Ivermectin systemic availability in adult volunteers treated with different oral pharmaceutical formulations
L Ceballos, L Alvarez, A Lifschitz, C Lanusse
Biomedicine & Pharmacotherapy, doi:10.1016/j.biopha.2023.114391
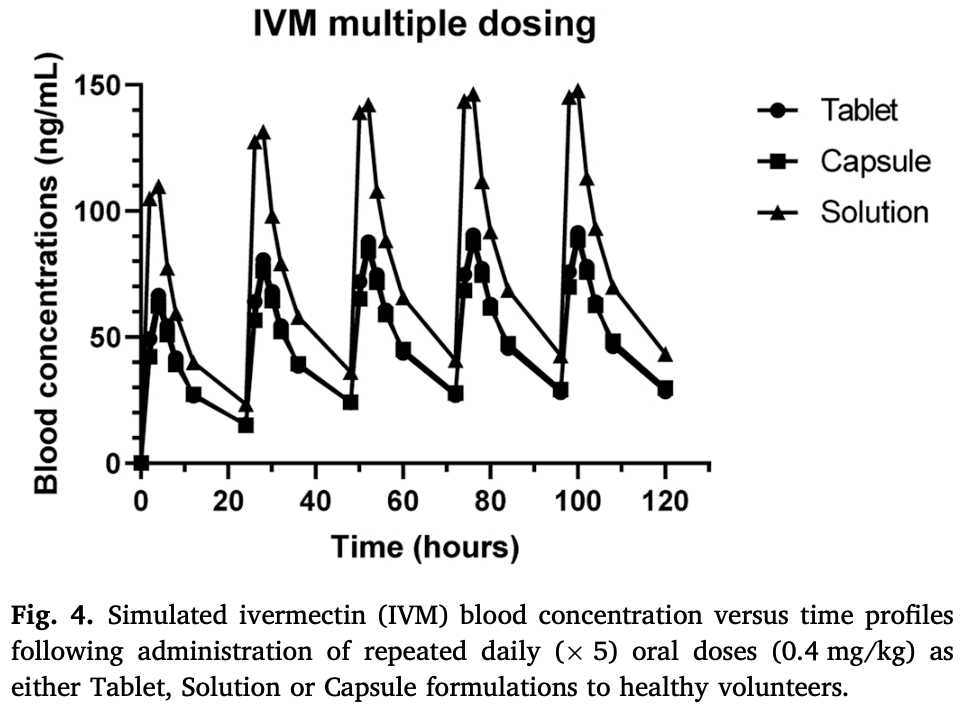
Ivermectin (IVM) is currently approved as an antiparasitic agent for human use in the treatment of onchocerciasis, lymphatic filariasis, strongyloidiasis, scabies, and pediculosis. Recent findings indicate that IVM may reach other pharmacological targets, which accounts for its proven anti-inflammatory/immunomodulatory, cytostatic, and antiviral effects. However, little is known about the assessment of alternative drug formulations for human use. Objective: To compare the systemic availability and disposition kinetics of IVM orally administered as different pharmaceutical formulations (tablet, solution, or capsule) to healthy adults. Experimental design/main findings: Volunteers were randomly assigned to 1 of 3 experimental groups and orally treated with IVM as either, a tablet, solution, or capsules at 0.4 mg/kg in a three-phase crossover design. Blood samples were taken as dried blood spots (DBS) between 2 and 48 h post-treatment and IVM was analyzed by HPLC with fluorescence detection. IVM Cmax value was higher (P < 0.05) after the administration of the oral solution compared to treatments with both solid preparations. The oral solution resulted in a significantly higher IVM systemic exposure (AUC: 1653 ng h/mL) compared to the tablet (1056 ng h/mL) and capsule (996 ng h/mL) formulations. The simulation of a 5-day repeated administration for each formulation did not show a significant systemic accumulation. Conclusion: Beneficial effects against systemically located parasitic infections as well as in any other potential therapeutic field of IVM application would be expected from its use in the form of oral solution. This pharmacokinetic-based therapeutic advantage without the risk of excessive accumulation needs to be corroborated in clinical trials specifically designed for each purpose.
Conflict of interest statement Please declare any financial or personal interests that might be potentially viewed to influence the work presented. Interests could include consultancies, honoraria, patent ownership or other. If there are none state 'there are none'.
References
Allen, MODFIT: a pharmacokinetics computer program, Biopharm. Drug Dispos
Alvarez, Imperiale, Sanchez, Murno, Lanusse, Uptake of albendazole and albendazole sulphoxide by Haemonchus contortus and Fasciola hepatica in sheep, Vet. Parasitol
Ashburn, Drug repositioning: identifying and developing new uses for existing drugs, Nat. Rev. Drug Discov
Ashraf, Prichard, IVM exhibits potent antimitotic activity, Vet. Parasitol
Baraka, Mahmoud, Marschke, Geary, Homeida et al., Ivermectin distribution in the plasma and tissues of patients infected with Onchocerca volvulus, Eur. J. Clin. Pharm
Botta, Rivara, Zuliani, Radi, Drug repurposing approaches to fight Dengue virus infection and related diseases, Front Biosci,
doi:10.2741/4630Campbell, Fisher, Stapley, Albersschonberg, Jacob, Ivermectin: a potent new antiparasitic agent, Science
Ceballos, Elissondo, Bruni, Denegri, Alvarez et al., Flubendazole in cystic echinococcosis therapy: pharmaco-parasitological evaluation in mice, Parasitol. Int,
doi:10.1016/j.parint.2009.07.006Chaccour, Lines, Whitty, Effect of ivermectin on Anopheles gambiae mosquitoes fed on humans; the potential of oral insecticides in malaria control, J. Infect. Dis
Dupuy, Derlon, Sutra, Cadiergues, Franc et al., Pharmacokinetics of selamectin in dogs after topical application, Vet. Res
Duthaler, Leisegang, Karlsson, Krähenbühl, Hammann, The effect of food on the pharmacokinetics of oral ivermectin, J. Antimicrob. Chemother,
doi:10.1093/jac/dkz466Echazú, Juarez, Vargas, Cajal, Cimino et al., Albendazole and ivermectin for the control of soil-transmitted helminths in an area with high prevalence of Strongyloides stercoralis and hookworm in northwestern Argentina: a community-based pragmatic study, PLoS Negl. Trop. Dis,
doi:10.1371/journal.pntd.0006003Edwards, Dingsdale, Helsby, Orme, Breckenridge, The relative systemic availability of ivermectin after administration as capsule, tablet, and oral solution, Eur. J. Clin. Pharm,
doi:10.1007/BF00637608Errecalde, Lifschitz, Vecchioli, Ceballos, Errecalde et al., Safety and pharmacokinetic assessments of a novel ivermectin nasal spray formulation in a pig model, J. Pharm. Sci,
doi:10.1016/j.xphs.2021.01.017Foy, Kobylinski, Da Silva, Rasgon, Sylla, Endectocides for malaria control, Trends Parasitol
González-Canga, Fernández-Martínez, Sahagún-Prieto, Diez-Liébana, Sierra-Vega et al., A review of the pharmacological interactions of ivermectin in several animal species, Curr. Drug Metab,
doi:10.2174/138920009788498969Guzzo, Furtek, Porras, Safety, tolerability, and pharmacokinetics of escalating high doses of ivermectin in healthy adult subjects, J. Clin. Pharm,
doi:10.1177/009127002401382731Health Organization, Summary of global update on implementation of preventive chemotherapy against neglected tropical diseases in 2019, Wkly. Epiemiol. Rec
Hennessy, Ali, Sillince, The effect of a short-term reduction in feed on the pharmacokinetics and efficacy of albendazole in sheep, Aust. Vet. J
Homeida, Malcolm, Eltayeb, Eversole, Elassad et al., The lack of influence of food and local alcoholic brew on the blood level of Mectizan(®) (ivermectin), Acta Trop,
doi:10.1016/j.actatropica.2013.03.019Ich, ICH Q2 (R1) Harmonised tripartite guideline validation of analytical procedures: text and methodology
Intuyod, Hahnvajanawong, Pinlaor, Pinlaor, Anti-parasitic drug ivermectin exhibits potent anticancer activity against gemcitabine-resistant cholangiocarcinoma in vitro, Anticancer Res,
doi:10.21873/anticanresJuarez, Schcolnik-Cabrera, Dominguez-Gomez, Chavez-Blanco, Diaz-Chavez et al., Antitumor effects of ivermectin at clinically feasible concentrations support its clinical development as a repositioned cancer drug, Cancer Chemother. Pharm,
doi:10.1007/s00280-020-04041-zKnopp, Mohammed, Speich, Hattendorf, Khamis et al., Albendazole and mebendazole administered alone or in combination with ivermectin against Trichuris trichiura: a randomized controlled trial, Clin. Infect. Dis,
doi:10.1086/657310Kobylinski, Sylla, Chapman, Sarr, Foy, Ivermectin mass drug administration for humans disrupts malaria parasite transmission in Senegalese villages, Am. J. Trop. Med. Hyg
Krolewiecki, Lifschitz, Moragas, Travacio, Valentini et al., Antiviral effect of high-dose ivermectin in adults with COVID-19: a proof-of-concept randomized trial, EClinicalMedicine,
doi:10.1016/j.eclinm.2021.100959Lanusse, Lifschitz, Virkel, Alvarez, Sánchez et al., Comparative plasma disposition kinetics of ivermectin, moxidectin and doramectin in cattle, J. Vet. Pharm. Ther,
doi:10.1046/j.1365-2885.1997.00825.xLanusse, Prichard, Relationship between pharmacological properties and clinical efficacy of ruminant anthelmintics, Vet. Parasitol,
doi:10.1016/0304-4017(93)90115-4Lifschitz, Ballent, Virkel, Sallovitz, Lanusse, Sex-related differences in the gastrointestinal disposition of ivermectin in the rat: P-glycoprotein involvement and itraconazole modulation, J. Pharm. Pharm,
doi:10.1211/jpp.58.8.0005Lifschitz, Virkel, Sallovitz, Sutra, Galtier et al., Comparative distribution of ivermectin and doramectin to parasite location tissues in cattle, Vet. Parasitol,
doi:10.1016/s0304-4017(99)00175-2Lim, Vilcheze, Ng, Jacobs, García et al., Anthelmintic avermectins kill Mycobacterium tuberculosis, including multidrugresistant clinical strains, Antimicrob. Agents Chemother
Lloberas, Alvarez, Entrocasso, Virkel, Lanusse et al., Measurement of ivermectin concentrations in target worms and host gastrointestinal tissues: influence of the route of administration on the activity against resistant Haemonchus contortus in lambs, Exp. Parasitol,
doi:10.1016/j.exppara.2012.04.014Lo, Fink, Williams, Blodinger, Pharmacokinetic studies of ivermectin: effects of formulation, Vet. Res. Commun,
doi:10.1007/BF02215150Long, Ren, Li, Zeng, Human pharmacokinetics of orally taken ivermectin, Chin. J. Clin. Pharm
Matamoros, Sánchez, Gabrie, Juárez, Ceballos et al., Efficacy and safety of albendazole and high-dose ivermectin co-administration in school-aged children infected with Trichuris trichiura in Honduras: a randomized controlled trial, Clin. Infect. Dis,
doi:10.1093/cid/ciab365Muñoz, Ballester, Antonijoan, Gich, Rodríguez et al., Safety and pharmacokinetic profile of fixed-dose ivermectin with an innovative 18mg tablet in healthy adult volunteers, PLoS Negl. Trop. Dis,
doi:10.1371/journal.pntd.0006020Navarro, Camprubí, Requena-Méndez, Buonfrate, Giorli et al., Safety of high-dose ivermectin: a systematic review and meta-analysis, J. Antimicrob. Chemother,
doi:10.1093/jac/dkz524Panchal, Rawat, Kumar, Kibria, Singh et al., Plasmodium falciparum signal recognition particle components and antiparasitic efect of ivermectin in blocking nucleocytoplasmic shuttling of SRP, Cell Death Dis
Rajter, Sherman, Fatteh, Vogel, Sacks et al., Use of ivermectin is associated with lower mortality in hospitalized patients with coronavirus disease 2019: the ivermectin in COVID nineteen study, Chest,
doi:10.1016/j.chest.2020.10.009Sajid, Iqbal, Muhammad, Immunomodulatory effect of various anti-parasitics: a review, Parasitology
Saumell, Lifschitz, Baroni, Fusé, Bistoletti et al., The route of administration drastically affects ivermectin activity against small strongyles in horses, Vet. Parasitol,
doi:10.1016/j.vetpar.2017.01.025Schinkel, Smit, Vantelligen, Beijnen, Wagenaar et al., Disruption of the mouse mdr1a P-glycoprotein gene leads to a deficiency in the bloodbrain barrier and to increased sensitivity to drugs, Cell
Schulz, Coulibaly, Schindler, Wimmersberger, Keiser, Pharmacokinetics of ascending doses of ivermectin in Trichuris trichiura-infected children aged 2-12 years, J. Antimicrob. Chemother,
doi:10.1093/jac/dkz083Sharmeen, Skrtic, Sukhai, Hurren, Gronda et al., The antiparasitic agent ivermectin induces chloridedependent membrane hyperpolarization and cell death in leukemia cells, Blood,
doi:10.1182/blood-2010-01-262675Smit, Ochomo, Waterhouse, Kwambai, Abong'o et al., Pharmacokinetics-pharmacodynamics of high-dose ivermectin with dihydroartemisinin-piperaquine on mosquitocidal activity and QT-prolongation (IVERMAL), Clin. Pharm. Ther,
doi:10.1002/cpt.1219Smit, Ochomo, Waterhouse, Kwambai, Abong'o et al., Pharmacokinetics-pharmacodynamics of high-dose ivermectin with dihydroartemisinin-piperaquine on mosquitocidal activity and QT-prolongation (IVERMAL), Clin. Pharm. Ther,
doi:10.1002/cpt.1219Suputtamongkol, Avirutnan, Mairiang, Angkasekwinai, Niwattayakul et al., Ivermectin accelerates circulating nonstructural protein NS1 clerance in adult dengue patients: a combined phase 2/3 randomized double-blinded placebo controlled trial, Clin. Infect. Dis,
doi:10.1093/cid/ciaa1332Tay, Fraser, Chan, Moreland, Rathore et al., Nuclear localization of dengue virus (DENV) 1-4 nonstructural protein 5; protection against all 4 DENV serotypes by the inhibitor IVM, Antivir. Res
Toutain, Upson, Terhune, Mckenzie, Comparative pharmacokinetics of doramectin and Ivermectin in cattle, Vet. Parasitol
Wicks, Kaye, Weatherley, Lewis, Davison et al., Effect of formulation on the pharmacokinetics and efficacy of doramectin, Vet. Parasitol
Yang, Atkinson, Wang, Lee, Bogoyevitch et al., The broad spectrum antiviral ivermectin targets the host nuclear transport importin α/β1 heterodimer, Antivir. Res,
doi:10.1016/j.antiviral.2020.104760Zabala, Vazquez-Villoldo, Rissiek, Gejo, Martin et al., P2 × 4 receptor controls microglia activation and favors remyelination in autoimmune encephalitis, EMBO Mol. Med,
doi:10.15252/emmm.201708743Zhang, Song, Ci, An, Ju et al., Ivermectin inhibits LPS-induced production of inflammatory cytokines and improves LPS-induced survival in mice, Inflamm. Res,
doi:10.1007/s00011-008-8007-8