-dose IVM use occurred at nearly identical rates in its IVM and placebo arms. The backdrop for these indicators of IVM use in study controls was widespread sales of IVM for COVID-19 in the Cali area during the study period, with 1.6 IVM doses sold over the counter for each case of COVID-19. The study compounded these risks of contamination of the control arm with critical errors in blinding and segregation of IVM v. placebo doses. A labeling error substituted IVM for placebo doses of 38 patients. Also, 5% dextrose solution was used for the first 64 patients in the placebo group, easily distinguishable from bitter tasting IVM. Given widespread availability and sales of IVM in Cali, lapses in segregation and blinding of IVM and control doses, and IVMcharacteristic AEs in controls, the integrity of the study's control arm was violated. Some useful information can nevertheless be salvaged from outcomes of this study's IVM treatment arm, which had 0 deaths and generally mild symptoms, with AEs typical for high-dose IVM (replicated in the control group) that were generally mild and transient.
Conflicts of Interest:
References
Ali, The effect of ivermectin on some haematological indices in rabbits: influence of vitamin K treatment, Clin Exp Pharmacol Physiol
Baraka, Mahmoud, Marschke, Ivermectin distribution in the plasma and tissues of patients infected with Onchocerca volvulus, Eur J Clin Pharmacol
Canga, Prieto, Liébana, The Pharmacokinetics and Interactions of Ivermectin in Humans-A Mini-review, The AAPS Journal
Chaccour, Hammann, Rabinovich, Ivermectin to reduce malaria transmission I. Pharmacokinetic and pharmacodynamic considerations regarding efficacy and safety, Malaria Journal
Chamie, Hibberd, Scheim, Ivermectin for COVID-19 in Peru: 14-fold reduction in nationwide excess deaths,
doi:10.31219/osf.io/9egh4Chen, Laurent, Onur, A systematic review of neurological symptoms and complications of COVID-19, Journal of Neurology
Email, Scheim, Lopez, Daniel, Connor, None
Goa, Mctavish, Clissold, Ivermectin, Drugs
Guzzo, Furtek, Porras, Safety, tolerability, and pharmacokinetics of escalating high doses of ivermectin in healthy adult subjects, J Clin Pharmacol
Hernández, Department of Valle del Cauca in Colombia will begin to give ivermectin to those infected with COVID-19
Hill, Abdulamir, Ahmed, Meta-analysis of randomized trials of ivermectin to treat SARS-CoV-2 infection, Research Square,
doi:10.21203/rs.3.rs-148845/v1Juarez, Schcolnik-Cabrera, Dueñas-Gonzalez, The multitargeted drug ivermectin: from an antiparasitic agent to a repositioned cancer drug, Am J Cancer Res
Kamgno, Gardon, Gardon-Wendel, Adverse systemic reactions to treatment of onchocerciasis with ivermectin at normal and high doses given annually or three-monthly, Trans R Soc Trop Med Hyg
Li, Dai, Wu, The occurrence of diarrhea in COVID-19 patients, Clin Res Hepatol Gastroenterol
López-Medina, López, Hurtado, Effect of Ivermectin on Time to Resolution of Symptoms Among Adults With Mild COVID-19,
doi:10.1001/jama.2021.3071López-Medina, López, Hurtado, Effect of Ivermectin on Time to Resolution of Symptoms Among Adults With Mild COVID-19: A Randomized Clinical Trial, JAMA
Munoz, Ballester, Antonijoan, Safety and pharmacokinetic profile of fixed-dose ivermectin with an innovative 18mg tablet in healthy adult volunteers, PLoS Negl Trop Dis
Pardhan, Vaughan, Zhang, Sore eyes as the most significant ocular symptom experienced by people with COVID-19: a comparison between pre-COVID-19 and during COVID-19 states, BMJ Open Ophthalmology
Pinzon, Wijaya, Buana, Neurologic Characteristics in Coronavirus Disease 2019 (COVID-19): A Systematic Review and Meta-Analysis, Frontiers in Neurology
Roche, Evaluation of the impact of the performance of the national competition authorities participating in the COMPAL Programme within their respective markets
Scott, Ivermectin Toxicity
Smit, Ochomo, Aljayyoussi, Safety and mosquitocidal efficacy of high-dose ivermectin when co-administered with dihydroartemisinin-piperaquine in Kenyan adults with uncomplicated malaria (IVERMAL): a randomised, double-blind, placebo-controlled trial, Lancet Infect Dis
Vanachayangkul, Im-Erbsin, Tungtaeng, Safety, pharmacokinetics, and liver-stage Plasmodium cynomolgi effect of high-dose ivermectin and chloroquine in Rhesus Macaques, bioRxiv,
doi:10.1101/2020.04.27.065409