Novel non-covalent ivermectin complex Didenectin is revolutionizing healthcare
Kirill Didenko
doi:10.5281/zenodo.10215620
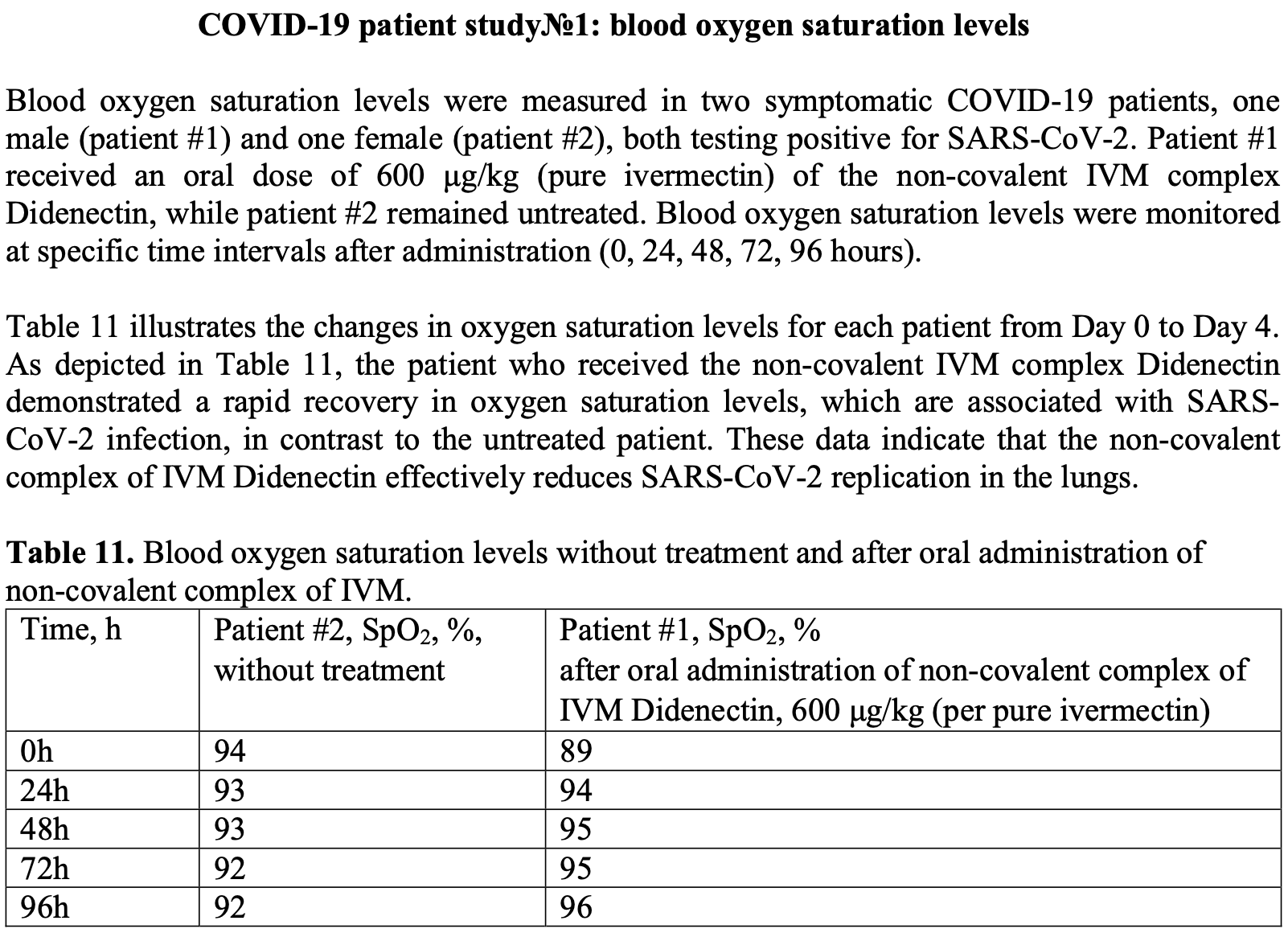
COVID-19 pandemic has accelerated scientific knowledge and led to groundbreaking advancements in virology, and it has also given rise development of new viruses based on the coronavirus both in governmental and private laboratories all over the world. Laboratory accidents, no matter how well-controlled, can happen. We need to explore a development of an effective treatment that can mitigate the potential misuse of the coronavirus as a foundation for new viruses. In this article, we introduce a revolutionary breakthrough in coronavirus treatment, new ivermectin-based complex Didenectin (antiviral ivermectin), which is proved to reduce virus load to 100 times during 24h, leading to revolutionary rapid recovery of SARS-CoV-2 patients and Dengue patients within a record 24-hour timeframe. The newly developed ivermectin-polymer complex, known as Didenectin, is derived from an innovative solid dispersion of ivermectin formed through the novel process of mechanochemical activation. This complex combines with arabinogalactan polymer to create a non-covalent interaction. The ivermectin-polymer complex Didenectin, due to its modified molecular structure, exhibits altered properties, including a 20-fold increase in solubility, increased bioavailability, enhanced permeability, and, simultaneously, a 3.4-fold reduction in oral toxicity compare to ordinary ivermectin. The substantial changes in drug parameters compared to the base compound result in a qualitatively new treatment outcome and results in a significant 100 times reduction in the viral load within the initial 24-hour period. Instead of merely improving treatment efficacy indicators, such as ventilation, ICU admissions, hospitalization, and recovery, the treatment led to the complete recovery of patients in the shortest time possible. The treatment duration for the infection is reduced to just 1 day at a single dose of more than 600-700 μg/kg (according to pure IVM). As ivermectin is under investigation as an anticancer agent, Didenectin emerges as a promising candidate for cancer treatment due to its low toxicity. * -Doses and concentrations highlighted in bold were used to construct a dose-C max linear regression. † -Theoretical calculated doses estimated based on linear regression. 1 -The ingested amount (in μg/kg/day) was derived from an estimated body weight of 75 kg for patients with unknown body weight. 2 -Calculated by plasma-lung ratio 2.67 [2]. ‡ -Maximum single dose have been used in a trial in healthy volunteers without clinically significant safety issues [6].
Table 15 . Documented in vitro antiviral action of ivermectin [13] .
Virus InhibitoryConcentration / Foldreduction According to studies, Ivermectin has broad anticancer activity and has antitumor effects in vitro and in vivo [19, 20, 21] . Didenectin emerges as a promising candidate for cancer treatment due to its low toxicity.
References
Chaccourc, Blanco-Dimatteoa, Pinedai, Fernandez-Monteroa, Ruiz-Castillop et al., The effect of early treatment with ivermectin on viral load, symptoms and humoral response in patients with non-severe COVID-19: A pilot, double-blind, placebo-controlled, randomized clinical trial, EClinicalMedicine,
doi:10.1016/j.eclinm.2020.100720Chung, Yang, Wu, Deng, Tsai, Agricultural avermectins: an uncommon but potentially fatal cause of pesticide poisoning, Ann Emerg Med,
doi:10.1016/s0196-0644(99)70271-4Crump, Ivermectin: enigmatic multifaceted 'wonder' drug continues to surprise and exceed expectations, J Antibiot,
doi:10.1038/ja.2017.11Guzzo, Furtek, Porras, Chen, Tipping et al., Safety, tolerability, and pharmacokinetics of escalating high doses of ivermectin in healthy adult subjects, J ClinPharmacol,
doi:10.1177/009127002401382731Heidary, Gharebaghi, Ivermectin: a systematic review from antiviral effects to COVID-19 complementary regimen, J Antibiot,
doi:10.1038/s41429-020-0336-zJermain, Hanafin, Cao, Lifschitz, Lanusse et al., Development of a Minimal Physiologically-Based Pharmacokinetic Model to Simulate Lung Exposure in Humans Following Oral Administration of Ivermectin for COVID-19 Drug Repurposing, J Pharm Sci,
doi:10.1016/j.xphs.2020.08.024Juarez, Schcolnik-Cabrera, Dueñas-Gonzalez, The multitargeted drug ivermectin: from an antiparasitic agent to a repositioned cancer drug, Am J Cancer Res
Kositz, Bradley, Hutchins, Last, 'alessandro et al., Broadening the range of use cases for ivermectin -a review of the evidence, Trans R Soc Trop Med Hyg,
doi:10.1093/trstmh/trab114Krolewieckia, Moragasm, Travaciom, Valentinir, Alonsodf et al., Antiviral effect of high-dose ivermectin in adults with COVID-19: A proof-of-concept randomized trial, EClinicalMedicine,
doi:10.1016/j.eclinm.2021.100959Lespine, Martin, Dupuy, Roulet, Pineau et al., Interaction of macrocyclic lactones with P-glycoprotein: structure-affinity relationship, Eur J Pharm Sci,
doi:10.1016/j.ejps.2006.10.004Lifschitz, Virkel, Sallovitz, Sutra, Galtier et al., Comparative distribution of ivermectin and doramectin to parasite location tissues in cattle, Vet Parasitol,
doi:10.1016/s0304-4017(99)00175-2Na-Bangchang, High-performance liquid chromatographic method for the determination of ivermectin in plasma, Southeast Asian J Trop Med Public Health
Navarrom, Camprubí, Requena-Méndeza, Buonfrated, Giorlig et al., Safety of high-dose ivermectin: a systematic review and meta-analysis, J AntimicrobChemother,
doi:10.1093/jac/dkz524Nihal, Ahmad, Dose translation from animal to human studies revisited, FASEB J
Pandi, Bulusu, Kommineni, Khan, Singh, Amorphous solid dispersions: An update for preparation, characterization, mechanism on bioavailability, stability, regulatory considerations and marketed products, Int J Pharm,
doi:10.1016/j.ijpharm.2020.119560Rojas-Oviedo, Retchkiman-Corona, Quirino-Barreda, Cárdenas, Schabes-Retchkiman, Solubility Enhancement of a Poorly Water Soluble Drug by Forming Solid Dispersions using Mechanochemical Activation, Indian J Pharm Sci,
doi:10.4103/0250-474X.110576Savjanikt, Gajjarak, Savjanijk, Drug solubility: importance and enhancement techniques, ISRN Pharm,
doi:10.5402/2012/195727Schmith, Zhou, Lohmer, The Approved Dose of Ivermectin Alone is not the Ideal Dose for the Treatment of COVID-19, ClinPharmacolTher,
doi:10.1002/cpt.1889Tangm, Hux, Wangy, Yaox, Zhangw et al., Ivermectin, a potential anticancer drug derived from an antiparasitic drug, Pharmacol Res,
doi:10.1016/j.phrs.2020.105207Vasconcelos, Sarmento, Costa, Solid dispersions as strategy to improve oral bioavailability of poor water soluble drugs, Drug Discov Today,
doi:10.1016/j.drudis.2007.09.005Wang, Pharmacology/Toxicology NDA review and evaluation
Yamasmith, Efficacy and Safety of Ivermectin against Dengue Infection: A Phase III, Randomized, Double-blind, Placebo-controlled Trial
DOI record:
{
"DOI": "10.5281/ZENODO.10215620",
"URL": "https://zenodo.org/doi/10.5281/zenodo.10215620",
"abstract": "In this article, we introduce a revolutionary breakthrough in coronavirus treatment, new ivermectin-based complex Didenectin (antiviral ivermectin), which is proved to reduce virus load to 100 times during 24h, leading to revolutionary rapid recovery of SARS-CoV-2 patients and Dengue patients within a record 24-hour timeframe.\nThe introduction of new non-covalent complex of ivermectin is changing the game in the healthcare industry. A novel non-covalent ivermectin-polymer complex Didenectin, already showed remarkable results on SARS-CoV-2 as well as on Dengue, a condition currently lacking any specific treatment. The complex should also be effective for the treatment of other viral infections that have shown in vitro sensitivity to ivermectin such as Chikungunya, Zika, Yellow fever, West Nile, avian influenza A (H7N7), HIV-1, Japanese encephalitis, tick-borne encephalitis, Epstein-Barr and others.\nIvermectin, a widely recognized anti-parasitic medication, exhibited remarkable efficacy in reducing viral load of SARS-CoV-2 by 93% and more within a single day under in vitro conditions, as demonstrated by Caly et al. However, translating these results to in vivo human studies was hindered by the substantial toxicity of ivermectin, preventing the achievement of the necessary IC50 2.4 μM and IC90 5 μM concentration of ivermectin in body tissues. The advent of the innovative Didenectin allowed to achieve a notable 3.4-fold reduction in oral toxicity and a remarkable 20-fold increase in solubility when compared to standard ivermectin. This led to the achievement of necessary IC50 2.4 μM and IC90 5 μM concentration of ivermectin and comparable reduction in viral load in vivo as previously demonstrated in vitro by Caly et al.\nDidenectin's rapid and effective action demonstrates its potential not only for SARS-CoV-2 but potentially for Dengue as well. The first-ever cure of a Dengue patient within a 24-hour timeframe using Didenectin represents a monumental achievement in the field of antiviral research.\nThis article delves into the complex's mechanism of action, pre-clinical results, as well as a detailed description of the method for obtaining the ivermectin-polymer complex Didenectin, along with the complete formula and table of dosages.",
"author": [
{
"family": "Didenko",
"given": "Kirill"
}
],
"copyright": "Creative Commons Attribution 4.0 International",
"id": "https://doi.org/10.5281/zenodo.10215620",
"issued": {
"date-parts": [
[
2023,
11,
29
]
]
},
"language": "en",
"publisher": "Zenodo",
"title": "Novel non-covalent ivermectin complex Didenectin is revolutionizing healthcare.",
"type": "article",
"version": "1"
}