Efficacy and safety of ivermectin in patients with mild COVID-19 in Japan and Thailand
M.D Hiroshige Mikamo, M.D Satoshi Takahashi, M.D Yuka Yamagishi, Ph.D Akihiro Hirakawa, M.D Toshiyuki Harada, M.D Hirotaka Nagashima, M.D Chiaki Noguchi, M.D Kentaro Masuko, M.D Hiromitsu Maekawa, M.D Tatsuhiko Kashii, Hiroyuki Ohbayashi, Shinichiro Hosokawa, Katsuyuki Maejima, Masaya Yamato, Weerawat Manosuthi, Supachai Paiboonpol, Hideki Suganami, Ryohei Tanigawa, Hitoshi Kawamura
Journal of Infection and Chemotherapy, doi:10.1016/j.jiac.2023.12.012
Background: Ivermectin is an antiparasitic drug administered to hundreds of millions of people worldwide. Fundamental research suggests that ivermectin is effective against coronavirus disease 2019 (COVID-19); therefore, we investigated the efficacy and safety of ivermectin as a COVID-19 treatment option.
Methods: This multi-regional (Japan and Thailand), multicenter, placebo-controlled, randomized, double-blind, parallel-group, Phase III study evaluated the efficacy and safety of ivermectin in patients with mild COVID-19 (IVERMILCO Study). The participants took a specified number of the investigational product (ivermectin or placebo) tablets of, adjusted to a dose of 0.3-0.4 mg/kg, orally on an empty stomach once daily for three days. The primary efficacy endpoint was the time at which clinical symptoms first showed an improving trend by 168 h after investigational product administration.
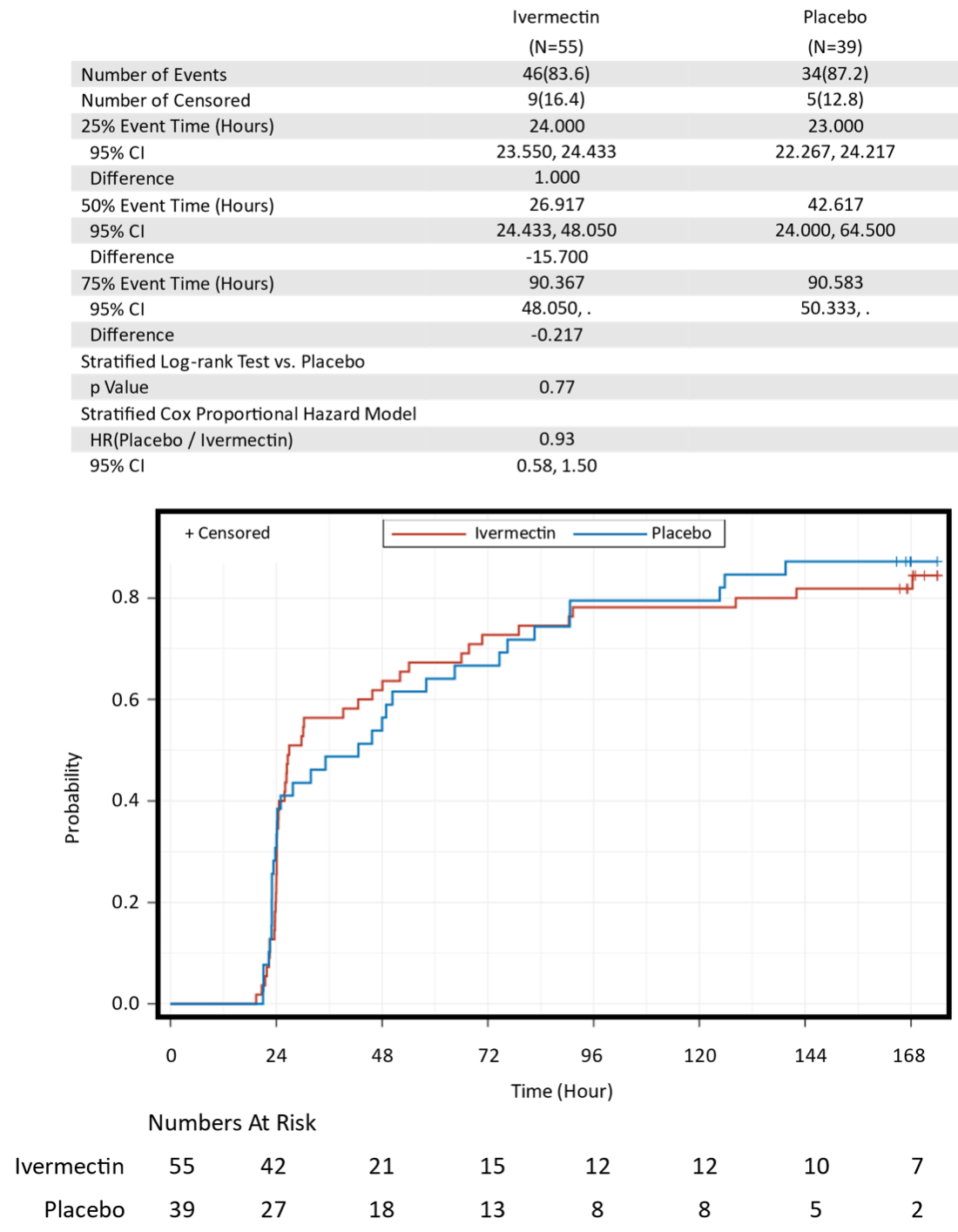
Results: A total of 1,030 eligible participants were assigned to receive the investigational product; 502 participants received ivermectin and 527 participants received a placebo. The primary efficacy endpoint was approximately 96 h (approximately four days) for both ivermectin and placebo groups, which did not show statistically significant difference (stratified log-rank test, p=0.61). The incidence of adverse events and adverse drug reactions did not show statistically significant differences between the ivermectin and placebo groups (chi-square test, p=0.97, p=0.59).
Conclusions: The results show that ivermectin(0.3-0.4 mg/kg), as a treatment for patients with mild COVID-19, is ineffective; however, its safety has been confirmed for participants, J o u r n a l P r e -p r o o f Confidential including minor participants of 12 years or older. (IVERMILCO Study ClinicalTrials.gov number, NCT05056883.
Conflict of Interest assistance with an earlier version of the manuscript and Kowa employees, Masaya Tanahashi and Tatsuya Muto for statistical analysis and interpretation of this study results. We also thank to Editage (www.editage.com) for English language editing.
References
Ashburn, Thor, Drug repositioning: Identifying and developing new uses for existing drugs, Nat Rev Drug Discov,
doi:10.1038/nrd1468Bernal, Da Silva, Musungaie, Kovalchuk, Gonzalez et al., Molnupiravir for oral treatment of Covid-19 in nonhospitalized patients, N Engl J Med,
doi:10.1056/NEJMoa2116044Bramante, Huling, Tignanelli, Buse, Liebovitz et al., Randomized trial of metformin, ivermectin, and fluvoxamine for Covid-19, N Engl J Med,
doi:10.1056/NEJMoa2201662Bryant, Lawrie, Dowswell, Fordham, Mitchell et al., Ivermectin for Prevention and Treatment of COVID-19 Infection: A Systematic Review, Metaanalysis, and Trial Sequential Analysis to Inform Clinical Guidelines, Am J Ther,
doi:10.1097/MJT.0000000000001402Chable-Bessia, Boullé, Neyret, Swain, Hénaut et al., Low Selectivity Indices of Ivermectin and Macrocyclic Lactones on SARS-CoV-2 Replication In Vitro, COVID,
doi:10.3390/covid2010005Crump, Ōmura, Ivermectin, 'wonder drug' from Japan: the human use perspective, Proc Jpn Acad Ser B Phys Biol Sci,
doi:10.2183/pjab.87.13Hammond, Leister-Tebbe, Gardner, Abreu, Wisemandle, Oral nirmatrelvir for high-risk, nonhospitalized adults with Covid-19, N Engl J Med,
doi:10.1056/NEJMoa2118542López-Medina, López, Hurtado I C, Dávalos, Ramirez, Martinez, Effect of ivermectin on time to resolution of symptoms among adults with mild COVID-19: A randomized clinical trial, JAMA,
doi:10.1001/jama.2021.3071Mathieu, Ritchie, Rodés-Guirao, Appel, Giattino et al., Coronavirus Pandemic (COVID-19)
Naggie, Boulware, Lindsell, Stewart, Gentile et al., Effect of ivermectin vs placebo on time to sustained recovery in outpatients with mild to moderate COVID-19: A randomized clinical trial, JAMA,
doi:10.1001/jama.2022.18590Naggie, Boulware, Lindsell, Stewart, Slandzicki et al., Effect of higher-dose ivermectin for 6 days vs placebo on time to sustained recovery in outpatients with COVID-19. A Randomized Clinical Trial, JAMA,
doi:10.1001/jama.2023.1650Reis, Silva, Silva D C M, Thabane, Milagres, Ferreira, r n a l P r e -p r o o f Effect of early treatment with ivermectin among patients with Covid-19, N Engl J Med,
doi:10.1056/NEJMoa2115869Smit, Ochomo, Aljayyoussi, Kwambai, Abong'o B O et al., Safety and mosquitocidal efficacy of high-dose ivermectin when coadministered with dihydroartemisinin-piperaquine in Kenyan adults with uncomplicated malaria (IVERMAL): a randomised, double-blind, placebo-controlled trial, Lancet,
doi:10.1016/S1473-3099(18)30163-4DOI record:
{
"DOI": "10.1016/j.jiac.2023.12.012",
"ISSN": [
"1341-321X"
],
"URL": "http://dx.doi.org/10.1016/j.jiac.2023.12.012",
"alternative-id": [
"S1341321X23003161"
],
"assertion": [
{
"label": "This article is maintained by",
"name": "publisher",
"value": "Elsevier"
},
{
"label": "Article Title",
"name": "articletitle",
"value": "Efficacy and safety of ivermectin in patients with mild COVID-19 in Japan and Thailand"
},
{
"label": "Journal Title",
"name": "journaltitle",
"value": "Journal of Infection and Chemotherapy"
},
{
"label": "CrossRef DOI link to publisher maintained version",
"name": "articlelink",
"value": "https://doi.org/10.1016/j.jiac.2023.12.012"
},
{
"label": "Content Type",
"name": "content_type",
"value": "article"
},
{
"label": "Copyright",
"name": "copyright",
"value": "© 2023 Japanese Society of Chemotherapy, Japanese Association for Infectious Diseases, and Japanese Society for Infection Prevention and Control. Published by Elsevier Ltd."
}
],
"author": [
{
"ORCID": "http://orcid.org/0000-0001-8876-6411",
"affiliation": [],
"authenticated-orcid": false,
"family": "Mikamo",
"given": "Hiroshige",
"sequence": "first"
},
{
"ORCID": "http://orcid.org/0000-0002-2121-1021",
"affiliation": [],
"authenticated-orcid": false,
"family": "Takahashi",
"given": "Satoshi",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Yamagishi",
"given": "Yuka",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hirakawa",
"given": "Akihiro",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-0825-5166",
"affiliation": [],
"authenticated-orcid": false,
"family": "Harada",
"given": "Toshiyuki",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Nagashima",
"given": "Hirotaka",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Noguchi",
"given": "Chiaki",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Masuko",
"given": "Kentaro",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Maekawa",
"given": "Hiromitsu",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kashii",
"given": "Tatsuhiko",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ohbayashi",
"given": "Hiroyuki",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hosokawa",
"given": "Shinichiro",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Maejima",
"given": "Katsuyuki",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Yamato",
"given": "Masaya",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Manosuthi",
"given": "Weerawat",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Paiboonpol",
"given": "Supachai",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Suganami",
"given": "Hideki",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tanigawa",
"given": "Ryohei",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0009-0009-3826-9842",
"affiliation": [],
"authenticated-orcid": false,
"family": "Kawamura",
"given": "Hitoshi",
"sequence": "additional"
}
],
"container-title": "Journal of Infection and Chemotherapy",
"container-title-short": "Journal of Infection and Chemotherapy",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"clinicalkey.fr",
"clinicalkey.jp",
"clinicalkey.es",
"clinicalkey.com.au",
"jiac-j.com",
"clinicalkey.com",
"elsevier.com",
"sciencedirect.com"
]
},
"created": {
"date-parts": [
[
2023,
12,
27
]
],
"date-time": "2023-12-27T02:42:02Z",
"timestamp": 1703644922000
},
"deposited": {
"date-parts": [
[
2023,
12,
27
]
],
"date-time": "2023-12-27T15:46:48Z",
"timestamp": 1703692008000
},
"funder": [
{
"DOI": "10.13039/501100003478",
"award": [
"HATSUKEN 0422–3",
"HATSUKEN 0430–8"
],
"doi-asserted-by": "publisher",
"name": "Government of Japan Ministry of Health Labour and Welfare"
}
],
"indexed": {
"date-parts": [
[
2023,
12,
28
]
],
"date-time": "2023-12-28T00:13:15Z",
"timestamp": 1703722395175
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2023,
12
]
]
},
"language": "en",
"license": [
{
"URL": "https://www.elsevier.com/tdm/userlicense/1.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
12,
1
]
],
"date-time": "2023-12-01T00:00:00Z",
"timestamp": 1701388800000
}
},
{
"URL": "http://creativecommons.org/licenses/by-nc-nd/4.0/",
"content-version": "vor",
"delay-in-days": 26,
"start": {
"date-parts": [
[
2023,
12,
27
]
],
"date-time": "2023-12-27T00:00:00Z",
"timestamp": 1703635200000
}
}
],
"link": [
{
"URL": "https://api.elsevier.com/content/article/PII:S1341321X23003161?httpAccept=text/xml",
"content-type": "text/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://api.elsevier.com/content/article/PII:S1341321X23003161?httpAccept=text/plain",
"content-type": "text/plain",
"content-version": "vor",
"intended-application": "text-mining"
}
],
"member": "78",
"original-title": [],
"prefix": "10.1016",
"published": {
"date-parts": [
[
2023,
12
]
]
},
"published-print": {
"date-parts": [
[
2023,
12
]
]
},
"publisher": "Elsevier BV",
"reference": [
{
"DOI": "10.1038/nrd1468",
"article-title": "Drug repositioning: identifying and developing new uses for existing drugs",
"author": "Ashburn",
"doi-asserted-by": "crossref",
"first-page": "673",
"journal-title": "Nat Rev Drug Discov",
"key": "10.1016/j.jiac.2023.12.012_bib1",
"volume": "3",
"year": "2004"
},
{
"DOI": "10.1016/j.pharmthera.2021.107930",
"article-title": "Drug repurposing for COVID-19: approaches, challenges and promising candidates",
"author": "Ng",
"doi-asserted-by": "crossref",
"journal-title": "Pharmacol Ther",
"key": "10.1016/j.jiac.2023.12.012_bib2",
"volume": "228",
"year": "2021"
},
{
"DOI": "10.2183/pjab.87.13",
"article-title": "Ivermectin, ‘wonder drug’ from Japan: the human use perspective",
"author": "Crump",
"doi-asserted-by": "crossref",
"first-page": "13",
"journal-title": "Proc Jpn Acad Ser B Phys Biol Sci",
"key": "10.1016/j.jiac.2023.12.012_bib3",
"volume": "87",
"year": "2011"
},
{
"DOI": "10.1016/j.antiviral.2020.104787",
"article-title": "The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro",
"author": "Caly",
"doi-asserted-by": "crossref",
"journal-title": "Antivir Res",
"key": "10.1016/j.jiac.2023.12.012_bib4",
"volume": "178",
"year": "2020"
},
{
"DOI": "10.1038/s41429-021-00491-6",
"article-title": "The mechanisms of action of ivermectin against SARS-CoV-2-an extensive review",
"author": "Zaidi",
"doi-asserted-by": "crossref",
"first-page": "60",
"journal-title": "J Antibiot (Tokyo)",
"key": "10.1016/j.jiac.2023.12.012_bib5",
"volume": "75",
"year": "2022"
},
{
"DOI": "10.1097/MJT.0000000000001402",
"article-title": "Ivermectin for prevention and treatment of COVID-19 infection: a systematic review, meta-analysis, and trial sequential analysis to inform clinical guidelines",
"author": "Bryant",
"doi-asserted-by": "crossref",
"first-page": "e434",
"issue": "4",
"journal-title": "Am J Therapeut",
"key": "10.1016/j.jiac.2023.12.012_bib6",
"volume": "28",
"year": "2021"
},
{
"key": "10.1016/j.jiac.2023.12.012_bib7",
"series-title": "Assessing COVID-19-related symptoms in outpatient adult and adolescent subjects in clinical trials of drugs and biological products for COVID-19 prevention or treatment guidance for industry",
"year": "2020"
},
{
"DOI": "10.1001/jama.2022.18590",
"article-title": "Effect of ivermectin vs placebo on time to sustained recovery in outpatients with mild to moderate COVID-19: a randomized clinical trial",
"author": "Naggie",
"doi-asserted-by": "crossref",
"first-page": "1595",
"journal-title": "JAMA",
"key": "10.1016/j.jiac.2023.12.012_bib8",
"volume": "328",
"year": "2022"
},
{
"DOI": "10.1056/NEJMoa2201662",
"article-title": "Randomized trial of metformin, ivermectin, and fluvoxamine for Covid-19",
"author": "Bramante",
"doi-asserted-by": "crossref",
"first-page": "599",
"journal-title": "N Engl J Med",
"key": "10.1016/j.jiac.2023.12.012_bib9",
"volume": "387",
"year": "2022"
},
{
"DOI": "10.1056/NEJMoa2115869",
"article-title": "Effect of early treatment with ivermectin among patients with Covid-19",
"author": "Reis",
"doi-asserted-by": "crossref",
"first-page": "1721",
"journal-title": "N Engl J Med",
"key": "10.1016/j.jiac.2023.12.012_bib10",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.1001/jama.2021.3071",
"article-title": "Effect of ivermectin on time to resolution of symptoms among adults with mild COVID-19: a randomized clinical trial",
"author": "López-Medina",
"doi-asserted-by": "crossref",
"first-page": "1426",
"journal-title": "JAMA",
"key": "10.1016/j.jiac.2023.12.012_bib11",
"volume": "325",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2116044",
"article-title": "Molnupiravir for oral treatment of Covid-19 in nonhospitalized patients",
"author": "Jayk Bernal",
"doi-asserted-by": "crossref",
"first-page": "509",
"journal-title": "N Engl J Med",
"key": "10.1016/j.jiac.2023.12.012_bib12",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.1056/NEJMoa2118542",
"article-title": "Oral nirmatrelvir for high-risk, nonhospitalized adults with Covid-19",
"author": "Hammond",
"doi-asserted-by": "crossref",
"first-page": "1397",
"journal-title": "N Engl J Med",
"key": "10.1016/j.jiac.2023.12.012_bib13",
"volume": "386",
"year": "2022"
},
{
"key": "10.1016/j.jiac.2023.12.012_bib14",
"series-title": "STROMECTOL® (ivermectin) tablets",
"year": "2022"
},
{
"DOI": "10.3390/covid2010005",
"article-title": "Low selectivity indices of ivermectin and macrocyclic lactones on SARS-CoV-2 replication in vitro",
"author": "Chable-Bessia",
"doi-asserted-by": "crossref",
"first-page": "60",
"issue": "1",
"journal-title": "COVID",
"key": "10.1016/j.jiac.2023.12.012_bib16",
"volume": "2",
"year": "2022"
},
{
"DOI": "10.1001/jama.2023.1650",
"article-title": "Effect of higher-dose ivermectin for 6 days vs placebo on time to sustained recovery in outpatients with COVID-19. A Randomized Clinical Trial",
"author": "Naggie",
"doi-asserted-by": "crossref",
"first-page": "888",
"journal-title": "JAMA",
"key": "10.1016/j.jiac.2023.12.012_bib17",
"volume": "329",
"year": "2023"
},
{
"article-title": "Coronavirus pandemic (COVID-19)",
"author": "Mathieu",
"journal-title": "Our World In Data",
"key": "10.1016/j.jiac.2023.12.012_bib18",
"year": "2020"
},
{
"DOI": "10.1016/S1473-3099(18)30163-4",
"article-title": "Safety and mosquitocidal efficacy of high-dose ivermectin when coadministered with dihydroartemisinin-piperaquine in Kenyan adults with uncomplicated malaria (IVERMAL): a randomised, double-blind, placebo-controlled trial",
"author": "Smit",
"doi-asserted-by": "crossref",
"first-page": "615",
"journal-title": "Lancet",
"key": "10.1016/j.jiac.2023.12.012_bib19",
"volume": "18",
"year": "2018"
},
{
"article-title": "MedinCell announces positive results for the SAIVE clinical study in prevention of Covid-19 infection in a contact-based population",
"journal-title": "MedinCell",
"key": "10.1016/j.jiac.2023.12.012_bib20",
"year": "2023"
}
],
"reference-count": 19,
"references-count": 19,
"relation": {},
"resource": {
"primary": {
"URL": "https://linkinghub.elsevier.com/retrieve/pii/S1341321X23003161"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Infectious Diseases",
"Pharmacology (medical)",
"Microbiology (medical)"
],
"subtitle": [],
"title": "Efficacy and safety of ivermectin in patients with mild COVID-19 in Japan and Thailand",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1016/elsevier_cm_policy"
}