Ivermectin for Treatment of Mild-to-Moderate COVID-19 in the Outpatient Setting: A Decentralized, Placebo-controlled, Randomized, Platform Clinical Trial
MD, MHS Susanna Naggie
doi:10.1101/2022.06.10.22276252
Background: The effectiveness of ivermectin to shorten symptom duration or prevent hospitalization among outpatients in the United States with mild-to-moderate symptomatic coronavirus disease 2019 (COVID-19) is unknown.
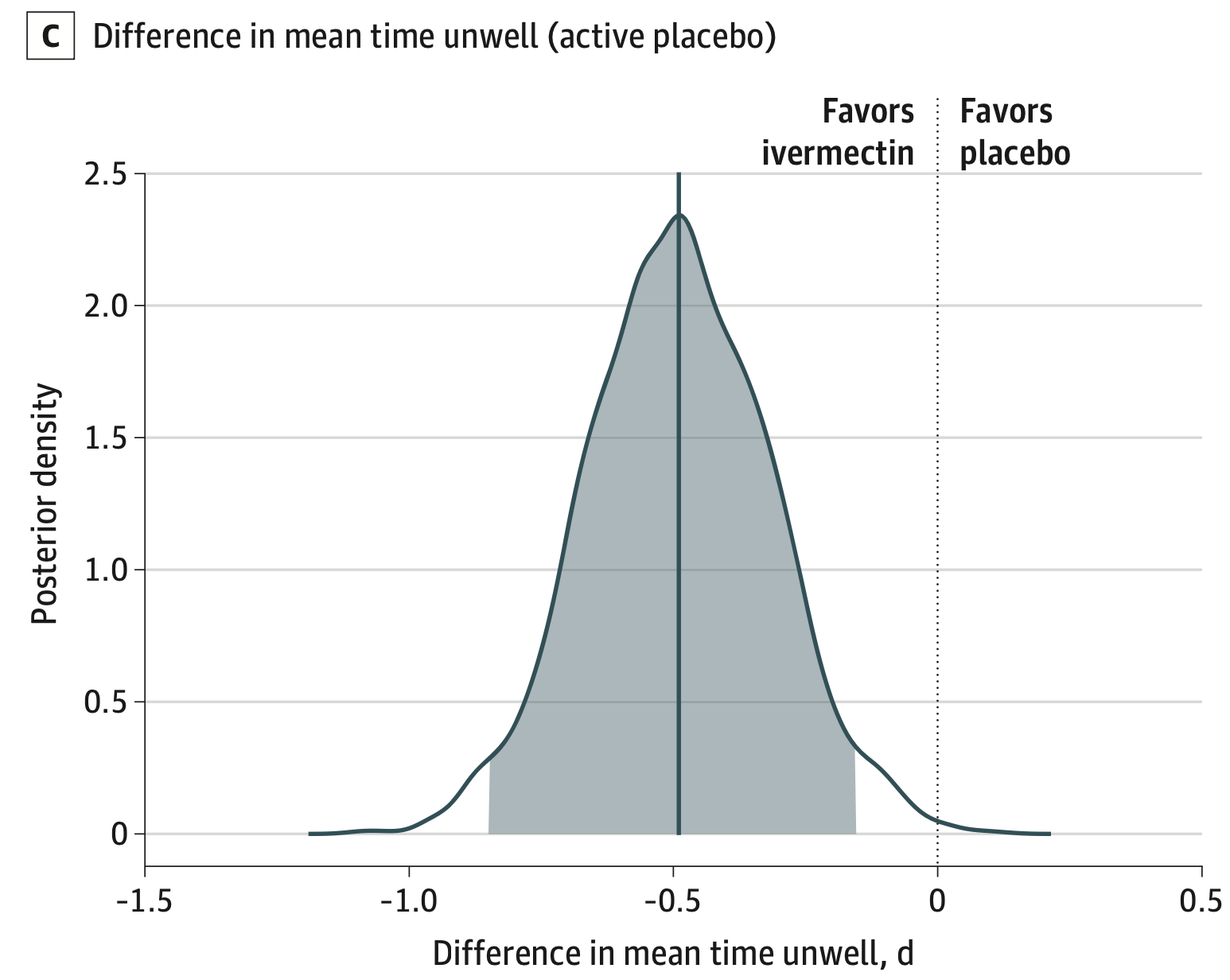
Objective: We evaluated the efficacy of ivermectin 400 µg/kg daily for 3 days compared with placebo for the treatment of early mild-to-moderate COVID-19. Methods: ACTIV-6 is an ongoing, decentralized, double-blind, randomized, placebo-controlled platform trial to evaluate repurposed therapies in outpatients with mild-to-moderate COVID-19. Non-hospitalized adults age ≥30 years with confirmed COVID-19, experiencing ≥2 symptoms of acute infection for ≤7 days were randomized to receive ivermectin 400 µg/kg daily for 3 days or placebo. The main outcome measure was time to sustained recovery, defined as achieving at least 3 consecutive days without symptoms. Secondary outcomes included a composite of hospitalization or death by day 28. Results: Of the 3457 participants who consented to be evaluated for inclusion in the ivermectin arm, 1591 were eligible for this study arm, randomized to receive ivermectin 400 µg/kg (n=817) or placebo (n=774), and received study drug. Of those enrolled, 47% reported receiving at least 2 doses of SARS-CoV-2 vaccination. The posterior probability for any improvement in time to recovery was 0.91 (hazard ratio 1.07, 95% credible interval 0.96-1.17). The posterior probability of this benefit exceeding 24 hours was less than 0.01, as measured by the difference in mean time unwell. Hospitalizations or deaths were uncommon (ivermectin [n=10]; placebo [n=9]). Ivermectin at 400 µg/kg was safe and without serious adverse events as compared with placebo (ivermectin [n=10]; placebo [n=9]). Conclusions: Ivermectin dosed at 400 µg/kg daily for 3 days resulted in less than one day of shortening of symptoms and did not lower incidence of hospitalization or death among outpatients with COVID-19 in the United States during the delta and omicron variant time periods.
Author Contributions Author and collaborator contributions, including responsibility for decision to submit the manuscript, drafting of the initial manuscript, study conceptualization, investigation, data curation, formal analysis, study supervision, and review and editing of the manuscript, are provided in the Online Supplement. CL and TS directly accessed and verified the underlying study data. SN and AFH had access to all the study data and had final responsibility for the decision to submit the paper for publication.
References
Bernal, Da Silva, Musungaie, Molnupiravir for Oral Treatment of Covid-19 in Nonhospitalized Patients, N Engl J Med
Bmi, >30 kg/m 2
Bmi, None, kg/m
Boulware, Pullen, Bangdiwala, A Randomized Trial of Hydroxychloroquine as Postexposure Prophylaxis for Covid-19, N Engl J Med
Caly, Druce, Catton, Jans, Wagstaff, The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro, Antiviral Res
Hammond, Leister-Tebbe, Gardner, Oral Nirmatrelvir for High-Risk, Nonhospitalized Adults with Covid-19, N Engl J Med
Hill, Mirchandani, Pilkington, Ivermectin for COVID-19: Addressing Potential Bias and Medical Fraud, Open Forum Infect Dis
Horby, Lim, Emberson, Dexamethasone in Hospitalized Patients with Covid-19, N Engl J Med
Kalil, Patterson, Mehta, Baricitinib plus Remdesivir for Hospitalized Adults with Covid-19, N Engl J Med
Pott-Junior, Paoliello, De, Miguel, Use of ivermectin in the treatment of Covid-19: A pilot trial, Toxicol Rep
Reis, Santos Moreira-Silva, Silva, Effect of early treatment with fluvoxamine on risk of emergency care and hospitalisation among patients with COVID-19: the TOGETHER randomised, platform clinical trial, Lancet Glob Health
Reis, Silva, Silva, Effect of Early Treatment with Ivermectin among Patients with Covid-19, N Engl J Med
Retraction Notice ; Elgazzar, Efficacy and safety of ivermectin for treatment and prophylaxis of COVID-19 pandemic, Res Square rs
Retraction Notice ; Kory, Meduri, Iglesias, Varon, Marik, Clinical and scientific rationale for the "MATH+" hospital treatment protocol for COVID-19, J Intensive Care Med,
doi:10.1177/08850666211049062Skipper, Pastick, Engen, Hydroxychloroquine in Nonhospitalized Adults With Early COVID-19 : A Randomized Trial, Ann Intern Med
Thorlund, Dron, Park, Hsu, Forrest et al., A real-time dashboard of clinical trials for COVID-19, Lancet Digit Health
Yu, Bafadhel, Dorward, Inhaled budesonide for COVID-19 in people at high risk of complications in the community in the UK (PRINCIPLE): a randomised, controlled, open-label, adaptive platform trial, Lancet
DOI record:
{
"DOI": "10.1001/jama.2022.18590",
"ISSN": [
"0098-7484"
],
"URL": "http://dx.doi.org/10.1001/jama.2022.18590",
"abstract": "<jats:sec><jats:title>Importance</jats:title><jats:p>The effectiveness of ivermectin to shorten symptom duration or prevent hospitalization among outpatients in the US with mild to moderate symptomatic COVID-19 is unknown.</jats:p></jats:sec><jats:sec><jats:title>Objective</jats:title><jats:p>To evaluate the efficacy of ivermectin, 400 μg/kg, daily for 3 days compared with placebo for the treatment of early mild to moderate COVID-19.</jats:p></jats:sec><jats:sec><jats:title>Design, Setting, and Participants</jats:title><jats:p>ACTIV-6, an ongoing, decentralized, double-blind, randomized, placebo-controlled platform trial, was designed to evaluate repurposed therapies in outpatients with mild to moderate COVID-19. A total of 1591 participants aged 30 years and older with confirmed COVID-19, experiencing 2 or more symptoms of acute infection for 7 days or less, were enrolled from June 23, 2021, through February 4, 2022, with follow-up data through May 31, 2022, at 91 sites in the US.</jats:p></jats:sec><jats:sec><jats:title>Interventions</jats:title><jats:p>Participants were randomized to receive ivermectin, 400 μg/kg (n = 817), daily for 3 days or placebo (n = 774).</jats:p></jats:sec><jats:sec><jats:title>Main Outcomes and Measures</jats:title><jats:p>Time to sustained recovery, defined as at least 3 consecutive days without symptoms. There were 7 secondary outcomes, including a composite of hospitalization or death by day 28.</jats:p></jats:sec><jats:sec><jats:title>Results</jats:title><jats:p>Among 1800 participants who were randomized (mean [SD] age, 48 [12] years; 932 women [58.6%]; 753 [47.3%] reported receiving at least 2 doses of a SARS-CoV-2 vaccine), 1591 completed the trial. The hazard ratio (HR) for improvement in time to recovery was 1.07 (95% credible interval [CrI], 0.96-1.17; posterior <jats:italic>P</jats:italic> value [HR &amp;gt;1] = .91). The median time to recovery was 12 days (IQR, 11-13) in the ivermectin group and 13 days (IQR, 12-14) in the placebo group. There were 10 hospitalizations or deaths in the ivermectin group and 9 in the placebo group (1.2% vs 1.2%; HR, 1.1 [95% CrI, 0.4-2.6]). The most common serious adverse events were COVID-19 pneumonia (ivermectin [n = 5]; placebo [n = 7]) and venous thromboembolism (ivermectin [n = 1]; placebo [n = 5]).</jats:p></jats:sec><jats:sec><jats:title>Conclusions and Relevance</jats:title><jats:p>Among outpatients with mild to moderate COVID-19, treatment with ivermectin, compared with placebo, did not significantly improve time to recovery. These findings do not support the use of ivermectin in patients with mild to moderate COVID-19.</jats:p></jats:sec><jats:sec><jats:title>Trial Registration</jats:title><jats:p>ClinicalTrials.gov Identifier: <jats:ext-link xmlns:xlink=\"http://www.w3.org/1999/xlink\" ext-link-type=\"uri\" xlink:href=\"https://clinicaltrials.gov/ct2/show/NCT04885530\">NCT04885530</jats:ext-link></jats:p></jats:sec>",
"author": [
{
"affiliation": [
{
"name": "Duke Clinical Research Institute, Duke University School of Medicine, Durham, North Carolina"
},
{
"name": "Department of Medicine, Duke University School of Medicine, Durham, North Carolina"
}
],
"family": "Naggie",
"given": "Susanna",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Division of Infectious Diseases and International Medicine, University of Minnesota, Minneapolis"
}
],
"family": "Boulware",
"given": "David R.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Biostatistics, Vanderbilt University Medical Center, Nashville, Tennessee"
}
],
"family": "Lindsell",
"given": "Christopher J.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Biostatistics, Vanderbilt University Medical Center, Nashville, Tennessee"
}
],
"family": "Stewart",
"given": "Thomas G.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Emergency Medicine, Lewis Katz School of Medicine at Temple University, Philadelphia, Pennsylvania"
}
],
"family": "Gentile",
"given": "Nina",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Biostatistics, Vanderbilt University Medical Center, Nashville, Tennessee"
}
],
"family": "Collins",
"given": "Sean",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medicine, Weill Cornell Medicine, New York, New York"
}
],
"family": "McCarthy",
"given": "Matthew William",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medicine, Miller School of Medicine, University of Miami, Miami, Florida"
}
],
"family": "Jayaweera",
"given": "Dushyantha",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Division of Pulmonary, Critical Care and Sleep Medicine, University of Missouri-Kansas City School of Medicine, Kansas City, Kansas"
}
],
"family": "Castro",
"given": "Mario",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Division of Infectious Diseases, Johns Hopkins University, Baltimore, Maryland"
}
],
"family": "Sulkowski",
"given": "Mark",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medicine, University of Pittsburgh Medical Center, Pittsburgh, Pennsylvania"
}
],
"family": "McTigue",
"given": "Kathleen",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Stakeholder Advisory Committee, Pittsburgh, Pennsylvania"
}
],
"family": "Thicklin",
"given": "Florence",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Duke Clinical Research Institute, Duke University School of Medicine, Durham, North Carolina"
},
{
"name": "Department of Medicine, Duke University School of Medicine, Durham, North Carolina"
}
],
"family": "Felker",
"given": "G. Michael",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Emergency Medicine, University of Colorado Denver-Anschutz, Denver"
}
],
"family": "Ginde",
"given": "Adit A.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Division of Infectious Diseases and International Medicine, University of Minnesota, Minneapolis"
}
],
"family": "Bramante",
"given": "Carolyn T.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Clinical Trials Center of Middle Tennessee, Franklin"
}
],
"family": "Slandzicki",
"given": "Alex J.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Focus Clinical Research Solutions, Charlotte, North Carolina"
}
],
"family": "Gabriel",
"given": "Ahab",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "NorthShore University HealthSystem, Evanston, Illinois"
}
],
"family": "Shah",
"given": "Nirav S.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medicine, Medical University of South Carolina, Charleston"
}
],
"family": "Lenert",
"given": "Leslie A.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "National Center for Advancing Translational Sciences, Bethesda, Maryland"
}
],
"family": "Dunsmore",
"given": "Sarah E.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Foundation for the National Institutes of Health, Bethesda, Maryland"
}
],
"family": "Adam",
"given": "Stacey J.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Duke Clinical Research Institute, Duke University School of Medicine, Durham, North Carolina"
}
],
"family": "DeLong",
"given": "Allison",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Biomedical Advanced Research and Development Authority, Washington, DC"
}
],
"family": "Hanna",
"given": "George",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Duke Clinical Research Institute, Duke University School of Medicine, Durham, North Carolina"
}
],
"family": "Remaly",
"given": "April",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Duke Clinical Research Institute, Duke University School of Medicine, Durham, North Carolina"
}
],
"family": "Wilder",
"given": "Rhonda",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Duke Clinical Research Institute, Duke University School of Medicine, Durham, North Carolina"
}
],
"family": "Wilson",
"given": "Sybil",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Health Outcomes & Biomedical Informatics, College of Medicine, University of Florida, Gainesville"
}
],
"family": "Shenkman",
"given": "Elizabeth",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Duke Clinical Research Institute, Duke University School of Medicine, Durham, North Carolina"
},
{
"name": "Department of Medicine, Duke University School of Medicine, Durham, North Carolina"
}
],
"family": "Hernandez",
"given": "Adrian F.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Vincent",
"given": "William (Kelly)",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Vincent",
"given": "Raina",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Bianchi",
"given": "Ray",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Premas",
"given": "Jen",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Cordero-Loperena",
"given": "Diana",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Rivera",
"given": "Evelyn",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Gupta",
"given": "Madhu",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Karawan",
"given": "Greg",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Ziomek",
"given": "Carey",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Arena",
"given": "Joseph",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "DeAlmeida",
"given": "Sonaly",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Ramin",
"given": "Soroush",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Nataraj",
"given": "Jaya",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Paasche-Orlow",
"given": "Michael",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Henault",
"given": "Lori",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Waite",
"given": "Katie",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Miller",
"given": "David",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Brounce",
"given": "Ginger",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "George-Adebayo",
"given": "Constance",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Adebayo",
"given": "Adeolu",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Wallan",
"given": "Jessica",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Slandzicki",
"given": "Alex",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Vogel",
"given": "Claudia",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Munoz",
"given": "Sebastian",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Kavtaradze",
"given": "David",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Watson",
"given": "Cassandra",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Singleton",
"given": "David",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Rivon",
"given": "Maria",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Sevier",
"given": "Amanda",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Del Pilar",
"given": "Arnold",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Spangler",
"given": "Amber",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Rao",
"given": "Sohail",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Cantu",
"given": "Luis",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Krishna",
"given": "Arvind",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Evans",
"given": "Kathy",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Falkner",
"given": "Tylene",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Kerr",
"given": "Brandi",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Spees",
"given": "Robert",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Marta",
"given": "Mailyn",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Felker",
"given": "G. Michael",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Harrington",
"given": "Amanda",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Dolor",
"given": "Rowena",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Frazier",
"given": "Madison",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Vergara",
"given": "Lorraine",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Wilson",
"given": "Jessica",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Burruss",
"given": "Valencia",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Hurst",
"given": "Terri",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Ofotokun",
"given": "Igho",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Bristow",
"given": "Laurel",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Prabhu",
"given": "Rajesh",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Klicka",
"given": "Krystal",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Lightfeather",
"given": "Amber",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "James",
"given": "Vicki",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Rogers",
"given": "Marcella",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Parihar",
"given": "Pradeep",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Torress",
"given": "De'Ambra",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Oragwu",
"given": "Chukwuemeka",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Oguego",
"given": "Ngozi",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Pillai",
"given": "Rajesh",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Juma",
"given": "Mustafa",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Gabriel",
"given": "Ahab",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Ghaly",
"given": "Emad",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Al-Haddadin",
"given": "Dafer",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Ramirez",
"given": "Courtney",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Hassanien",
"given": "Gammal",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Ismail",
"given": "Samah",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Meltzer",
"given": "Andrew",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Moran",
"given": "Seamus",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Brehaut",
"given": "Scott",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Roche",
"given": "Angelina",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Mehta",
"given": "Manisha",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Koppinger",
"given": "Nicole",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Baez",
"given": "Jose",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Pagan",
"given": "Ivone",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Abdelsayed",
"given": "Dallal",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Aziz",
"given": "Mina",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Robinson",
"given": "Philip",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Nguyen",
"given": "Julie",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Pardue",
"given": "Victoria",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Hammons",
"given": "Llisa",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Ruiz-Unger",
"given": "Juan",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Gonzalez",
"given": "Susan",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Reyes",
"given": "Lionel",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Cienki",
"given": "John",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Jimenez",
"given": "Gisselle",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Cohen",
"given": "Jonathan",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Wong",
"given": "Matthew",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Yuan",
"given": "Ying",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Szeto",
"given": "Jeremy",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Sulkowski",
"given": "Mark",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Stelmash",
"given": "Lauren",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Amon",
"given": "Arch",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Haight",
"given": "Daniel",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Lamb",
"given": "Deryl",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Harper",
"given": "Amron",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Pyram-Bernard",
"given": "Nancy",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Quintero",
"given": "Arlen",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Adhami",
"given": "Eftim",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Maria",
"given": "Josette",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Paudel",
"given": "Diksha",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Raymond",
"given": "Oksana",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Summers",
"given": "Jeffrey",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Turner",
"given": "Tammy",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Lenert",
"given": "Leslie",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Gallegos",
"given": "Sam",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Szwast",
"given": "Elizabeth Ann",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Abdulghani",
"given": "Ahsan",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Vasoya",
"given": "Pravin",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Miller",
"given": "Conrad",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Wiley",
"given": "Hawa",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Shah",
"given": "Nirav",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Klein",
"given": "Tovah",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Castex",
"given": "Julie",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Feliciano",
"given": "Phillip",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Olivo",
"given": "Jacqueline",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Ghaly",
"given": "Marian",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Javed",
"given": "Zainub",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Nawrocki",
"given": "Alexandra",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Vecchiarelli",
"given": "Anthony",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Vigil",
"given": "Nikki",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Cherukuri",
"given": "Vijaya",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Burden",
"given": "Erica",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Linn",
"given": "Dawn",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Fisher",
"given": "Laura",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Patel",
"given": "Vijay",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Patel",
"given": "Praksha",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Patel",
"given": "Yuti",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Ellison",
"given": "Leonard",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Harrison",
"given": "Jeffrey",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Shah",
"given": "Binod",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Shah",
"given": "Sugata",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Singh",
"given": "Upinder",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Donahue",
"given": "Julia",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Jazayeri",
"given": "Yasmin",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Gupta",
"given": "Anita",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Chandrasekar",
"given": "N",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Moritz",
"given": "Beth",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Fortt",
"given": "Tabitha",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Fortt",
"given": "Anisa",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Jones-Ince",
"given": "Ingrid",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "McKee",
"given": "Alix",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Schattinger",
"given": "Christy",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Wilson",
"given": "Jason",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Farlow",
"given": "Brenda",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Gentile",
"given": "Nina",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Finlaw",
"given": "Lillian",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Richwine",
"given": "Randall",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Williams",
"given": "Tearani",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Paizer",
"given": "Penny",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Carson",
"given": "Lisa",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Michelson",
"given": "Edward",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Austin",
"given": "Danielle",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Khetpal",
"given": "Sangeeta",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Cantrell",
"given": "Tiffany",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Franklin",
"given": "Drew",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Marshall",
"given": "Karissa",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Mahadevan",
"given": "Arvind",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Rosequist",
"given": "Madelyn",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Gnoni",
"given": "Martin",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Daffner",
"given": "Crystal",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "VandeWeerd",
"given": "Carla",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Roberts",
"given": "Mitchell",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "D'Andrea",
"given": "Mark",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Lim",
"given": "Stephen",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Swink",
"given": "Wayne",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Powers-Fletcher",
"given": "Margaret",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Mukunzi",
"given": "Sylvere",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Shenkman",
"given": "Elizabeth",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Hensley",
"given": "Jamie",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Manning",
"given": "Brittney",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Isache",
"given": "Carmen",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Bowman",
"given": "Jennifer",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Callaghan-Brown",
"given": "Angelique",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Scott",
"given": "Taylor",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Schwasinger-Schmidt",
"given": "Tiffany",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Cornejo",
"given": "Ashlie",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Jayaweera",
"given": "Dushyantha",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Almanzar",
"given": "Maria",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Ginsburg",
"given": "Letty",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Hajaz",
"given": "Americo",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Bramante",
"given": "Carolyn",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Robinson",
"given": "Matthew",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Seithel",
"given": "Michelle",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Sekikawa",
"given": "Akira",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Klawson",
"given": "Emily",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Ostrosky",
"given": "Luis",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Umana",
"given": "Virginia",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Patterson",
"given": "Thomas",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Tragus",
"given": "Robin",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Jackson",
"given": "Patrick",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Hallowell",
"given": "Caroline",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Haughey",
"given": "Heather",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Vaidya-Tank",
"given": "Bhavna",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Gould",
"given": "Cameron",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Goyal",
"given": "Parul",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Gatewood",
"given": "Carly",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Williamson",
"given": "John",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Seagle",
"given": "Hannah",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "McCarthy",
"given": "Matthew",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Salsgiver",
"given": "Elizabeth",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Armas",
"given": "Eddie",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Cheng",
"given": "Jhonsai",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Huerta",
"given": "Priscilla",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Garcia-Diaz",
"given": "Julia",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Aamodt",
"given": "David",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Ayers",
"given": "JaMario",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Collins",
"given": "Jess",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Graves",
"given": "John",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Grindstaff",
"given": "James",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Harrell",
"given": "Frank",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Lai",
"given": "Jessica",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Lopez",
"given": "Itzel",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Marlin",
"given": "Jessica",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Merkel",
"given": "Alyssa",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Nwosu",
"given": "Sam",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Obregon",
"given": "Savannah",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Orozco",
"given": "Dirk",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Perez-Torres",
"given": "Yoli",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Prato",
"given": "Nelson",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Ratcliff",
"given": "Colleen",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Rhode",
"given": "Max",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Rothman",
"given": "Russell",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Shirey-Rice",
"given": "Jana",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Vermillion",
"given": "Krista",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Tan",
"given": "Hsi-Nien",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Tregoning",
"given": "Seibert",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Vance",
"given": "Meghan",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Vongsamphanh",
"given": "Amber",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Weir",
"given": "Maria",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators"
}
],
"family": "Zaleski",
"given": "Nicole",
"sequence": "additional"
},
{
"affiliation": [],
"name": "Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators",
"sequence": "additional"
}
],
"container-title": "JAMA",
"container-title-short": "JAMA",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2022,
10,
21
]
],
"date-time": "2022-10-21T18:30:36Z",
"timestamp": 1666377036000
},
"deposited": {
"date-parts": [
[
2023,
8,
15
]
],
"date-time": "2023-08-15T15:45:11Z",
"timestamp": 1692114311000
},
"indexed": {
"date-parts": [
[
2024,
4,
8
]
],
"date-time": "2024-04-08T20:34:38Z",
"timestamp": 1712608478563
},
"is-referenced-by-count": 51,
"issue": "16",
"issued": {
"date-parts": [
[
2022,
10,
25
]
]
},
"journal-issue": {
"issue": "16",
"published-print": {
"date-parts": [
[
2022,
10,
25
]
]
}
},
"language": "en",
"link": [
{
"URL": "https://jamanetwork.com/journals/jama/articlepdf/2797483/jama_naggie_2022_oi_220112_1692105760.87877.pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "10",
"original-title": [],
"page": "1595",
"prefix": "10.1001",
"published": {
"date-parts": [
[
2022,
10,
25
]
]
},
"published-print": {
"date-parts": [
[
2022,
10,
25
]
]
},
"publisher": "American Medical Association (AMA)",
"reference": [
{
"DOI": "10.1056/NEJMoa2116044",
"article-title": "Molnupiravir for oral treatment of COVID-19 in nonhospitalized patients.",
"author": "Jayk Bernal",
"doi-asserted-by": "publisher",
"first-page": "509",
"issue": "6",
"journal-title": "N Engl J Med",
"key": "joi220112r1",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.1001/jama.2020.22760",
"article-title": "Fluvoxamine vs placebo and clinical deterioration in outpatients with symptomatic COVID-19: a randomized clinical trial.",
"author": "Lenze",
"doi-asserted-by": "publisher",
"first-page": "2292",
"issue": "22",
"journal-title": "JAMA",
"key": "joi220112r2",
"volume": "324",
"year": "2020"
},
{
"DOI": "10.1016/S2214-109X(21)00448-4",
"article-title": "Effect of early treatment with fluvoxamine on risk of emergency care and hospitalisation among patients with COVID-19: the TOGETHER randomised, platform clinical trial.",
"author": "Reis",
"doi-asserted-by": "publisher",
"first-page": "e42",
"issue": "1",
"journal-title": "Lancet Glob Health",
"key": "joi220112r3",
"volume": "10",
"year": "2022"
},
{
"DOI": "10.1016/S0140-6736(21)01744-X",
"article-title": "Inhaled budesonide for COVID-19 in people at high risk of complications in the community in the UK (PRINCIPLE): a randomised, controlled, open-label, adaptive platform trial.",
"author": "Yu",
"doi-asserted-by": "publisher",
"first-page": "843",
"issue": "10303",
"journal-title": "Lancet",
"key": "joi220112r4",
"volume": "398",
"year": "2021"
},
{
"DOI": "10.1001/jama.2021.3071",
"article-title": "Effect of ivermectin on time to resolution of symptoms among adults with mild COVID-19: a randomized clinical trial.",
"author": "López-Medina",
"doi-asserted-by": "publisher",
"first-page": "1426",
"issue": "14",
"journal-title": "JAMA",
"key": "joi220112r5",
"volume": "325",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2021436",
"article-title": "Dexamethasone in hospitalized patients with COVID-19.",
"author": "Horby",
"doi-asserted-by": "publisher",
"first-page": "693",
"issue": "8",
"journal-title": "N Engl J Med",
"key": "joi220112r6",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1001/jamainternmed.2022.0189",
"article-title": "Efficacy of ivermectin treatment on disease progression among adults with mild to moderate COVID-19 and comorbidities: the I-TECH randomized clinical trial.",
"author": "Lim",
"doi-asserted-by": "publisher",
"first-page": "426",
"issue": "4",
"journal-title": "JAMA Intern Med",
"key": "joi220112r7",
"volume": "182",
"year": "2022"
},
{
"DOI": "10.1016/S0140-6736(21)00676-0",
"article-title": "Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial.",
"author": "RECOVERY Collaborative Group",
"doi-asserted-by": "publisher",
"first-page": "1637",
"issue": "10285",
"journal-title": "Lancet",
"key": "joi220112r8",
"volume": "397",
"year": "2021"
},
{
"DOI": "10.1016/j.antiviral.2020.104787",
"article-title": "The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro.",
"author": "Caly",
"doi-asserted-by": "publisher",
"journal-title": "Antiviral Res",
"key": "joi220112r9",
"volume": "178",
"year": "2020"
},
{
"DOI": "10.1016/S2589-7500(20)30086-8",
"article-title": "A real-time dashboard of clinical trials for COVID-19.",
"author": "Thorlund",
"doi-asserted-by": "publisher",
"first-page": "e286",
"issue": "6",
"journal-title": "Lancet Digit Health",
"key": "joi220112r10",
"volume": "2",
"year": "2020"
},
{
"DOI": "10.1093/ofid/ofab645",
"article-title": "Ivermectin for COVID-19: addressing potential bias and medical fraud.",
"author": "Hill",
"doi-asserted-by": "publisher",
"issue": "2",
"journal-title": "Open Forum Infect Dis",
"key": "joi220112r11",
"volume": "9",
"year": "2022"
},
{
"DOI": "10.1177/0885066620973585",
"article-title": "Retracted article: Clinical and scientific rationale for the “MATH+” hospital treatment protocol for COVID-19.",
"author": "Kory",
"doi-asserted-by": "publisher",
"first-page": "135",
"issue": "2",
"journal-title": "J Intensive Care Med",
"key": "joi220112r13",
"volume": "36",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2115869",
"article-title": "Effect of early treatment with ivermectin among patients with COVID-19.",
"author": "Reis",
"doi-asserted-by": "publisher",
"first-page": "1721",
"issue": "18",
"journal-title": "N Engl J Med",
"key": "joi220112r14",
"volume": "386",
"year": "2022"
},
{
"key": "joi220112r12",
"unstructured": "Research Square. Retraction notice: Elgazzar A. Efficacy and safety of ivermectin for treatment and prophylaxis of COVID-19 pandemic. Preprint posted July 14, 2021, withdrawn November 16, 2021. Accessed June 8, 2022. https://www.researchsquare.com/article/rs-100956/v4"
},
{
"key": "joi220112r15",
"unstructured": "R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing. Accessed September 19, 2022. https://www.R-project.org/"
}
],
"reference-count": 15,
"references-count": 15,
"relation": {},
"resource": {
"primary": {
"URL": "https://jamanetwork.com/journals/jama/fullarticle/2797483"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"General Medicine"
],
"subtitle": [
"A Randomized Clinical Trial"
],
"title": "Effect of Ivermectin vs Placebo on Time to Sustained Recovery in Outpatients With Mild to Moderate COVID-19",
"type": "journal-article",
"volume": "328"
}