Ivermectin Use Associated with Reduced Duration of Covid-19 Febrile Illness in a Community Setting
Muhammad Ishaq Ghauri2, Ali Nasir, Muslim Afsar1, Abbas1, Shariq Muhammad, Mukarram2, Yahya Muhammad, Khizra Ishaq1 Peracha1, Ishaq1, Muhammad Shariq Mukarram, Muhammad Abbas1, Shariq Mukarram, Iver
International Journal of Clinical Studies and Medical Case Reports, doi:10.46998/ijcmcr.2021.13.000320
Background: SARS-CoV-2 infection (COVID-19) is a potentially lethal disease that may progress into severe respiratory distress syndrome requiring ventilatory support. While azithromycin (AZI) and hydroxychloroquine (HCQ) are considered similar to placebo in COVID-19, other drugs such as ivermectin (IVER), are being repurposed to treat this pandemic. This study was designed to assess the effects of ivermectin on duration of febrile illness and disease outcomes in mild-to-moderate COVID-19 infection in a community setting.
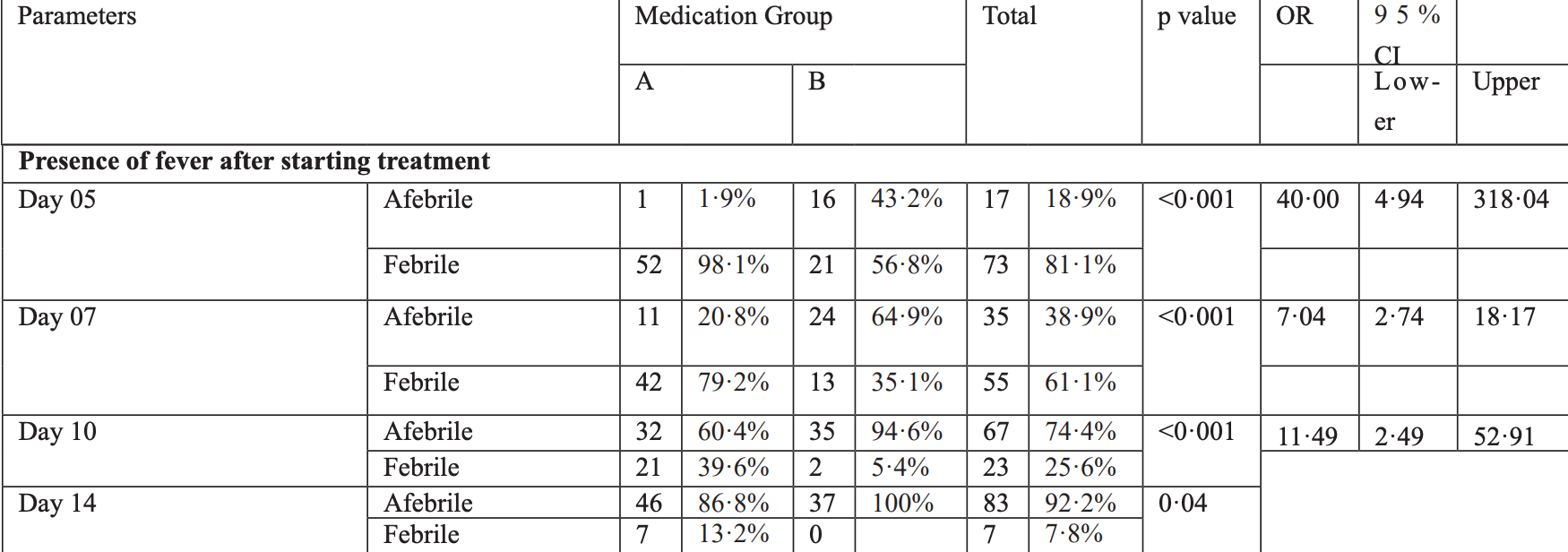
Methods: In this case-control study 95 suspected patients of mild-to-moderate COVID-19 were included. The controls (Group-A) received AZI+HCQ for seven days while the cases (Group-B) received IVER+AZI+HCQ for six days. Results: A total of 41 patients were in Group-B, while 54 patients were in Group-A. Group-B had consistently and significantly shorter span of fever on days 5, 7, 10 and 14, where the logistic regression showed IVER as the major (Exp B 49•55; p<0•001) underlying factor. The Kaplan-Meier survival analysis showed that Group-A had a prolonged febrile illness (p<0•001). Conclusions: Ivermectin use is associated with reduced duration of febrile illness in COVID-19 in outpatient setting, thus potentially saving precious lives, reducing direct load on healthcare facilities and preventing high cost of management in a community setting.
Supplementary Table: Logistic Regression Analysis
Conflict of Interest The authors state that there is no financial or any other conflict of interest in relation to this research.
Authors' Contribution NAA and MIG contributed equally. Both contributed in study design, data analysis, writing up the manuscript, overall supervision at their participating institutions respectively, and final approval. MA contributed in data entry and manuscript writing. MSM contributed in data collection and manuscript writing. MYP contributed in data entry and manuscript writing. KI contributed in data collection.
References
Anser, Yousaf, Khan, Social and administrative issues related to the COVID-19 pandemic in Pakistan: better late than never, Environ Sci Pollut Res Int,
doi:10.1007/s11356-020-10008-7Atif, Malik, Why is Pakistan vulnerable to COVID-19 associated morbidity and mortality? A scoping review, Int J Health Plann Manage,
doi:10.1002/hpm.3016Cavalcanti, Zampieri, Rosa, Hydroxychloroquine with or without Azithromycin in Mild-to-Moderate Covid-19, N Engl J Med,
doi:10.1056/NE-JMoa2019014Crump, Ivermectin: enigmatic multifaceted 'wonder' drug continues to surprise and exceed expectations, J Antibiot,
doi:10.1038/ja.2017.11Gabler, Keller, Prescriptions Surged as Trump Praised Drugs in Coronavirus Fight, NY Times
Heidary, Gharebaghi, Ivermectin: a systematic review from antiviral effects to COVID-19 complementary regimen, J Antibiot,
doi:10.1038/s41429-020-0336-zHozhabri, Sparascio, Sohrabi, The Global Emergency of Novel Coronavirus (SARS-CoV-2): An Update of the Current Status and Forecasting, Int J Environ Res Public Health,
doi:10.3390/ijerph17165648Hung, Lung, Tso, Triple combination of interferon beta-1b, lopinavir-ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: an open-label, randomised, phase 2 trial, Lancet,
doi:10.1016/S0140-6736(20)31042-4Kelleni, Nitazoxanide/azithromycin combination for COV-ID-19: A suggested new protocol for early management, Pharmacol Res,
doi:10.1016/j.phrs.2020.104874Kucirka, Lauer, Laeyendecker, Variation in False-Negative Rate of Reverse Transcriptase Polymerase Chain Reaction-Based SARS-CoV-2 Tests by Time Since Exposure, Ann Intern Med,
doi:10.7326/M20-1495Li, Zhao, Zhan, Quantitative proteomics reveals a broad-spectrum antiviral property of ivermectin, benefiting for COVID-19 treatment, J Cell Physiol,
doi:10.1002/jcp.30055Mahévas, Tran, Roumier, Clinical efficacy of hydroxychloroquine in patients with covid-19 pneumonia who require oxygen: observational comparative study using routine care data, BMJ,
doi:10.1136/bmj.m1844Malik, Ishraquzzaman, Kalimuddin, Clinical Presentation, Management and In-Hospital Outcome of Healthcare Personnel With COVID-19 Disease, Cureus,
doi:10.7759/cureus.10004Molina, Delaugerre, Goff, No evidence of rapid antiviral clearance or clinical benefit with the combination of hydroxychloroquine and azithromycin in patients with severe COVID-19 infection, Med Mal Infect,
doi:10.1016/j.medmal.2020.03.006Muñoz, Ballester, Antonijoan, Safety and pharmacokinetic profile of fixed-dose ivermectin with an innovative 18mg tablet in healthy adult volunteers, PLoS Negl Trop Dis,
doi:10.1371/journal.pntd.0006020Pandey, Nikam, Shreya, Potential therapeutic targets for combating SARS-CoV-2: Drug repurposing, clinical trials and recent advancements, Life Sci,
doi:10.1016/j.lfs.2020.117883Patrì, Fabbrocini, Hydroxychloroquine and ivermectin: A synergistic combination for COVID-19 chemoprophylaxis and treatment?, J Am Acad Dermatol,
doi:10.1016/j.jaad.2020.04.017Rajter, Sherman, Fatteh, Use of Ivermectin is Associated with Lower Mortality in Hospitalized Patients with COVID-19 (ICON study), Chest,
doi:10.1016/j.chest.2020.10.009Rizzo, Ivermectin, antiviral properties and COVID-19: a possible new mechanism of action, Naunyn Schmiedebergs Arch Pharmacol,
doi:10.1007/s00210-020-01902-5Savarino, Trani, Donatelli, Design and derivatives with antimalarial and antiviral activities, Infect Dis,
doi:10.1021/jm0601856Sciama, Is France's president fueling the hype over an unproven coronavirus treatment?, Science
Stokes, Zambrano, Anderson, Coronavirus Disease 2019 Case Surveillance -United States, January 22, Morb Mortal Wkly Rep,
doi:10.15585/mmwr.mm6924e2Tang, Cao, Han, Hydroxychloroquine in patients with mainly mild to moderate coronavirus disease 2019: open label, randomised controlled trial, BMJ,
doi:10.1136/bmj.m1849Wamae, Mass Drug Administration and Worms Experience in Africa: Envisage Repurposing Ivermectin for SARS-COV-2, Am J Trop Med Hyg,
doi:10.4269/ajtmh.20-0295Worldometers, Info, None
DOI record:
{
"DOI": "10.46998/ijcmcr.2021.13.000320",
"ISSN": [
"2692-5877"
],
"URL": "http://dx.doi.org/10.46998/IJCMCR.2021.13.000320",
"author": [
{
"affiliation": [],
"family": "Mukarram",
"given": "Muhammad Shariq ",
"sequence": "first"
}
],
"container-title": "International Journal of Clinical Studies and Medical Case Reports",
"container-title-short": "IJCMCR",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2021,
10,
29
]
],
"date-time": "2021-10-29T06:23:56Z",
"timestamp": 1635488636000
},
"deposited": {
"date-parts": [
[
2021,
10,
29
]
],
"date-time": "2021-10-29T06:23:57Z",
"timestamp": 1635488637000
},
"indexed": {
"date-parts": [
[
2024,
3,
28
]
],
"date-time": "2024-03-28T16:44:31Z",
"timestamp": 1711644271480
},
"is-referenced-by-count": 1,
"issue": "4",
"issued": {
"date-parts": [
[
2021,
10,
7
]
]
},
"journal-issue": {
"issue": "4",
"published-online": {
"date-parts": [
[
2021,
10,
7
]
]
}
},
"member": "26450",
"original-title": [],
"prefix": "10.46998",
"published": {
"date-parts": [
[
2021,
10,
7
]
]
},
"published-online": {
"date-parts": [
[
2021,
10,
7
]
]
},
"publisher": "International Journal of Clinical Studies and Medical Case Reports",
"reference-count": 0,
"references-count": 0,
"relation": {},
"resource": {
"primary": {
"URL": "https://ijclinmedcasereports.com/ijcmcr-ra-id-00320/"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Electrical and Electronic Engineering",
"Atomic and Molecular Physics, and Optics"
],
"subtitle": [],
"title": "Ivermectin Use Associated with Reduced Duration of Covid-19 Febrile Illness in a Community Setting",
"type": "journal-article",
"volume": "13"
}