Efficacy and safety of oral ivermectin in the treatment of mild to moderate Covid-19 patients: a multi-centre double-blind randomized controlled clinical trial
Ananda Wijewickrema, Hasini Banneheke, Arunasalam Pathmeswaran, Fathima Wardha Refai, Malika Kauranaratne, Neelika Malavige, Chandima Jeewandara, Mahendra Ekanayake, Dilhar Samaraweera, Dhanusha Thambavita, Priyadarshani Galappatthy
BMC Infectious Diseases, doi:10.1186/s12879-024-09563-y
Background Evidence on ivermectin as a treatment for Covid-19 is controversial. A Cochrane review concluded that the efficacy and safety of ivermectin is uncertain (evidence up to April 2022) and WHO recommended its use only in the setting of clinical trials. This study aimed to assess the efficacy and safety of oral ivermectin in hospitalized patients with mild to moderate Covid-19. Trial design and methods A double-blind, randomized placebo-controlled clinical trial was conducted among RT-PCR-confirmed, adults, hospitalised within the first four days of symptoms. Patients received oral ivermectin 24 mg or placebo daily for five days. RT-PCR was repeated on days five and ten. Clinical progression was monitored using the World Health Organization Clinical Progression Scale. Serum ivermectin levels were measured on days three, five, and seven. The primary outcome was the difference in the viral load between day zero and ten in the two groups.
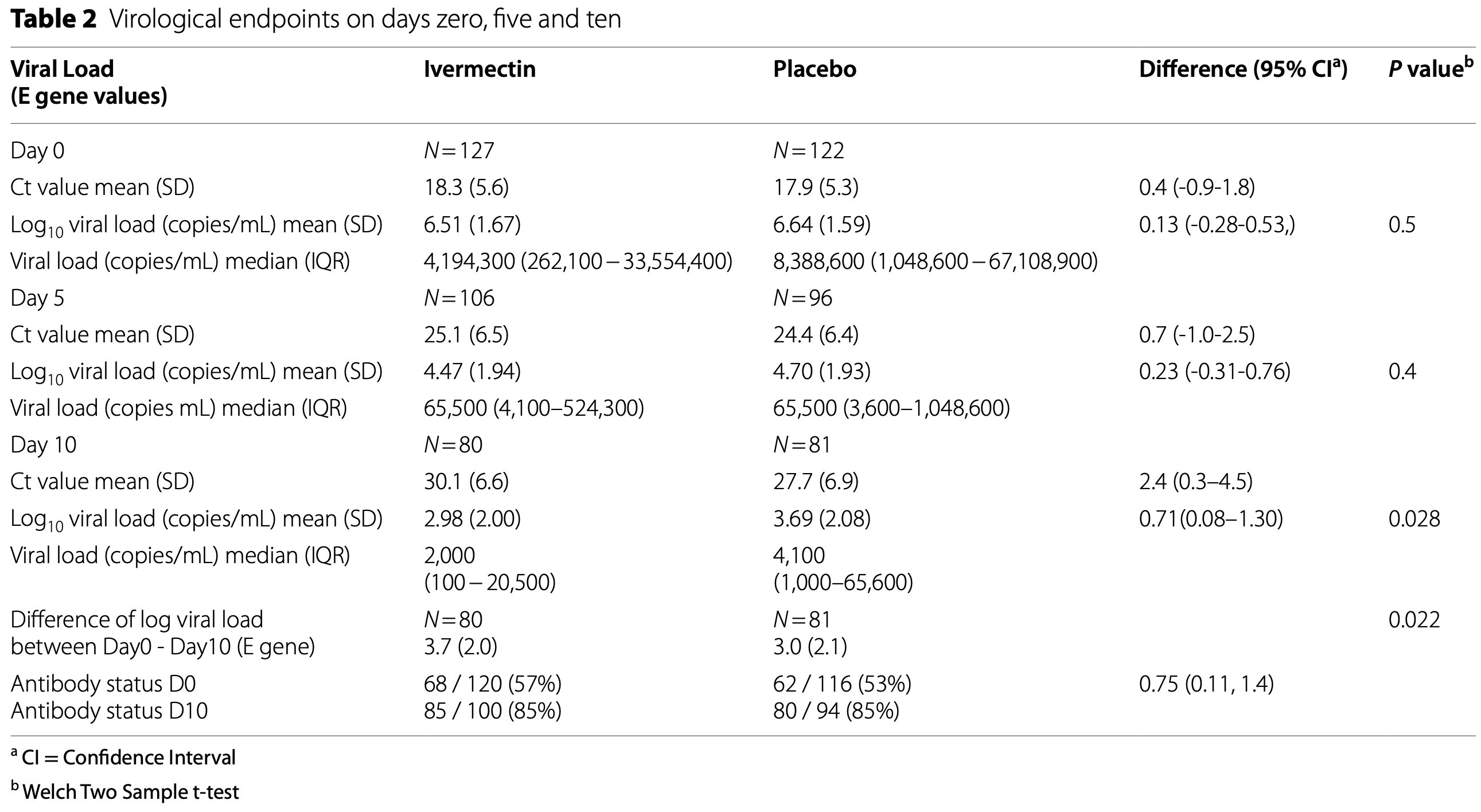
Results Out of 1699 patients screened, 249 underwent randomization and 127 received ivermectin, and 122 placebo. D10 median viral load for E gene (IQR) was 2,000 copies/mL (100 -20,500) with ivermectin (n = 80) and 4,100 copies/ mL (1,000-65,600) with placebo (n = 81, p = 0.028), per protocol analysis. The difference in Log viral load between day zero and ten between ivermectin and placebo was 3.72 and 2.97 respectively (p = 0.022). There was no significant difference in the WHO clinical progression scale or the adverse effects. Ivermectin blood levels taken before or with meals were not significantly different. Only 7 and 17 patients achieved blood levels above 160ng/ML and 100ng/ML respectively and they did not achieve a significantly lower viral load.
Conclusion Although ivermectin resulted in statistically significant lower viral load in patients with mild to moderate Covid-19, it had no significant effect on clinical symptoms.
and 81 (placebo) patients. Table 2 presents the difference in Log 10 viral load (copies/mL) on day 10; the post hoc power is 60%, which is lower than the planned conventional 80%, as we did not achieve the calculated sample size. However, this is unlikely to have affected the findings of this trial as we have observed a significant reduction in the viral load in the ivermectin arm. A proportion of patients did not have day 28 followup clinical information as they were not contactable over the phone after discharge. Since all patients were
Availability of data and materials The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations Ethics approval and consent to participate Ethics approval was obtained from the Ethics Review Committee of the Faculty of Medicine, University of Colombo (EC-21-EM02). All methods were carried out in accordance with relevant guidelines and regulations stated in the Declaration of Helsinki
Consent for publication Not applicable.
Competing interests The authors declare no competing interests.
Publisher's Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Ahmed, Karim, Ross, Hossain, Clemens et al., A five-day course of ivermectin for the treatment of COVID-19 may reduce the duration of illness, Int J Infect Dis IJID Off Publ Int Soc Infect Dis
Bernigaud, Guillemot, Ahmed-Belkacem, Grimaldi-Bensouda, Lespine et al., Oral ivermectin for a scabies outbreak in a longterm care facility: potential value in preventing COVID-19 and associated mortality, Br J Dermatol
Biber, Harmelin, Ram, Shaham, Nemet, The effect of ivermectin on the viral load and culture viability in early treatment of nonhospitalized patients with mild COVID-19 -a double-blind, randomized placebo-controlled trial, Int J Infect Dis IJID off Publ Int Soc Infect Dis
Bryant, Lawrie, Dowswell, Fordham, Mitchell et al., Ivermectin for Prevention and Treatment of COVID-19 Infection: A Systematic Review, Meta-analysis, and Trial Sequential Analysis to Inform Clinical Guidelines, Am J Ther
Buonfrate, Chesini, Martini, Roncaglioni, Fernandez et al., High-dose ivermectin for early treatment of COVID-19 (COVER study): a randomised, double-blind, multicentre, phase II, dose-finding, proof-of-concept clinical trial, Int J Antimicrob Agents
Caly, Druce, Catton, Jans, Wagstaff, The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro, Antiviral Res
Castañeda-Sabogal, Chambergo-Michilot, Toro-Huamanchumo, Silva-Rengifo, Gonzales-Zamora et al., Outcomes of Ivermectin in the treatment of COVID-19: a systematic review and meta-analysis
Chaccour, Casellas, Blanco-Di Matteo, Pineda, Fernandez-Montero et al., The effect of early treatment with ivermectin on viral load, symptoms and humoral response in patients with non-severe COVID-19: a pilot, double-blind, placebo-controlled, randomized clinical trial, EClinicalMedicine
Chen, Nirula, Heller, Gottlieb, Boscia et al., SARS-CoV-2 neutralizing antibody LY-CoV555 in outpatients with Covid-19, N Engl J Med
De La Rocha, Cid-López, Venegas-López, Sc, Sánchez-Ortiz et al., Ivermectin compared with placebo in the clinical course in Mexican patients with asymptomatic and mild COVID-19: a randomized clinical trial, BMC Infect Dis
Elgazzar, Hany, Youssef, Hany, Hafez et al., Efficacy and safety of Ivermectin for treatment and prophylaxis of COVID-19 pandemic, Res Sq,
doi:10.21203/rs.3.rs-100956/v1Gonzalez, Gámez, Enciso, Maldonado, Palacios et al., Efficacy and Safety of Ivermectin and Hydroxychloroquine in Patients with Severe COVID-19: A Randomized Controlled Trial, Infect Dis Rep
Guzzo, Furtek, Porras, Chen, Tipping et al., Safety, tolerability, and pharmacokinetics of escalating high doses of ivermectin in healthy adult subjects, J Clin Pharmacol
Heidary, Gharebaghi, Ivermectin: a systematic review from antiviral effects to COVID-19 complementary regimen, J Antibiot
Krolewiecki, Lifschitz, Moragas, Travacio, Valentini et al., Antiviral effect of high-dose ivermectin in adults with COVID-19: a proof-of-concept randomized trial, EClinicalMedicine
Lamontagne, Stegemann, Agarwal, Agoritsas, Siemieniuk et al., A living WHO guideline on drugs to prevent covid-19, BMJ
Lim, Hor, Tay, Jelani, Tan et al., Efficacy of ivermectin treatment on disease progression among adults with mild to moderate COVID-19 and comorbidities: the I-TECH randomized clinical trial, JAMA Intern Med
López-Medina, López, Hurtado, Dávalos, Ramirez et al., Effect of ivermectin on time to resolution of symptoms among adults with mild COVID-19: a randomized clinical trial, JAMA
Marshall, Murthy, Diaz, Adhikari, Angus et al., A minimal common outcome measure set for COVID-19 clinical research, Lancet Infect Dis
Mohan, Tiwari, Suri, Mittal, Patel et al., Ivermectin in mild and moderate COVID-19 (RIVET-COV): a randomized, placebo-controlled trial, Res Sq,
doi:10.21203/rs.3.rs-191648/v1Naggie, Boulware, Lindsell, Stewart, Gentile et al., Effect of ivermectin vs placebo on time to sustained recovery in outpatients with mild to moderate COVID-19: a randomized clinical trial, JAMA
Navarro, Camprubí, Requena-Méndez, Buonfrate, Giorli et al., Safety of high-dose ivermectin: a systematic review and meta-analysis, J Antimicrob Chemother
Niaee, Gheibi, Namdar, Allami, Zolghadr et al., Ivermectin as an adjunct treatment for hospitalized adult COVID-19 patients: a randomized multi-center clinical trial, Res Sq,
doi:10.21203/rs.3.rs-109670/v1Pushpakumara, Jeewandara, Bary, Madushanka, Perera et al., Identification of differences in the magnitude and specificity of SARS-CoV-2 nucleocapsid antibody responses in naturally infected and vaccinated individuals, Clin Exp Immunol
Ranasinghe, Jayathilaka, Jeewandara, Gunasinghe, Ariyaratne et al., Molecular epidemiology of AY.28 and AY.104 delta sublineages in Sri Lanka, Front Public Heal
Reis, Silva, Silva, Thabane, Milagres et al., Effect of early treatment with ivermectin among patients with Covid-19, N Engl J Med
Thakur, Datusalia, Kumar, Use of steroids in COVID-19 patients: a meta-analysis, Eur J Pharmacol
Vallejos, Zoni, Bangher, Villamandos, Bobadilla et al., Ivermectin to prevent hospitalizations in patients with COVID-19 (IVERCOR-COVID19) a randomized, double-blind, placebo-controlled trial, BMC Infect Dis
Wagner, Griesel, Mikolajewska, Metzendorf, Fischer et al., Systemic corticosteroids for the treatment of COVID-19: Equity-related analyses and update on evidence, Cochrane Database Syst Rev,
doi:10.1002/14651858.CD014963.pub2Who, WHO advises that ivermectin only be used to treat COVID-19 within clinical trials