COVID-19 In-Hospital Mortality Rate is Reduced by Prophylactic Use of Ivermectin: Findings From a City-Wide, Prospective Observational Study Using Propensity Score Matching (PSM)
MD, ARDMS Lucy Kerr, PhD Fernando Baldi, PhD Raysildo Barbosa Lôbo, Washington Luiz, Olivato Assagra, Fernando Carlos Proença, DDS, DPD, MRCDC Jennifer A Hibberd, Juan J Chamie-Quintero, MD, MPA Pierre Kory, MD, MSc, PhD Flavio A Cadegiani, MD, MSc Flávio A Cadegiani
doi:10.13140/RG.2.2.26793.52327
Background: Based on ivermectin safety profile and lack of alternative options and vaccines, a medical-based citywide governmental program offered ivermectin prophylaxis for COVID-19 (city of Itajaí, state of Santa Catarina, Southern Brazi). The aim of the present study was to evaluate the impact of prophylactic ivermectin use in inhospital COVID-19 mortality rate.
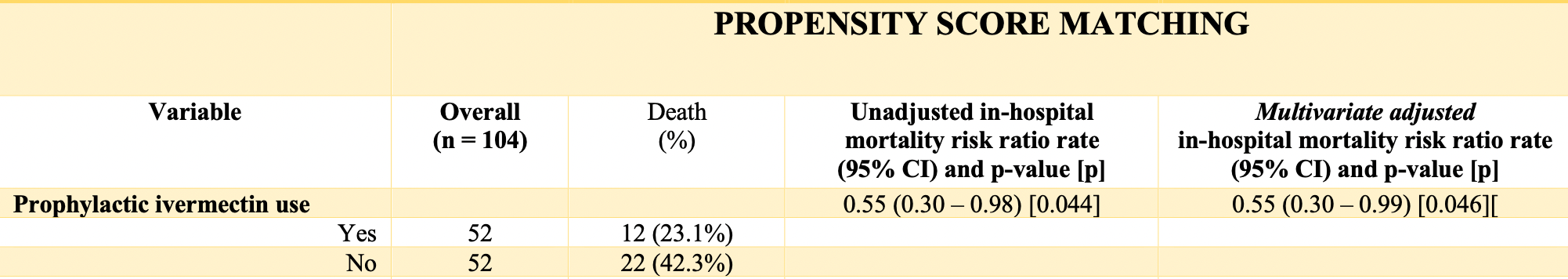
Materials and methods: We analyzed data from hospitalized COVID-19 patients of a major COVID-19 reference hospital during a city-wide program of ivermectin prophylaxis for COVID-19 (Hospital Marieta Konder Bornhausen, Itajaí, Santa Catarina, Brazil), between July and December 2020. We compared in-hospital mortality rates between patients that used ivermectin regularly prior to COVID-19 and those that did not use ivermectin. Comparisons were performed before and after propensity score matching (PSM). Groups were balanced for sex, age, hypertension, type 2 diabetes (T2D) and cancer. Liver [alanine transferase (ALT)], kidney (creatinine), and inflammatory [highsensitivity sensitive C-reactive protein (hs-CRP)] parameters were compared between ivermectin users and non-users through inverse PSM. Results: A total of 378 hospitalized subjects were included. In propensity score matched groups, two cohorts of 52 subjects were compared. There were 12 deaths among pre-COVID ivermectin users and 22 deaths among non-users (23.1% and 42.3% in-hospital all-cause mortality rate, respectively), a 45% reduction in in-hospital mortality rate ratio of (RR, 0.55; 95%CI, 0.30 -0.98; p = 0.044). Creatinine, hs-CRP and ALT levels were significantly lower among pre-COVID ivermectin users than non-users (p = 0.03, p = 0.0013 and p = 0.019).
Conclusion : Prophylactic use of ivermectin for COVID-19 reduced in-hospital all-cause mortality rate due to COVID-19, irrespective of age, sex and comorbidities, and should be become a predictor of good outcomes among hospitalized subjects, in addition to the already known protective factors, including earlier age, female sex and absence of metabolic comorbidities.
Final discussion The potential benefits of ivermectin to prevent COVID-19 infection and risk of death due to COVID-19 in a population-level analysis 26 can be extended to those subjects that did not respond to ivermectin prophylaxis apparently, since they were infected by COVID-19 and the disease progressed to the need of hospitalization. This unexpected residual benefits of ivermectin were detected through reduction of in-hospital mortality, confirmed by unadjusted and multivariate Poisson adjusted analysis, and further confirmed through the employment of PSM. In addition, regular, chronic use of ivermectin seemed to provide protection for kidney and liver functions, and reduce the dysfunctional inflammatory response. Despite the limitations, the present findings are highly relevant for public health decision-making, since ivermectin has a well-established safety profile, potential benefits for other diseases, may reduce COVID-19-related health costs, and is unexpensive, with a favorable cost-effectiveness.
Conclusion
Conflict of Interest The authors declare no conflict of interest regarding the drug, ivermectin, and potential commercial benefits of the expansion of its use for COVID-19, or any other related gains. Dr Lucy Kerr received funding from Vitamedic, that manufactures ivermectin, unrelated to this study. Dr. Flavio A. Cadegiani was contracted by Vitamedic for consulting services unrelated to this study, and donated the full budget for COVID-19 patient care..
References
Andersson, Ottestad, Tracey, Extracellular HMGB1: a therapeutic target in severe pulmonary inflammation including COVID-19?, Mol Med
Behera, Patro, Singh, Chandanshive, R R, Pradhan et al., Role of Ivermectin in the prevention of SARS-CoV-2 infection among healthcare workers in India: A matched case-control study, PLoS One,
doi:10.1371/journal.pone.0247163Chen, Kubo, Ivermectin and its target molecules: shared and unique modulation mechanisms of ion channels and receptors by Ivermectin, J Physiol,
doi:10.1113/JP275236Crump, Ivermectin: enigmatic multifaceted 'wonder' drug continues to surprise and exceed expectations, J Antibiot,
doi:10.1038/ja.2017.11Heidary, Gharebaghi, Ivermectin: a systematic review from antiviral effects to COVID-19 complementary regimen, J Antibiot,
doi:10.1038/s41429-020-0336-zJin, Feng, Rong, The antiparasitic drug Ivermectin is a novel FXR ligand that regulates metabolism, Nat Commun,
doi:10.1038/ncomms2924Juarez, Schcolnik-Cabrera, Dueñas-Gonzalez, The multitargeted drug Ivermectin: from an antiparasitic agent to a repositioned cancer drug, Am J Cancer Res
Juarez, Schcolnik-Cabrera, Dueñas-Gonzalez, The multitargeted drug Ivermectin: from an antiparasitic agent to a repositioned cancer drug, J Immunol Balt Md,
doi:10.4049/jimmunol.1700965Kaur, Shekhar, Sharma, Sarma, Prakash et al., Ivermectin as a potential drug for treatment of COVID-19: an in-sync review with clinical and computational attributes, Pharmacol Rep,
doi:10.1007/s43440-020-00195-yLayhadi, Turner, Crossman, Fountain, ATP evokes Ca2+ responses and CXCL5 secretion via P2X4 receptor activation in human monocyte-derived 23 macrophages, J Immunol Balt Md,
doi:10.4049/jimmunol.1700965Li, Zhao, Zhan, Quantitative proteomics reveals a broadspectrum antiviral property of Ivermectin, benefting for COVID19 treatment, J Cell Physiol
Mastrangelo, Ivermectin is a potent inhibitor of flavivirus replication specifically targeting NS3 helicase activity: new prospects for an old drug, J. Antimicrob. Chemother
Park, Iwasaki, Type I. and type III interferons-induction, signaling, evasion, and application to combat COVID-19, Cell Host Microbe
Scheim, Ivermectin for COVID 19 treatment Clinical response at quasithreshold doses via hypothesized alleviation of CD147 mediated vascular occlusive
Wagstaff, Ivermectin is a specific inhibitor of importin α/βmediated nuclear import able to inhibit replication of HIV-1 and dengue virus, Biochem. J
Yan, Ci, Chen, Anti-Inflammatory effects of Ivermectin in mouse model of allergic asthma, athway. Fundam Clin Pharm
Yang, Permethrin and ivermectin modulate lipid metabolism in steatosis-induced HepG2 hepatocyte, Food and Chemical Toxicology,
doi:10.1016/j.fct.2019.02.005Zaidi, Dehgani-Mobaraki, The mechanisms of action of Ivermectin against SARS-CoV-2: An evidence-based clinical review article, J Antibiot,
doi:10.1038/s41429-021-00430-5Zaidi, Dehgani-Mobaraki, The mechanisms of action of Ivermectin against SARS-CoV-2: An evidence-based clinical review article, J Antibiot,
doi:10.1038/s41429-021-00430-5Zhang, Song, Ci, Ivermectin inhibits LPS-induced production of inflammatory cytokines and improves LPS-induced survival in mice, Inflamm Res,
doi:10.1007/s00011-008-8007-8DOI record:
{
"DOI": "10.13140/RG.2.2.26793.52327",
"URL": "http://rgdoi.net/10.13140/RG.2.2.26793.52327",
"author": [
{
"family": "Kerr",
"given": "Lucy"
},
{
"family": "Baldi",
"given": "Fernando"
},
{
"literal": "Raysildo Barbosa Lôbo"
},
{
"literal": "Washington Luiz Olivato Assagra"
},
{
"family": "Proença",
"given": "Fernando Carlos"
},
{
"family": "Hibberd",
"given": "Jennifer A"
},
{
"family": "Chamie",
"given": "Juan"
},
{
"family": "Kory",
"given": "Pierre"
},
{
"literal": "Cadegiani Flávio"
}
],
"container-title": "Unpublished",
"id": "https://doi.org/10.13140/rg.2.2.26793.52327",
"issued": {
"date-parts": [
[
2021
]
]
},
"language": "en",
"publisher": "Unpublished",
"title": "COVID-19 In-Hospital Mortality Rate is Reduced by Prophylactic Use of Ivermectin: Findings From a City-Wide, Prospective Observational Study Using Propensity Score Matching (PSM)",
"type": "article-journal"
}