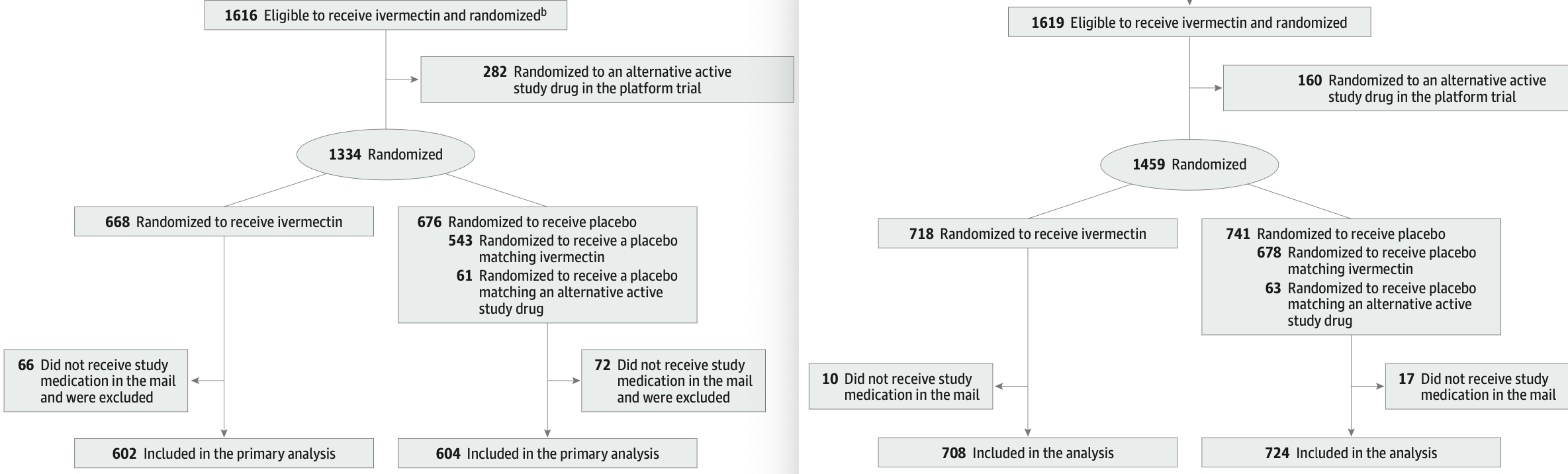
Partial correction to ACTIV-6 600 confirming that 16% of patients were missing in the analysis.
It's not clear how the trial could have such a large error for the number of patients randomized, why the correction took over a year, or why the many other errors have not been corrected
1.
The revised data exhibits additional concerns:
138 did not receive the study medication in the first version while only 27 did not receive it in the second version.
8.0% of the original patients reported had an adverse event while only 0.4% of the newly added patients did (eTable 2).
2.4% of the original patients had missing symptom severity while 32% of the newly added patients did (eFigure 4).
3 patients had missing symptom onset dates in the original set, while none are reported for the larger set (eFigure 4).
There are many anomolies in the CONSORT diagrams. Although both start with the same initial number of patients assessed:
10 had a drug allergy to ivermectin in the first version while only 7 do in the second version.
11 were on warfarin in the first version while only 4 were in the second version.
9 were hospitalized in the last 10 days in the first version while only one was in the second version.
7 had current use of ivermectin in the first version while only 4 had current or recent use in the second version.
The second version claims 78 were exluded for symptom onset >7 days which is not in the first version, and both versions show patients up to 10 days from onset included (eFigure 2).
In the second version, 17 fewer patients were eligible but elected not to continue.
There are many more anomalies in the CONSORT diagrams.
For many additional errors and concerns see
1.
Naggie et al., 16 May 2024, peer-reviewed, 1 author.
DOI record:
{
"DOI": "10.1001/jama.2024.8723",
"ISSN": [
"0098-7484"
],
"URL": "http://dx.doi.org/10.1001/jama.2024.8723",
"author": [
{
"affiliation": [
{
"name": "Duke Clinical Research Institute, Duke University School of Medicine, Durham, North Carolina"
}
],
"family": "Naggie",
"given": "Susanna",
"sequence": "first"
}
],
"container-title": "JAMA",
"container-title-short": "JAMA",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2024,
5,
16
]
],
"date-time": "2024-05-16T19:30:35Z",
"timestamp": 1715887835000
},
"deposited": {
"date-parts": [
[
2024,
5,
16
]
],
"date-time": "2024-05-16T19:30:37Z",
"timestamp": 1715887837000
},
"indexed": {
"date-parts": [
[
2024,
5,
17
]
],
"date-time": "2024-05-17T00:36:05Z",
"timestamp": 1715906165438
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2024,
5,
16
]
]
},
"language": "en",
"link": [
{
"URL": "https://jamanetwork.com/journals/jama/articlepdf/2818991/jama_naggie_2024_le_240062_1715708612.16601.pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "10",
"original-title": [],
"prefix": "10.1001",
"published": {
"date-parts": [
[
2024,
5,
16
]
]
},
"published-online": {
"date-parts": [
[
2024,
5,
16
]
]
},
"publisher": "American Medical Association (AMA)",
"reference": [
{
"DOI": "10.1001/jama.2023.1650",
"article-title": "Effect of higher-dose ivermectin for 6 days vs placebo on time to sustained recovery in outpatients with COVID-19: a randomized clinical trial.",
"author": "Naggie",
"doi-asserted-by": "publisher",
"first-page": "888",
"issue": "11",
"journal-title": "JAMA",
"key": "jle240062r1",
"volume": "329",
"year": "2023"
},
{
"article-title": "Errors in results from erroneous exclusion of participants in analysis.",
"journal-title": "JAMA",
"key": "jle240062r2"
}
],
"reference-count": 2,
"references-count": 2,
"relation": {},
"resource": {
"primary": {
"URL": "https://jamanetwork.com/journals/jama/fullarticle/2818991"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Error in the Exclusion of Participants From Analysis in the ACTIV-6 Platform Randomized Clinical Trial",
"type": "journal-article"
}