Clinical efficacy and safety of ivermectin (400 μg/kg, single dose) in patients with severe COVID-19: a randomized clinical trial
Francisco Ochoa-Jaramillo, Nora Cardona-Castro, Federico Rodriguez-Vega, Veronica Posada-Velez, Diego Rojas-Gual- Dron, Heidy Contreras-Martinez, Ana Romero-Millan, Jessica Porras-Mansilla
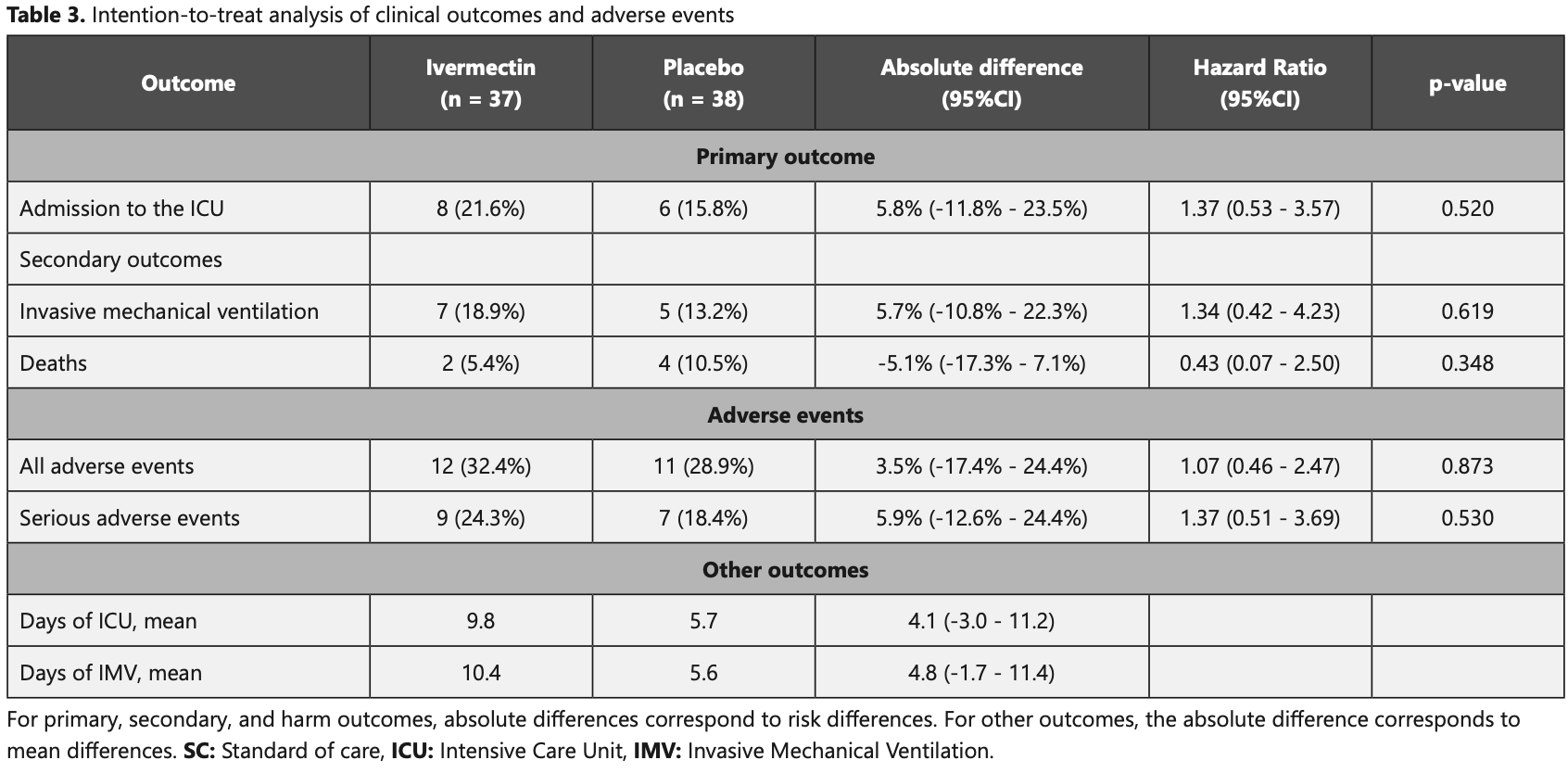
Purpose: To evaluate the clinical efficacy of including Ivermectin (single dose on day 1 of 400 μg/kg PO) in the standard of care in hospitalized adults with severe COVID-19. Methods: Double-blinded, parallel, placebo-controlled, single-center, randomized clinical trial. Seventy-five patients were randomly assigned (1:1) to receive standard of care plus ivermectin or placebo and were followed up for 21 days. Primary outcome measure was admission to ICU and secondary outcomes were the requirement of intensive mechanical ventilation (IMV) and in-hospital death. Intention-to-treat analyses, estimated risk differences (RD), and Hazard ratios (HR) with Cox regression were performed. Results: Enrollment stopped due to the lack of eligible patients. Thirty-seven patients were assigned to intervention and 38 to placebo. Patients in the ivermectin group were 54.5 years on average, 62.2% were male. Comorbidities were more prevalent in the control group (78.9% vs. 56.8%). There was no difference in the 21-day risk of admission to the ICU between ivermectin (21.6%) and placebo (15.8%) (RD= 5.8%; 95%CI: -11.8%-23.5%); neither in the risk of requirement of IMV (18.9% vs 13.2%), mortality (5.4% vs 10.5%) or in adverse events (32.4% vs. 28.9%). Discussion: Ivermectin showed no significant benefit in reducing the requirement of ICU, IMV, or mortality for severe COVID-19 patients.
Financial Support. The study was supported by Fundación Cerro Matoso, Mineros SA, Servicios Generales Suramericana S.A.S., and the Direction of Research CES University. None of the funding sources had any direct or indirect involvement in the study's design, conduct, and completion.
Declarations of interest. None. Author statement.
References
Arshad, Pertinez, Box, Tatham, Rajoli et al., Prioritization of Anti-SARS-Cov-2 Drug Repurposing Opportunities Based on Plasma and Target Site Concentrations Derived from their Established Human Pharmacokinetics, Clin Pharmacol Ther,
doi:10.1002/cpt.1909Canga, Prieto, Liébana, Martínez, Vega et al., The Pharmacokinetics and Interactions of Ivermectin in Humans-A Mini-review, AAPS J,
doi:10.1208/s12248-007-9000-9Chaccour, Casellas, Blanco-Di Matteo, Pineda, Fernandez-Montero et al., The effect of early treatment with ivermectin on viral load, symptoms and humoral response in patients with nonsevere COVID-19: A pilot, double-blind, placebo-controlled, randomized clinical trial, EClinicalMedicine,
doi:10.1016/j.eclinm.2020.100720Ci, Li, Yu, Zhang, Yu et al., Avermectin exerts antiinflammatory effect by downregulating the nuclear transcription factor kappa-B and mitogen-activated protein kinase activation pathway, Fundam Clin Pharmacol,
doi:10.1111/j.1472-8206.2009.00684Crump, Ivermectin: enigmatic multifaceted 'wonder' drug continues to surprise and exceed expectations, J Antibiot,
doi:10.1038/ja.2017.11De Melo, Lazarini, Larrous, Feige, Kergoat et al., Anti-COVID-19 efficacy of ivermectin in the golden hamster, Immunology,
doi:10.1101/2020.11.21.392639Gonzalez, Gámez, Enciso, Maldonado, Palacios et al., Efficacy and safety of Ivermectin and Hydroxychloroquine in patients with severe COVID-19. A randomized controlled trial,
doi:10.1101/2021.02.18.21252037Hill, Mirchandani, Pilkington, Ivermectin for COVID-19: Addressing Potential Bias and Medical Fraud, Open Forum Infectious Diseases,
doi:10.1093/ofid/ofab645Jermain, Hanafin, Cao, Lifschitz, Lanusse et al., Development of a minimal physiologically-based pharmacokinetic model to simulate lung exposure in humans following oral administration of ivermectin for COVID-19 Drug Repurposing, J Pharm Sci,
doi:10.1016/j.xphs.2020.08.024Kircik, Rosso, Layton, Schauber, Over 25 Years of Clinical Experience With Ivermectin: An Overview of Safety for an Increasing Number of Indications, J Drugs Dermatol
Lehrer, Rheinstein, Ivermectin Docks to the SARS-CoV-2 Spike Receptor-binding Domain Attached to ACE2, Vivo,
doi:10.21873/invivo.12134Mohan, Tiwari, Suri, Mittal, Patel et al., Single-dose oral ivermectin in mild and moderate COVID-19 (RIVET-COV): A singlecentre randomized, placebo-controlled trial, J Infect Chemother,
doi:10.1016/j.jiac.2021.08.021Rizzo, Ivermectin, antiviral properties and COVID-19: a possible new mechanism of action, Naunyn Schmiedebergs Arch Pharm,
doi:10.1007/s00210-020-01902-5Schulz, Altman, Moher, Statement: updated guidelines for reporting parallel group randomised trials, BMC Med,
doi:10.1186/1741-7015-8-18Trujillo, Consenso colombiano de atención, diagnóstico y manejo de la infección por SARS-COV-2/COVID 19 en establecimientos de atención de la salud. Recomendaciones basadas en consenso de expertos e informadas en la evidencia, Infectio,
doi:10.22354/in.v24i3.851Ventre, Rozières, Lenief, Albert, Rossio et al., Topical ivermectin improves allergic skin inflammation, Allergy,
doi:10.1111/all.13118Wehbe, Wehbe, Iratni, Pintus, Zaraket et al., Repurposing Ivermectin for COVID-19: Molecular Aspects and Therapeutic Possibilities, Front Immunol,
doi:10.3389/fimmu.2021.663586Who, Inchem, International Peer Reviewed Chemical Safety Information
Yan, Ci, Chen, Chen, Li et al., Anti-inflammatory effects of ivermectin in mouse model of allergic asthma, Inflamm Res,
doi:10.1007/s00011-011-0307-8Zhang, Song, Ci, Ju, Li, Ivermectin inhibits LPSinduced production of inflammatory cytokines and improves LPS-induced survival in mice, Inflamm Res,
doi:10.1007/s00011-008-8007-8Zhang, Song, Xiong, Ci, Li et al., Inhibitory effects of ivermectin on nitric oxide and prostaglandin E2 production in LPS-stimulated RAW 264.7 macrophages, Int Immunopharmacol,
doi:10.1016/j.intimp.2008.12.016DOI record:
{
"DOI": "10.22354/24223794.1105",
"ISSN": [
"2422-3794"
],
"URL": "http://dx.doi.org/10.22354/24223794.1105",
"abstract": "<jats:p>Purpose: To evaluate the clinical efficacy of including Ivermectin (single dose on day 1 of 400 ug/kg PO) in the standard of care in hospitalized adults with severe COVID-19. Methods: Double-blinded, parallel, placebo-controlled, single-center, randomized clinical trial. Seventy-five patients were randomly assigned (1:1) to receive standard of care plus ivermectin or placebo and were followed up for 21 days. Primary outcome measure was admission to ICU and secondary outcomes were the requirement of intensive mechanical ventilation (IMV) and in-hospital death. Intention-to-treat analyses, estimated risk differences (RD), and Hazard ratios (HR) with Cox regression were performed. Results: Enrollment stopped due to the lack of eligible patients. Thirty-seven patients were assigned to intervention and 38 to placebo. Patients in the ivermectin group were 54.5 years on average, 62.2% were male. Comorbidities were more prevalent in the control group (78.9% vs. 56.8%). There was no difference in the 21-day risk of admission to the ICU between ivermectin (21.6%) and placebo (15.8%) (RD= 5.8%; 95%CI: -11.8%-23.5%); neither in the risk of requirement of IMV (18.9% vs 13.2%), mortality (5.4% vs 10.5%) or in adverse events (32.4% vs. 28.9%). Discussion: Ivermectin showed no significant benefit in reducing the requirement of ICU, IMV, or mortality for severe COVID-19 patients.</jats:p>",
"author": [
{
"affiliation": [],
"family": "Ochoa-Jaramillo",
"given": "Francisco",
"sequence": "first"
},
{
"affiliation": [],
"family": "Rodriguez-Vega",
"given": "Federico",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cardona-Castro",
"given": "Nora",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Posada-Velez",
"given": "Veronica",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rojas-Gualdron",
"given": "Diego",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Contreras-Martinez",
"given": "Heidy",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Romero-Millan",
"given": "Ana",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Porras-Mansilla",
"given": "Jessica",
"sequence": "additional"
}
],
"container-title": "Infectio",
"container-title-short": "Infect",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2022,
10,
25
]
],
"date-time": "2022-10-25T17:04:04Z",
"timestamp": 1666717444000
},
"deposited": {
"date-parts": [
[
2022,
10,
25
]
],
"date-time": "2022-10-25T17:04:04Z",
"timestamp": 1666717444000
},
"indexed": {
"date-parts": [
[
2022,
10,
26
]
],
"date-time": "2022-10-26T05:02:49Z",
"timestamp": 1666760569004
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2022,
10,
21
]
]
},
"link": [
{
"URL": "http://revistainfectio.org/P_OJS/index.php/infectio/article/download/1105/1247",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "http://revistainfectio.org/P_OJS/index.php/infectio/article/download/1105/1247",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "9620",
"original-title": [],
"prefix": "10.22354",
"published": {
"date-parts": [
[
2022,
10,
21
]
]
},
"published-online": {
"date-parts": [
[
2022,
10,
21
]
]
},
"publisher": "Asociacion Colombiana de Infectologia - ACIN",
"reference-count": 0,
"references-count": 0,
"relation": {},
"resource": {
"primary": {
"URL": "http://revistainfectio.org/P_OJS/index.php/infectio/article/view/1105"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Clinical efficacy and safety of ivermectin (400 ug/kg, single dose) in patients with severe COVID-19: a randomized clinical trial",
"type": "journal-article"
}