Ivermectin A Potential Treatment In Covid-19, Related to Critical Illness
Rashid Qadeer, Syed Muhmmad Kashif, Dr Darshan Kumar, Madiha Mehmmood, Jawahar Lal
Pakistan Journal of Medical and Health Sciences, doi:10.53350/pjmhs2216824
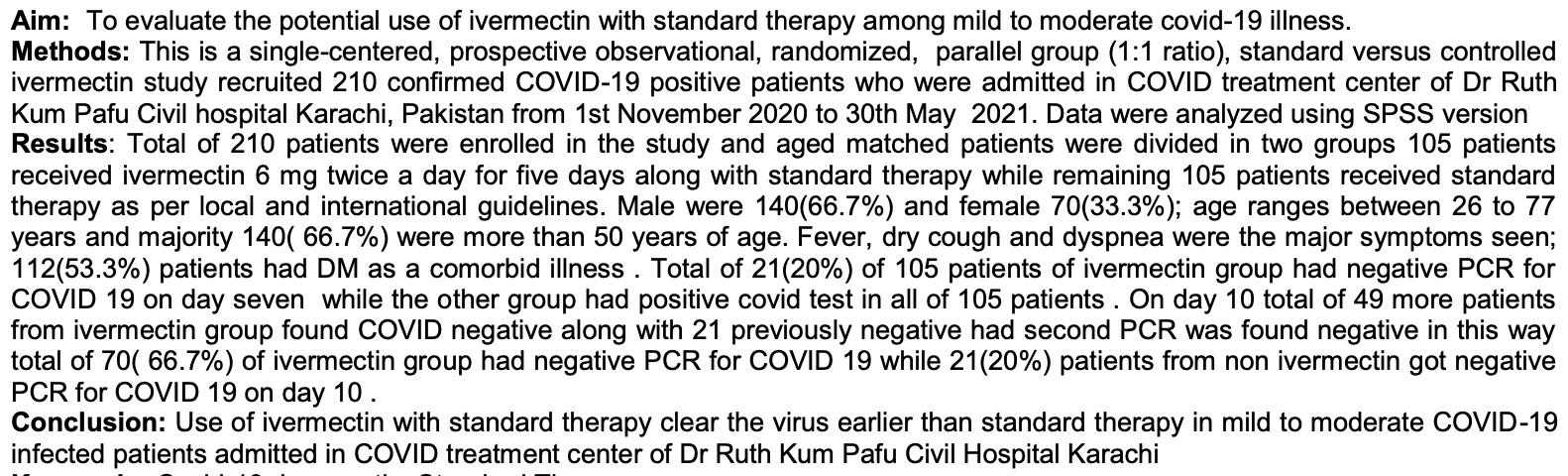
Aim: To evaluate the potential use of ivermectin with standard therapy among mild to moderate covid-19 illness. Methods: This is a single-centered, prospective observational, randomized, parallel group (1:1 ratio), standard versus controlled ivermectin study recruited 210 confirmed COVID-19 positive patients who were admitted in COVID treatment center of Dr Ruth Kum Pafu Civil hospital Karachi, Pakistan from 1st November 2020 to 30th May 2021. Data were analyzed using SPSS version Results: Total of 210 patients were enrolled in the study and aged matched patients were divided in two groups 105 patients received ivermectin 6 mg twice a day for five days along with standard therapy while remaining 105 patients received standard therapy as per local and international guidelines. Male were 140(66.7%) and female 70(33.3%); age ranges between 26 to 77 years and majority 140( 66.7%) were more than 50 years of age. Fever, dry cough and dyspnea were the major symptoms seen; 112(53.3%) patients had DM as a comorbid illness . Total of 21(20%) of 105 patients of ivermectin group had negative PCR for COVID 19 on day seven while the other group had positive covid test in all of 105 patients . On day 10 total of 49 more patients from ivermectin group found COVID negative along with 21 previously negative had second PCR was found negative in this way total of 70( 66.7%) of ivermectin group had negative PCR for COVID 19 while 21(20%) patients from non ivermectin got negative PCR for COVID 19 on day 10 . Conclusion: Use of ivermectin with standard therapy clear the virus earlier than standard therapy in mild to moderate COVID-19 infected patients admitted in COVID treatment center of Dr Ruth Kum Pafu Civil Hospital Karachi
References
Ahmed, Karim, Ross, A five-day course of ivermectin for the treatment of COVID-19 may reduce the duration of illness, Int J Infect Dis,
doi:10.1016/j.ijid.2020.11.191Formiga, Leblanc, De Souza Rebouças, Farias, De Oliveira et al., Ivermectin: an award-winning drug with expected antiviral activity against COVID-19, J Control Release,
doi:10.1016/j.jconrel.2020.10.009Jans, Wagstaff, The broad spectrum host-directed agent ivermectin as an antiviral for SARS-CoV-2 ?, Biochem Biophys Res Commun,
doi:10.1016/j.bbrc.2020.10.042Kaur, Shekhar, Sharma, Sarma, Prakash et al., Ivermectin as a potential drug for treatment of COVID-19: an in-sync review with clinical and computational attributes, Pharmacol Rep,
doi:10.1007/s43440-020-00195-yKinobe, Owens, A systematic review of experimental evidence for antiviral effects of ivermectin and an in silico analysis of ivermectin's possible mode of action against SARS-CoV-2, Fundam Clin Pharmacol,
doi:10.1111/fcp.12644Kory, Meduri, Varon, Iglesias, Marik, Review of the Emerging Evidence Demonstrating the Efficacy of Ivermectin in the Prophylaxis and Treatment of COVID-19, Am J Ther,
doi:10.1097/MJT.0000000000001377Marian, Current state of vaccine development and targeted therapies for COVID-19: impact of basic science discoveries, Cardiovasc Pathol,
doi:10.1016/j.carpath.2020.107278Mohamadian, Chiti, Shoghli, Biglari, Parsamanesh et al., COVID-19: Virology, biology and novel laboratory diagnosis, J Gene Med,
doi:10.1002/jgm.3303Navarro, Camprubí, Requena-Méndez, Safety of high-dose ivermectin: a systematic review and meta-analysis, J Antimicrob Chemother,
doi:10.1093/jac/dkz524Padhy, Mohanty, Das, Meher, Therapeutic potential of ivermectin as add on treatment in COVID 19: A systematic review and meta-analysis, J Pharm Pharm Sci,
doi:10.18433/jpps31457Phelan, Katz, Lo, The novel coronavirus originating in Wuhan, China: challenges for global health governance, Jama
Rajter, Sherman, Fatteh, Vogel, Sacks et al., Use of Ivermectin Is Associated With Lower Mortality in Hospitalized Patients With Coronavirus Disease 2019: The Ivermectin in COVID Nineteen Study, Chest,
doi:10.1016/j.chest.2020.10.009Taiub, Chowdhury, Shahbaz, Karim, Islam et al., A randomized trial of ivermectin-doxycycline and hydroxychloroquine-azithromycin therapy on COVID19 patients n
Vallejos, Zoni, Bangher, Ivermectin to prevent hospitalizations in patients with COVID-19 (IVERCOR-COVID19): a structured summary of a study protocol for a randomized controlled trial, Cochrane Database Syst Rev,
doi:10.1186/s13063-020-04813-1Wu, Ho, Huang, Jin, Li et al., SARS-CoV-2 is an appropriate name for the new coronavirus, The Lancet
DOI record:
{
"DOI": "10.53350/pjmhs2216824",
"URL": "http://dx.doi.org/10.53350/pjmhs2216824",
"abstract": "<jats:p>Aim: To evaluate the potential use of ivermectin with standard therapy among mild to moderate covid-19 illness. Methods: This is a single-centered, prospective observational, randomized, parallel group (1:1 ratio), standard versus controlled ivermectin study recruited 210 confirmed COVID-19 positive patients who were admitted in COVID treatment center of Dr Ruth Kum Pafu Civil hospital Karachi, Pakistan from 1st November 2020 to 30th May 2021. Data were analyzed using SPSS version Results: Total of 210 patients were enrolled in the study and aged matched patients were divided in two groups 105 patients received ivermectin 6 mg twice a day for five days along with standard therapy while remaining 105 patients received standard therapy as per local and international guidelines.</jats:p>",
"author": [
{
"affiliation": [],
"family": "Qadeer",
"given": "Rashid",
"sequence": "first"
},
{
"affiliation": [],
"family": "Kashif",
"given": "Syed Muhmmad",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kumar",
"given": "Darshan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mehmmood",
"given": "Madiha",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lal",
"given": "Jawahar",
"sequence": "additional"
},
{
"affiliation": [],
"family": ".",
"given": "Faizan",
"sequence": "additional"
}
],
"container-title": "Pakistan Journal of Medical and Health Sciences",
"container-title-short": "PJMHS",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2022,
9,
6
]
],
"date-time": "2022-09-06T09:18:30Z",
"timestamp": 1662455910000
},
"deposited": {
"date-parts": [
[
2022,
9,
6
]
],
"date-time": "2022-09-06T09:18:43Z",
"timestamp": 1662455923000
},
"indexed": {
"date-parts": [
[
2022,
9,
6
]
],
"date-time": "2022-09-06T09:41:49Z",
"timestamp": 1662457309628
},
"is-referenced-by-count": 0,
"issue": "8",
"issued": {
"date-parts": [
[
2022,
8,
31
]
]
},
"journal-issue": {
"issue": "8",
"published-print": {
"date-parts": [
[
2022,
8,
31
]
]
}
},
"member": "31137",
"original-title": [],
"page": "24-26",
"prefix": "10.53350",
"published": {
"date-parts": [
[
2022,
8,
31
]
]
},
"published-print": {
"date-parts": [
[
2022,
8,
31
]
]
},
"publisher": "Lahore Medical and Dental College",
"reference-count": 0,
"references-count": 0,
"relation": {},
"resource": {
"primary": {
"URL": "https://pjmhsonline.com/index.php/pjmhs/article/view/2153"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subtitle": [],
"title": "Ivermectin A Potential Treatment In Covid-19, Related to Critical Illness",
"type": "journal-article",
"volume": "16"
}