THE THERAPEUTIC POTENTIAL OF IVERMECTIN FOR COVID-19: A SYSTEMATIC REVIEW OF MECHANISMS AND EVIDENCE
MD Stefanie Kalfas, MBBS FRACP PhD Kumar Visvanathan, DSc Kim Chan, MBBS FRACP PhD John Drago
doi:10.1101/2020.11.30.20236570
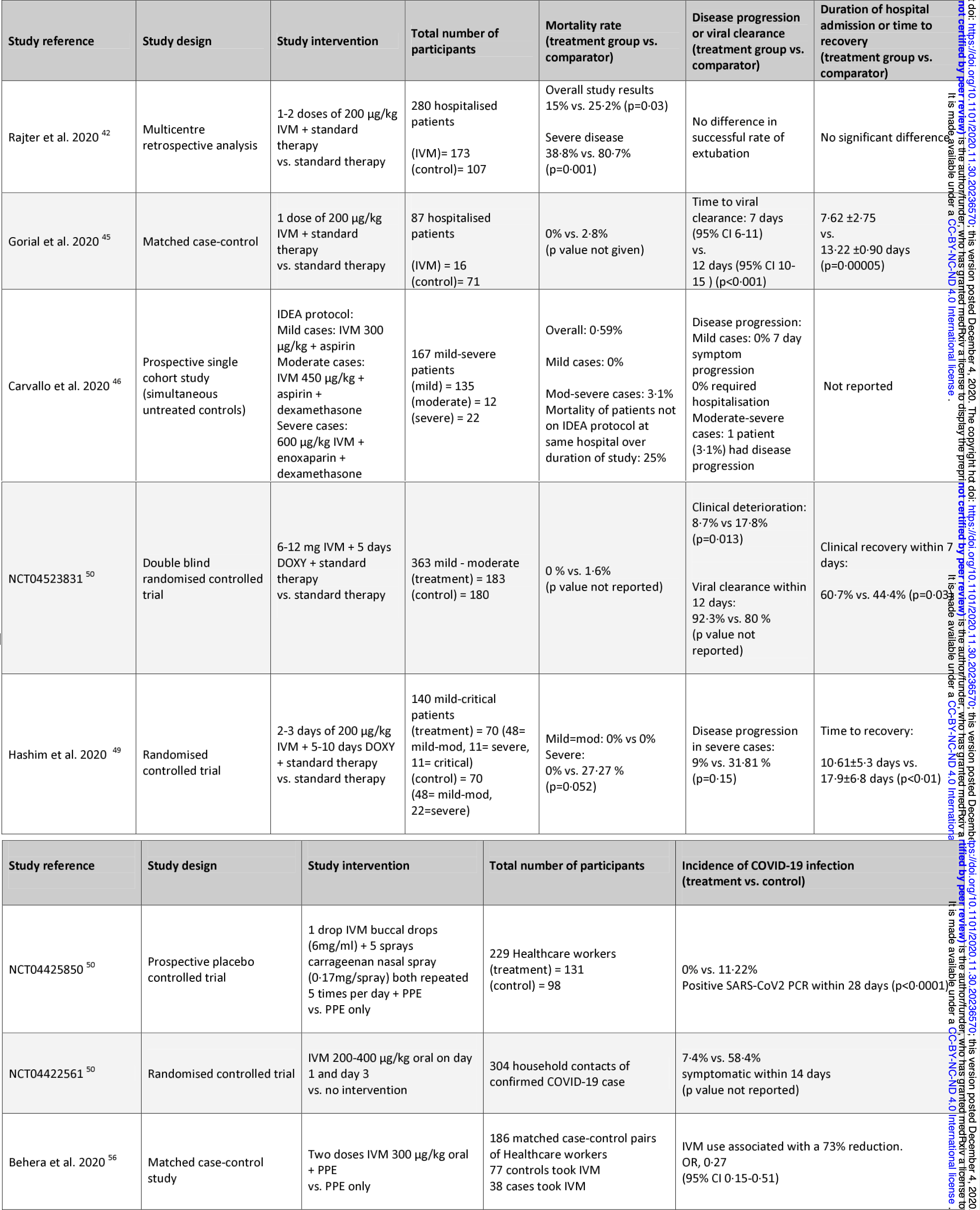
Introduction: Ivermectin is a commonly used antihelminthic agent with over 35 years of established safety data in humans. Recent data demonstrates antiviral activity in vitro against SARS-CoV-2, in addition to a range of viruses. In vitro and animal models also provide evidence of immunomodulatory action. These additional modes of action are supported by in silico modelling, which propose a number of viral and host targets that would mediate these effects. Objectives: The aim of this study is to systematically review the published and preprint clinical literature and study results that assessed the potential role of ivermectin as a COVID-19 therapeutic and prophylactic agent. Methods: We conducted a comprehensive review of PubMed, medRxiv, ClinicalTrials.gov, Global Coronavirus COVID-19 Clinical Trial Tracker, World Health Organization International Clinical Trials Registry Platform, EU Clinical Trials Register, ANZ clinical trials registry, and references from relevant articles. Results: Search keywords-"COVID-19 (and synonyms) AND ivermectin"-generated 86 articles on PubMed, 48 on medRvix and 37 on clinicaltrials.gov at the time of writing. Twelve of these were listed as completed clinical trials and of these, 8 were included as investigators had released results. Positive mortality benefit, reduced time to clinical recovery, reduced incidence of disease progression and decreased duration of hospital admission were reported in patients across all stages of clinical severity. Limitations: Due to the time-critical nature of the COVID-19 pandemic our review included preprint data, which must be interpreted with caution while it awaits peer review.
References
Baudou, Lespine, Durrieu, Serious Ivermectin Toxicity and Human ABCB1 Nonsense Mutations, N Engl J Med
Behera, Patro, Singh, Role of ivermectin in the prevention of COVID-19 infection among healthcare workers in India: A matched case-control study, medRxiv
Beigel, Tomashek, Dodd, Remdesivir for the Treatment of Covid-19 -Final Report, New England Journal of Medicine
Beigel, Tomashek, Dodd, Remdesivir for the Treatment of Covid-19 -Preliminary Report, N Engl J Med
Bhattacharya, Chowdhury, Mukherjee, Pre exposure hydroxychloroquine prophylaxis for covid-19 in healthcare workers: a retrospective cohort, medRxiv
Boulware, Pullen, Bangdiwala, A Randomized Trial of Hydroxychloroquine as Postexposure Prophylaxis for Covid-19, N Engl J Med
Caly, Druce, Catton, Jans, Wagstaff, The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro, Antiviral Res
Caly, Wagstaff, Jans, Nuclear trafficking of proteins from RNA viruses: Potential target for antivirals?, Antiviral Research
Campbell, Burg, Fisher, Dybas, The Discovery of Ivermectin and Other Avermectins
Carvallo, Hirsch, Farinella, Safety and Efficacy of the combined use of ivermectin, dexamethasone, enoxaparin and aspirin against COVID-19, medRxiv
Cazalis, Bodet, Gagnon, Grenier, Doxycycline reduces lipopolysaccharide-induced inflammatory mediator secretion in macrophage and ex vivo human whole blood models, J Periodontol
Chaccour, Abizanda, Irigoyen-Barrio, Nebulized ivermectin for COVID-19 and other respiratory diseases, a proof of concept, dose-ranging study in rats, Scientific Reports
Ci, Li, Yu, Avermectin exerts anti-inflammatory effect by downregulating the nuclear transcription factor kappa-B and mitogen-activated protein kinase activation pathway, Fundam Clin Pharmacol
Clinicaltrials, Search Results for: ivermectin | Completed Studies | Studies With Results | Covid19
Crump, Ōmura, Ivermectin, 'wonder drug' from Japan: the human use perspective, Proc Jpn Acad Ser B Phys Biol Sci
Dinicolantonio, Barroso, Mccarty, Ivermectin may be a clinically useful anti-inflammatory agent for late-stage COVID-19, Open Heart
Errecalde, Lifschitz, Vecchioli, Ivermectin repurposing for COVID-19 therapy: Safety and pharmacokinetic assessment of a novel nasal spray formulation in a pig model, bioRxiv
Fisher, Mrozik, The chemistry and pharmacology of avermectins, Annu Rev Pharmacol Toxicol
Fulcher, Jans, Regulation of nucleocytoplasmic trafficking of viral proteins: an integral role in pathogenesis?, Biochim Biophys Acta
Geary, Ivermectin 20 years on: maturation of a wonder drug, Trends Parasitol
Gendrot, Andreani, Jardot, In Vitro Antiviral Activity of Doxycycline against SARS-CoV-2, Molecules
Gilbert, Slechta, A Case of Ivermectin-Induced Warfarin Toxicity: First Published Report, Hosp Pharm
Gorial, Mashhadani, Sayaly, Effectiveness of Ivermectin as addon Therapy in COVID-19 Management (Pilot Trial), medRxiv
Guzzo, Furtek, Porras, Safety, tolerability, and pharmacokinetics of escalating high doses of ivermectin in healthy adult subjects, J Clin Pharmacol
Götz, Magar, Dornfeld, Influenza A viruses escape from MxA restriction at the expense of efficient nuclear vRNP import, Sci Rep
Hashim, Maulood, Rasheed, Fatak, Kabah et al., None
Heidary, Gharebaghi, Ivermectin: a systematic review from antiviral effects to COVID-19 complementary regimen, J Antibiot
Horby, Lim, Emberson, Dexamethasone in Hospitalized Patients with Covid-19 -Preliminary Report, N Engl J Med
Janabi, Effective Anti-SARS-CoV-2 RNA Dependent RNA Polymerase Drugs Based on Docking Methods: The Case of Milbemycin, Ivermectin, and Baloxavir Marboxil, Avicenna J Med Biotechnol
Jans, Martin, Wagstaff, Inhibitors of nuclear transport, Curr Opin Cell Biol
Jans, Wagstaff, Ivermectin as a Broad-Spectrum Host-Directed Antiviral: The Real Deal?, Cells
Ji, Cen, Lin, Study on the subacute inhalation toxicity of ivermectin in TC rats, Chinese Journal of Comparative Medicine
Kalhor, Sadeghi, Abolhasani, Kalhor, Rahimi, Repurposing of the approved small molecule drugs in order to inhibit SARS-CoV-2QS protein and human ACE2 interaction through virtual screening approaches, J Biomol Struct Dyn
Kircik, Rosso, Layton, Schauber, Over 25 Years of Clinical Experience With Ivermectin: An Overview of Safety for an Increasing Number of Indications, J Drugs Dermatol
Lehrer, Rheinstein, Ivermectin Docks to the SARS-CoV-2 Spike Receptor-binding Domain Attached to ACE2, Vivo
Li, Zhao, Zhan, Quantitative proteomics reveals a broad-spectrum antiviral property of ivermectin, benefiting for COVID-19 treatment, J Cell Physiol
Lundberg, Pinkham, Baer, Nuclear import and export inhibitors alter capsid protein distribution in mammalian cells and reduce Venezuelan Equine Encephalitis Virus replication, Antiviral Res
Lv, Liu, Wang, Ivermectin inhibits DNA polymerase UL42 of pseudorabies virus entrance into the nucleus and proliferation of the virus in vitro and vivo, Antiviral Res
Marty, Lowry, Rodriguez, Treatment of human disseminated strongyloidiasis with a parenteral veterinary formulation of ivermectin, Clin Infect Dis
Mastrangelo, Pezzullo, Burghgraeve, Ivermectin is a potent inhibitor of flavivirus replication specifically targeting NS3 helicase activity: new prospects for an old drug, J Antimicrob Chemother
Matthay, Thompson, Dexamethasone in hospitalised patients with COVID-19: addressing uncertainties, The Lancet Respiratory Medicine
Nicolas, Maia, Bassat, Safety of oral ivermectin during pregnancy: a systematic review and meta-analysis, Lancet Glob Health
Omura, Crump, Ivermectin: panacea for resource-poor communities?, Trends Parasitol
Omura, Ivermectin: 25 years and still going strong, Int J Antimicrob Agents
Pan, Peto, Karim, Repurposed antiviral drugs for COVID-19 -interim WHO SOLIDARITY trial results, medRxiv
Parvez, Karim, Hasan, Prediction of potential inhibitors for RNA-dependent RNA polymerase of SARS-CoV-2 using comprehensive drug repurposing and molecular docking approach, Int J Biol Macromol
Pastor-Barriuso, Perez-Gomez, Hernan, SARS-CoV-2 infection fatality risk in a nationwide seroepidemiological study, medRxiv
Perišić, Recognition of Potential COVID-19 Drug Treatments through the Study of Existing Protein-Drug and Protein-Protein Structures: An Analysis of Kinetically Active Residues, Biomolecules
Quah, Li, Phua, Mortality rates of patients with COVID-19 in the intensive care unit: a systematic review of the emerging literature, Crit Care
Rajasingham, Bangdiwala, Nicol, Hydroxychloroquine as preexposure prophylaxis for COVID-19 in healthcare workers: a randomized trial, medRxiv
Rajter, Sherman, Fatteh, Vogel, Sacks et al., Use of Ivermectin Is Associated With Lower Mortality in Hospitalized Patients With Coronavirus Disease, Chest
Sharp, Dohme, Stromectol (Ivermectin): Product Information
Steinhoff, Vocanson, Voegel, Hacini-Rachinel, Schäfer, Topical Ivermectin 10 mg/g and Oral Doxycycline 40 mg Modified-Release: Current Evidence on the Complementary Use of Anti-Inflammatory Rosacea Treatments, Adv Ther
Ventre, Rozières, Lenief, Topical ivermectin improves allergic skin inflammation, Allergy
Wagstaff, Sivakumaran, Heaton, Harrich, Jans, Ivermectin is a specific inhibitor of importin α/β-mediated nuclear import able to inhibit replication of HIV-1 and dengue virus, Biochem J
Wolstenholme, Glutamate-gated chloride channels, J Biol Chem
Yamasmith, Saleh-Arong, Avirutnan, Efficacy and Safety of Ivermectin against Dengue Infection: A Phase III, Randomized, Double-blind, Placebo-controlled Trial
Yan, Ci, Chen, Anti-inflammatory effects of ivermectin in mouse model of allergic asthma, Inflamm Res
Yang, Atkinson, Wang, The broad spectrum antiviral ivermectin targets the host nuclear transport importin α/β1 heterodimer, Antiviral Res
Zhang, Song, Ci, Ivermectin inhibits LPS-induced production of inflammatory cytokines and improves LPS-induced survival in mice, Inflamm Res
DOI record:
{
"DOI": "10.1101/2020.11.30.20236570",
"URL": "http://dx.doi.org/10.1101/2020.11.30.20236570",
"abstract": "<jats:title>ABSTRACT</jats:title><jats:sec><jats:title>Introduction</jats:title><jats:p>Ivermectin is a commonly used antihelminthic agent with over 35 years of established safety data in humans. Recent data demonstrates antiviral activity in vitro against SARS-CoV-2, in addition to a range of viruses. In vitro and animal models also provide evidence of immunomodulatory action. These additional modes of action are supported by in silico modelling, which propose a number of viral and host targets that would mediate these effects.</jats:p></jats:sec><jats:sec><jats:title>Objectives</jats:title><jats:p>The aim of this study is to systematically review the published and preprint clinical literature and study results that assessed the potential role of ivermectin as a COVID-19 therapeutic and prophylactic agent.</jats:p></jats:sec><jats:sec><jats:title>Methods</jats:title><jats:p>We conducted a comprehensive review of PubMed, medRxiv, ClinicalTrials.gov, Global Coronavirus COVID-19 Clinical Trial Tracker, World Health Organization International Clinical Trials Registry Platform, EU Clinical Trials Register, ANZ clinical trials registry, and references from relevant articles.</jats:p></jats:sec><jats:sec><jats:title>Results</jats:title><jats:p>Search keywords- “COVID-19 (and synonyms) AND ivermectin”- generated 86 articles on PubMed, 48 on medRvix and 37 on clinicaltrials.gov at the time of writing. Twelve of these were listed as completed clinical trials and of these, 8 were included as investigators had released results. Positive mortality benefit, reduced time to clinical recovery, reduced incidence of disease progression and decreased duration of hospital admission were reported in patients across all stages of clinical severity.</jats:p></jats:sec><jats:sec><jats:title>Limitations</jats:title><jats:p>Due to the time-critical nature of the COVID-19 pandemic our review included preprint data, which must be interpreted with caution while it awaits peer review.</jats:p></jats:sec>",
"accepted": {
"date-parts": [
[
2020,
12,
4
]
]
},
"author": [
{
"affiliation": [],
"family": "Kalfas",
"given": "Stefanie",
"sequence": "first"
},
{
"ORCID": "http://orcid.org/0000-0002-1176-5442",
"affiliation": [],
"authenticated-orcid": false,
"family": "Visvanathan",
"given": "Kumar",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-7054-3137",
"affiliation": [],
"authenticated-orcid": false,
"family": "Chan",
"given": "Kim",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-0335-1562",
"affiliation": [],
"authenticated-orcid": false,
"family": "Drago",
"given": "John",
"sequence": "additional"
}
],
"container-title": [],
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2020,
12,
5
]
],
"date-time": "2020-12-05T02:25:59Z",
"timestamp": 1607135159000
},
"deposited": {
"date-parts": [
[
2022,
12,
2
]
],
"date-time": "2022-12-02T14:17:56Z",
"timestamp": 1669990676000
},
"group-title": "Public and Global Health",
"indexed": {
"date-parts": [
[
2023,
8,
10
]
],
"date-time": "2023-08-10T17:35:09Z",
"timestamp": 1691688909490
},
"institution": [
{
"name": "medRxiv"
}
],
"is-referenced-by-count": 6,
"issued": {
"date-parts": [
[
2020,
12,
4
]
]
},
"link": [
{
"URL": "https://syndication.highwire.org/content/doi/10.1101/2020.11.30.20236570",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "246",
"original-title": [],
"posted": {
"date-parts": [
[
2020,
12,
4
]
]
},
"prefix": "10.1101",
"published": {
"date-parts": [
[
2020,
12,
4
]
]
},
"publisher": "Cold Spring Harbor Laboratory",
"reference": [
{
"key": "2020120616300677000_2020.11.30.20236570v1.1",
"unstructured": "World Health Organization Coronavirus disease 2019 (COVID–19) Weekly epidemiological update 24 August, 2020. 2020. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports (accessed August 29 2020)."
},
{
"key": "2020120616300677000_2020.11.30.20236570v1.2",
"unstructured": "The World Bank. The Global Economic Outlook During the COVID–19 Pandemic: A Changed World. 2020. https://www.worldbank.org/en/news/feature/2020/06/08/the–global–economic–outlook–during–the–covid–19–pandemic–a–changed–world (accessed August 31 2020)."
},
{
"key": "2020120616300677000_2020.11.30.20236570v1.3",
"unstructured": "Pastor–Barriuso R , Perez–Gomez B , Hernan MA , et al. SARS–CoV–2 infection fatality risk in a nationwide seroepidemiological study. medRxiv 2020: 2020.08.06.20169722."
},
{
"DOI": "10.1016/j.antiviral.2020.104787",
"article-title": "The FDA–approved drug ivermectin inhibits the replication of SARS–CoV–2 in vitro",
"doi-asserted-by": "crossref",
"first-page": "104787",
"journal-title": "Antiviral Res",
"key": "2020120616300677000_2020.11.30.20236570v1.4",
"volume": "178",
"year": "2020"
},
{
"DOI": "10.1021/bk-1984-0255.ch001",
"doi-asserted-by": "crossref",
"key": "2020120616300677000_2020.11.30.20236570v1.5",
"unstructured": "Campbell WC , Burg RW , Fisher MH , Dybas RA . The Discovery of Ivermectin and Other Avermectins. In: Magee PS , Kohn GK , Menn JJ , eds. Pesticide synthesis through rational approaches. Washington, DC: American Chemical Society; 1984: 5– 20."
},
{
"DOI": "10.1146/annurev.pa.32.040192.002541",
"doi-asserted-by": "publisher",
"key": "2020120616300677000_2020.11.30.20236570v1.6"
},
{
"DOI": "10.2183/pjab.87.13",
"doi-asserted-by": "publisher",
"key": "2020120616300677000_2020.11.30.20236570v1.7"
},
{
"DOI": "10.1074/jbc.R112.406280",
"doi-asserted-by": "publisher",
"key": "2020120616300677000_2020.11.30.20236570v1.8"
},
{
"DOI": "10.1056/NEJMc1917344",
"article-title": "Serious Ivermectin Toxicity and Human ABCB1 Nonsense Mutations",
"doi-asserted-by": "crossref",
"first-page": "787",
"issue": "8",
"journal-title": "N Engl J Med",
"key": "2020120616300677000_2020.11.30.20236570v1.9",
"volume": "383",
"year": "2020"
},
{
"DOI": "10.1086/430827",
"doi-asserted-by": "publisher",
"key": "2020120616300677000_2020.11.30.20236570v1.10"
},
{
"DOI": "10.1016/j.pt.2005.08.014",
"doi-asserted-by": "publisher",
"key": "2020120616300677000_2020.11.30.20236570v1.11"
},
{
"article-title": "Over 25 Years of Clinical Experience With Ivermectin: An Overview of Safety for an Increasing Number of Indications",
"first-page": "325",
"issue": "3",
"journal-title": "J Drugs Dermatol",
"key": "2020120616300677000_2020.11.30.20236570v1.12",
"volume": "15",
"year": "2016"
},
{
"DOI": "10.1016/j.ijantimicag.2007.08.023",
"doi-asserted-by": "publisher",
"key": "2020120616300677000_2020.11.30.20236570v1.13"
},
{
"DOI": "10.1177/009127002401382731",
"doi-asserted-by": "publisher",
"key": "2020120616300677000_2020.11.30.20236570v1.14"
},
{
"DOI": "10.1016/S2214-109X(19)30453-X",
"article-title": "Safety of oral ivermectin during pregnancy: a systematic review and meta–analysis",
"doi-asserted-by": "crossref",
"first-page": "e92",
"issue": "1",
"journal-title": "Lancet Glob Health",
"key": "2020120616300677000_2020.11.30.20236570v1.15",
"volume": "8",
"year": "2020"
},
{
"key": "2020120616300677000_2020.11.30.20236570v1.16",
"unstructured": "Merck Sharp & Dohme BV . Stromectol (Ivermectin): Product Information. Netherlands: Merck, Sharp & Dohme Corp.; 2018."
},
{
"DOI": "10.1177/0018578718758972",
"article-title": "A Case of Ivermectin–Induced Warfarin Toxicity: First Published Report",
"doi-asserted-by": "crossref",
"first-page": "393",
"issue": "6",
"journal-title": "Hosp Pharm",
"key": "2020120616300677000_2020.11.30.20236570v1.17",
"volume": "53",
"year": "2018"
},
{
"DOI": "10.1016/j.pt.2014.07.005",
"doi-asserted-by": "publisher",
"key": "2020120616300677000_2020.11.30.20236570v1.18"
},
{
"DOI": "10.1038/srep23138",
"doi-asserted-by": "publisher",
"key": "2020120616300677000_2020.11.30.20236570v1.19"
},
{
"DOI": "10.1016/j.antiviral.2013.10.004",
"doi-asserted-by": "publisher",
"key": "2020120616300677000_2020.11.30.20236570v1.20"
},
{
"DOI": "10.1016/j.antiviral.2018.09.010",
"doi-asserted-by": "publisher",
"key": "2020120616300677000_2020.11.30.20236570v1.21"
},
{
"DOI": "10.1093/jac/dks147",
"doi-asserted-by": "publisher",
"key": "2020120616300677000_2020.11.30.20236570v1.22"
},
{
"DOI": "10.1042/BJ20120150",
"doi-asserted-by": "publisher",
"key": "2020120616300677000_2020.11.30.20236570v1.23"
},
{
"DOI": "10.1016/j.antiviral.2020.104760",
"doi-asserted-by": "publisher",
"key": "2020120616300677000_2020.11.30.20236570v1.24"
},
{
"DOI": "10.1038/s41429-020-0336-z",
"article-title": "Ivermectin: a systematic review from antiviral effects to COVID–19 complementary regimen",
"doi-asserted-by": "crossref",
"first-page": "593",
"issue": "9",
"journal-title": "J Antibiot (Tokyo)",
"key": "2020120616300677000_2020.11.30.20236570v1.25",
"volume": "73",
"year": "2020"
},
{
"DOI": "10.1016/j.ceb.2019.01.001",
"doi-asserted-by": "publisher",
"key": "2020120616300677000_2020.11.30.20236570v1.26"
},
{
"DOI": "10.1016/j.bbamcr.2011.03.019",
"doi-asserted-by": "publisher",
"key": "2020120616300677000_2020.11.30.20236570v1.27"
},
{
"DOI": "10.1016/j.antiviral.2012.06.008",
"doi-asserted-by": "publisher",
"key": "2020120616300677000_2020.11.30.20236570v1.28"
},
{
"key": "2020120616300677000_2020.11.30.20236570v1.29",
"unstructured": "Yamasmith E , A–hamad Saleh–arong F , Avirutnan P , et al. Efficacy and Safety of Ivermectin against Dengue Infection: A Phase III, Randomized, Double–blind, Placebo–controlled Trial. The 34th Annual Meeting The Royal College of Physicians of Thailand Pattaya, Chonburi, Thailand The Royal College of Physicians of Thailand; 2018."
},
{
"DOI": "10.3390/cells9092100",
"doi-asserted-by": "crossref",
"key": "2020120616300677000_2020.11.30.20236570v1.30",
"unstructured": "Jans DA , Wagstaff KM . Ivermectin as a Broad–Spectrum Host–Directed Antiviral: The Real Deal? Cells 2020; 9(9)."
},
{
"DOI": "10.1007/s12325-016-0380-z",
"article-title": "Topical Ivermectin 10 mg/g and Oral Doxycycline 40 mg Modified–Release: Current Evidence on the Complementary Use of Anti–Inflammatory Rosacea Treatments",
"doi-asserted-by": "crossref",
"first-page": "1481",
"issue": "9",
"journal-title": "Adv Ther",
"key": "2020120616300677000_2020.11.30.20236570v1.31",
"volume": "33",
"year": "2016"
},
{
"DOI": "10.1002/jcp.30055",
"doi-asserted-by": "crossref",
"key": "2020120616300677000_2020.11.30.20236570v1.32",
"unstructured": "Li N , Zhao L , Zhan X. Quantitative proteomics reveals a broad–spectrum antiviral property of ivermectin, benefiting for COVID–19 treatment. J Cell Physiol 2020."
},
{
"DOI": "10.1111/j.1472-8206.2009.00684.x",
"doi-asserted-by": "publisher",
"key": "2020120616300677000_2020.11.30.20236570v1.33"
},
{
"DOI": "10.1007/s00011-008-8007-8",
"doi-asserted-by": "publisher",
"key": "2020120616300677000_2020.11.30.20236570v1.34"
},
{
"DOI": "10.1136/openhrt-2020-001350",
"doi-asserted-by": "crossref",
"key": "2020120616300677000_2020.11.30.20236570v1.35",
"unstructured": "DiNicolantonio JJ , Barroso J , McCarty M. Ivermectin may be a clinically useful anti–inflammatory agent for late–stage COVID–19. Open Heart 2020; 7(2)."
},
{
"DOI": "10.1007/s00011-011-0307-8",
"article-title": "Anti–inflammatory effects of ivermectin in mouse model of allergic asthma",
"doi-asserted-by": "crossref",
"first-page": "589",
"issue": "6",
"journal-title": "Inflamm Res",
"key": "2020120616300677000_2020.11.30.20236570v1.36",
"volume": "60",
"year": "2011"
},
{
"DOI": "10.1111/all.13118",
"article-title": "Topical ivermectin improves allergic skin inflammation",
"doi-asserted-by": "crossref",
"first-page": "1212",
"issue": "8",
"journal-title": "Allergy",
"key": "2020120616300677000_2020.11.30.20236570v1.37",
"volume": "72",
"year": "2017"
},
{
"DOI": "10.1080/07391102.2020.1824816",
"doi-asserted-by": "crossref",
"key": "2020120616300677000_2020.11.30.20236570v1.38",
"unstructured": "Kalhor H , Sadeghi S , Abolhasani H , Kalhor R , Rahimi H. Repurposing of the approved small molecule drugs in order to inhibit SARS–CoV–21S protein and human ACE2 interaction through virtual screening approaches. J Biomol Struct Dyn 2020: 1–16."
},
{
"DOI": "10.21873/invivo.12134",
"doi-asserted-by": "publisher",
"key": "2020120616300677000_2020.11.30.20236570v1.39"
},
{
"DOI": "10.3390/biom10091346",
"doi-asserted-by": "crossref",
"key": "2020120616300677000_2020.11.30.20236570v1.40",
"unstructured": "Perišić O. Recognition of Potential COVID–19 Drug Treatments through the Study of Existing Protein–Drug and Protein–Protein Structures: An Analysis of Kinetically Active Residues. Biomolecules 2020; 10(9)."
},
{
"article-title": "Effective Anti–SARS–CoV–2 RNA Dependent RNA Polymerase Drugs Based on Docking Methods: The Case of Milbemycin, Ivermectin, and Baloxavir Marboxil",
"first-page": "246",
"issue": "4",
"journal-title": "Avicenna J Med Biotechnol",
"key": "2020120616300677000_2020.11.30.20236570v1.41",
"volume": "12",
"year": "2020"
},
{
"DOI": "10.1016/j.ijbiomac.2020.09.098",
"article-title": "Prediction of potential inhibitors for RNA–dependent RNA polymerase of SARS–CoV–2 using comprehensive drug repurposing and molecular docking approach",
"doi-asserted-by": "crossref",
"first-page": "1787",
"journal-title": "Int J Biol Macromol",
"key": "2020120616300677000_2020.11.30.20236570v1.42",
"volume": "163",
"year": "2020"
},
{
"DOI": "10.1016/j.chest.2020.10.009",
"doi-asserted-by": "crossref",
"key": "2020120616300677000_2020.11.30.20236570v1.43",
"unstructured": "Rajter JC , Sherman MS , Fatteh N , Vogel F , Sacks J , Rajter JJ . Use of Ivermectin Is Associated With Lower Mortality in Hospitalized Patients With Coronavirus Disease 2019: The ICON Study. Chest 2020."
},
{
"key": "2020120616300677000_2020.11.30.20236570v1.44",
"unstructured": "Horby P , Lim WS , Emberson JR , et al. Dexamethasone in Hospitalized Patients with Covid–19 – Preliminary Report. N Engl J Med 2020."
},
{
"key": "2020120616300677000_2020.11.30.20236570v1.45",
"unstructured": "Matthay MA , Thompson BT . Dexamethasone in hospitalised patients with COVID– 19:p addressing uncertainties. The Lancet Respiratory Medicine."
},
{
"DOI": "10.1101/2020.07.07.20145979",
"doi-asserted-by": "crossref",
"key": "2020120616300677000_2020.11.30.20236570v1.46",
"unstructured": "Gorial FI , Mashhadani S , Sayaly HM , et al. Effectiveness of Ivermectin as add– on Therapy in COVID–19 Management (Pilot Trial). medRxiv 2020: 2020.07.07.20145979."
},
{
"DOI": "10.1101/2020.09.10.20191619",
"doi-asserted-by": "crossref",
"key": "2020120616300677000_2020.11.30.20236570v1.47",
"unstructured": "Carvallo HE , Hirsch RR , Farinella ME . Safety and Efficacy of the combined use of ivermectin, dexamethasone, enoxaparin and aspirin against COVID–19. medRxiv 2020: 2020.09.10.20191619."
},
{
"key": "2020120616300677000_2020.11.30.20236570v1.48",
"unstructured": "Pan H , Peto R , Karim QA , et al. Repurposed antiviral drugs for COVID–19 – interim WHO SOLIDARITY trial results. medRxiv 2020: 2020.10.15.20209817."
},
{
"DOI": "10.1056/NEJMoa2007764",
"doi-asserted-by": "publisher",
"key": "2020120616300677000_2020.11.30.20236570v1.49"
},
{
"DOI": "10.3390/molecules25215064",
"doi-asserted-by": "crossref",
"key": "2020120616300677000_2020.11.30.20236570v1.50",
"unstructured": "Gendrot M , Andreani J , Jardot P , et al. In Vitro Antiviral Activity of Doxycycline against SARS–CoV–2. Molecules 2020; 25(21)."
},
{
"DOI": "10.1902/jop.2008.080051",
"doi-asserted-by": "publisher",
"key": "2020120616300677000_2020.11.30.20236570v1.51"
},
{
"DOI": "10.1101/2020.10.26.20219345",
"doi-asserted-by": "crossref",
"key": "2020120616300677000_2020.11.30.20236570v1.52",
"unstructured": "Hashim HA , Maulood MF , Rasheed AM , Fatak DF , Kabah KK , Abdulamir AS . Controlled randomized clinical trial on using Ivermectin with Doxycycline for treating COVID–19 patients in Baghdad, Iraq. medRxiv 2020: 2020.10.26.20219345."
},
{
"key": "2020120616300677000_2020.11.30.20236570v1.53",
"unstructured": "ClinicalTrials.gov. Search Results for: ivermectin | Completed Studies | Studies With Results | Covid19. 2020. https://clinicaltrials.gov/ct2/results?term=ivermectin&cond=Covid19&recrs=e&age_v=&gndr=&type=&rslt=With&Search=Apply (accessed November 5 2020)."
},
{
"DOI": "10.1186/s13054-020-03006-1",
"doi-asserted-by": "publisher",
"key": "2020120616300677000_2020.11.30.20236570v1.54"
},
{
"key": "2020120616300677000_2020.11.30.20236570v1.55",
"unstructured": "Beigel JH , Tomashek KM , Dodd LE , et al. Remdesivir for the Treatment of Covid–19 — Preliminary Report. N Engl J Med 2020."
},
{
"DOI": "10.1056/NEJMoa2016638",
"doi-asserted-by": "publisher",
"key": "2020120616300677000_2020.11.30.20236570v1.56"
},
{
"DOI": "10.1101/2020.06.09.20116806",
"doi-asserted-by": "crossref",
"key": "2020120616300677000_2020.11.30.20236570v1.57",
"unstructured": "Bhattacharya R , Chowdhury S , Mukherjee R , et al. Pre exposure hydroxychloroquine prophylaxis for covid–19 in healthcare workers: a retrospective cohort. medRxiv 2020: 2020.06.09.20116806."
},
{
"DOI": "10.1101/2020.09.18.20197327",
"doi-asserted-by": "crossref",
"key": "2020120616300677000_2020.11.30.20236570v1.58",
"unstructured": "Rajasingham R , Bangdiwala AS , Nicol MR , et al. Hydroxychloroquine as pre– exposure prophylaxis for COVID–19 in healthcare workers: a randomized trial. medRxiv 2020: 2020.09.18.20197327."
},
{
"DOI": "10.1101/2020.10.29.20222661",
"doi-asserted-by": "crossref",
"key": "2020120616300677000_2020.11.30.20236570v1.59",
"unstructured": "Behera P , Patro BK , Singh AK , et al. Role of ivermectin in the prevention of COVID–19 infection among healthcare workers in India: A matched case–control study. medRxiv 2020: 2020.10.29.20222661."
},
{
"article-title": "Study on the subacute inhalation toxicity of ivermectin in TC rats",
"first-page": "70",
"issue": "3",
"journal-title": "Chinese Journal of Comparative Medicine",
"key": "2020120616300677000_2020.11.30.20236570v1.60",
"volume": "26",
"year": "2016"
},
{
"DOI": "10.1101/2020.10.23.352831",
"doi-asserted-by": "crossref",
"key": "2020120616300677000_2020.11.30.20236570v1.61",
"unstructured": "Errecalde J , Lifschitz A , Vecchioli G , et al. Ivermectin repurposing for COVID– 19 therapy: Safety and pharmacokinetic assessment of a novel nasal spray formulation in a pig model. bioRxiv 2020: 2020.10.23.352831."
},
{
"DOI": "10.1038/s41598-020-74084-y",
"article-title": "Nebulized ivermectin for COVID–19 and other respiratory diseases, a proof of concept, dose–ranging study in rats",
"doi-asserted-by": "crossref",
"first-page": "17073",
"issue": "1",
"journal-title": "Scientific Reports",
"key": "2020120616300677000_2020.11.30.20236570v1.62",
"volume": "10",
"year": "2020"
}
],
"reference-count": 62,
"references-count": 62,
"relation": {},
"resource": {
"primary": {
"URL": "http://medrxiv.org/lookup/doi/10.1101/2020.11.30.20236570"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"subtype": "preprint",
"title": "THE THERAPEUTIC POTENTIAL OF IVERMECTIN FOR COVID-19: A SYSTEMATIC REVIEW OF MECHANISMS AND EVIDENCE",
"type": "posted-content"
}