The Effect of Ivermectin on Reducing Viral Symptoms in Patients with Mild COVID-19
K U Abbas, S Muhammad, S F Ding
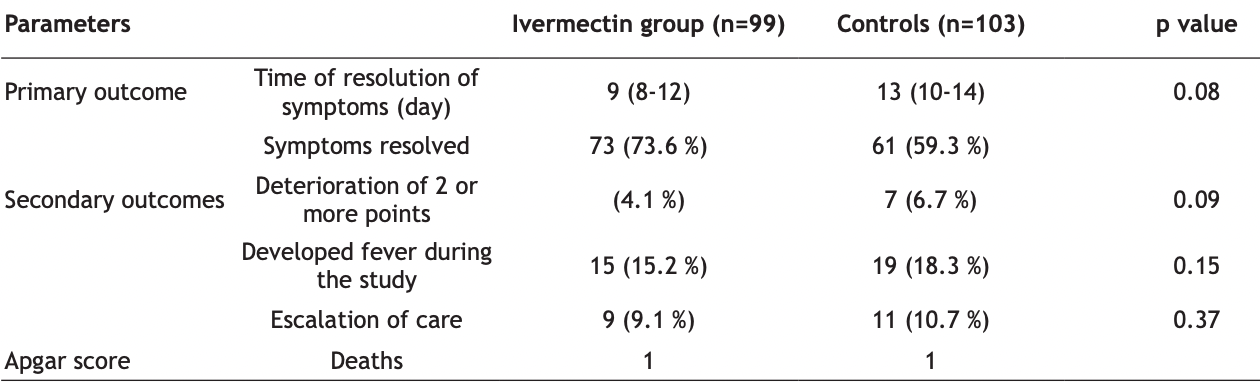
Ivermectin is widely prescribed as a potential treatment for coronavirus disease 2019, despite uncertainty about its clinical benefit. To determine whether ivermectin is an efficacious treatment for mild coronavirus disease 2019 is the objective of the study. A total of 476 adult patients with mild symptoms for 7 d or fewer in Jinan, China, were enrolled and followed up. Patients were randomly selected to receive ivermectin, 300 μg/ kg body weight per day for 5 d or placebo. The median time to resolution of symptoms was 10 d (interquartile range, 9-13) in the ivermectin group whereas it was 12 d (interquartile range, 9-13) in the placebo group (hazard ratio for resolution of symptoms, 1.07 [95 % confidence interval, 0.87 to 1.32]; p=0.53 by log-rank test). By d 21, 82 % in the ivermectin group and 79 % in the placebo group had resolved symptoms. The most common solicited adverse event was headache, reported by 104 patients (52 %) who received ivermectin and 111 patients (56 %) who received placebo. The most serious adverse event was multiorgan failure. Among adults with mild coronavirus disease 2019, a 5 d course of ivermectin, compared with placebo, did not significantly improve the time to resolution of symptoms. The findings do not support the use of ivermectin for treatment of mild coronavirus disease 2019.
Conflict of interests: The authors declared no conflict of interest.
References
Ansems, Grundeis, Dahms, Mikolajewska, Thieme et al., Remdesivir for the treatment of Covid-19, Cochrane Database Syst Rev
Beigel, Tomashek, Dodd, Mehta, Zingman et al., Remdesivir for the treatment of Covid-19, N Engl J Med
Bernal, Andrews, Gower, Gallagher, Simmons et al., Effectiveness of COVID-19 vaccines against the B. 1.617. 2 (Delta) variant, N Engl J Med
Bray, Rayner, Noël, Jans, Wagstaff, Ivermectin and COVID-19: A report in Antiviral Research, widespread interest, an FDA warning, two letters to the editor and the authors' responses, Antiviral Res
Caly, Druce, Catton, Jans, Wagstaff, The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro, Antiviral Res
Cao, Wang, Wen, Liu, Wang et al., A trial of lopinavir-ritonavir in adults hospitalized with severe Covid-19, N Engl J Med
De Melo, Lazarini, Larrous, Feige, Kergoat et al., Anti-COVID-19 efficacy of ivermectin in the golden hamster, BioRxiv
Diazgranados-Sánchez, Mejía-Fernández, Ls, Valencia-Artunduaga, Costa, Ivermectina como coadyuvante en la epilepsia refractaria, Rev Neurol
Elgazzar, Hany, Youssef, Hafez, Moussa et al., efficacy and safety of ivermectin for treatment and prophylaxis of COVID-19 pandemic
Hashim, Maulood, Rasheed, Fatak, Kabah et al., Controlled randomized clinical trial on using ivermectin with doxycycline for treating COVID-19 patients in Baghdad, Iraq
Huang, Wang, Li, Ren, Zhao et al., Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China, Lancet
Khan, Misdary, Yegya-Raman, Kim, Narayanan et al., Montelukast in hospitalized patients diagnosed with COVID-19, J Asthma
López-Medina, López, Hurtado, Dávalos, Ramirez et al., Effect of ivermectin on time to resolution of symptoms among adults with mild COVID-19: A randomized clinical trial, JAMA
Mastrangelo, Pezzullo, De Burghgraeve, Kaptein, Pastorino et al., Ivermectin is a potent inhibitor of flavivirus replication specifically targeting NS3 helicase activity: New prospects for an old drug, J Antimicrob Chemother
Momekov, Momekova, Ivermectin as a potential COVID-19 treatment from the pharmacokinetic point of view: Antiviral levels are not likely attainable with known dosing regimens, Biotechnol Biotechnol Equip
Niaee, Namdar, Allami, Zolghadr, Javadi et al., Ivermectin as an adjunct treatment for hospitalized adult COVID-19 patients: A randomized multicenter clinical trial, Asian Pac J Trop Biomed
Rajter, Sherman, Fatteh, Vogel, Sacks et al., Use of ivermectin is associated with lower mortality in hospitalized patients with coronavirus disease 2019: The ivermectin in COVID nineteen study, Chest
Schmith, Zhou, Lohmer, The approved dose of ivermectin alone is not the ideal dose for the treatment of COVID-19, Clin Pharmacol Ther
Smit, Ochomo, Aljayyoussi, Kwambai, Abong et al., Safety and mosquitocidal efficacy of high-dose ivermectin when co-administered with dihydroartemisininpiperaquine in Kenyan adults with uncomplicated malaria (IVERMAL): A randomised, double-blind, placebo-controlled trial, Lancet Infect Dis
Spuch, López-García, Rivera-Baltanás, Rodrígues-Amorím, Olivares, Does lithium deserve a place in the treatment against COVID-19? A preliminary observational study in six patients, case report, Front Pharmacol
Tay, Fraser, Chan, Moreland, Rathore et al., Nuclear localization of dengue virus (DENV) 1-4 nonstructural protein 5; protection against all 4 DENV serotypes by the inhibitor Ivermectin, Antiviral Res
Wagstaff, Sivakumaran, Heaton, Harrich, Jans, Ivermectin is a specific inhibitor of importin α/β-mediated nuclear import able to inhibit replication of HIV-1 and dengue virus, Biochem J
Wang, Wang, Chen, Qin, Unique epidemiological and clinical features of the emerging 2019 novel coronavirus pneumonia (COVID-19) implicate special control measures, J Med Virol
Xu, Shi, Wang, Zhang, Huang et al., Pathological findings of COVID-19 associated with acute respiratory distress syndrome, Lancet Respir Med
Yang, Atkinson, Wang, Lee, Bogoyevitch et al., The broad spectrum antiviral ivermectin targets the host nuclear transport importin α/β1 heterodimer, Antiviral Res
DOI record:
{
"DOI": "10.36468/pharmaceutical-sciences.spl.416",
"URL": "http://dx.doi.org/10.36468/pharmaceutical-sciences.spl.416",
"author": [
{
"affiliation": [],
"family": "Abbas",
"given": "K. U.",
"sequence": "first"
},
{
"affiliation": [],
"family": "Muhammad",
"given": "S.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ding",
"given": "S. F.",
"sequence": "additional"
}
],
"container-title": "Indian Journal of Pharmaceutical Sciences",
"container-title-short": "IJPS",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2022,
3,
18
]
],
"date-time": "2022-03-18T12:23:08Z",
"timestamp": 1647606188000
},
"deposited": {
"date-parts": [
[
2022,
3,
18
]
],
"date-time": "2022-03-18T12:23:10Z",
"timestamp": 1647606190000
},
"indexed": {
"date-parts": [
[
2024,
3,
28
]
],
"date-time": "2024-03-28T16:36:44Z",
"timestamp": 1711643804015
},
"is-referenced-by-count": 2,
"issue": "S1",
"issued": {
"date-parts": [
[
2022
]
]
},
"journal-issue": {
"issue": "S1",
"published-online": {
"date-parts": [
[
2022
]
]
}
},
"member": "22282",
"original-title": [],
"prefix": "10.36468",
"published": {
"date-parts": [
[
2022
]
]
},
"published-online": {
"date-parts": [
[
2022
]
]
},
"publisher": "Indian Pharmaceutical Association - IPA",
"reference-count": 0,
"references-count": 0,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.ijpsonline.com/articles/the-effect-of-ivermectin-on-reducing-viral-symptoms-in-patients-with-mild-covid19-4455.html?aid=4455"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subtitle": [],
"title": "The Effect of Ivermectin on Reducing Viral Symptoms in Patients with Mild COVID-19",
"type": "journal-article",
"volume": "84"
}