Self-prescribed Ivermectin use is associated with a lower rate of seroconversion in health care workers diagnosed with COVID, in a dose-dependent response
Célia Pedroso, Sara Vaz, Eduardo Martins Netto, Daniele Souza, Felice Deminco, Rafaela Mayoral, Eliana Menezes, Ana Patricia Amancio Da Cunha, Andres Moreira-Soto, Jan Felix Drexler, Carlos Brites
The Brazilian Journal of Infectious Diseases, doi:10.1016/j.bjid.2021.101603
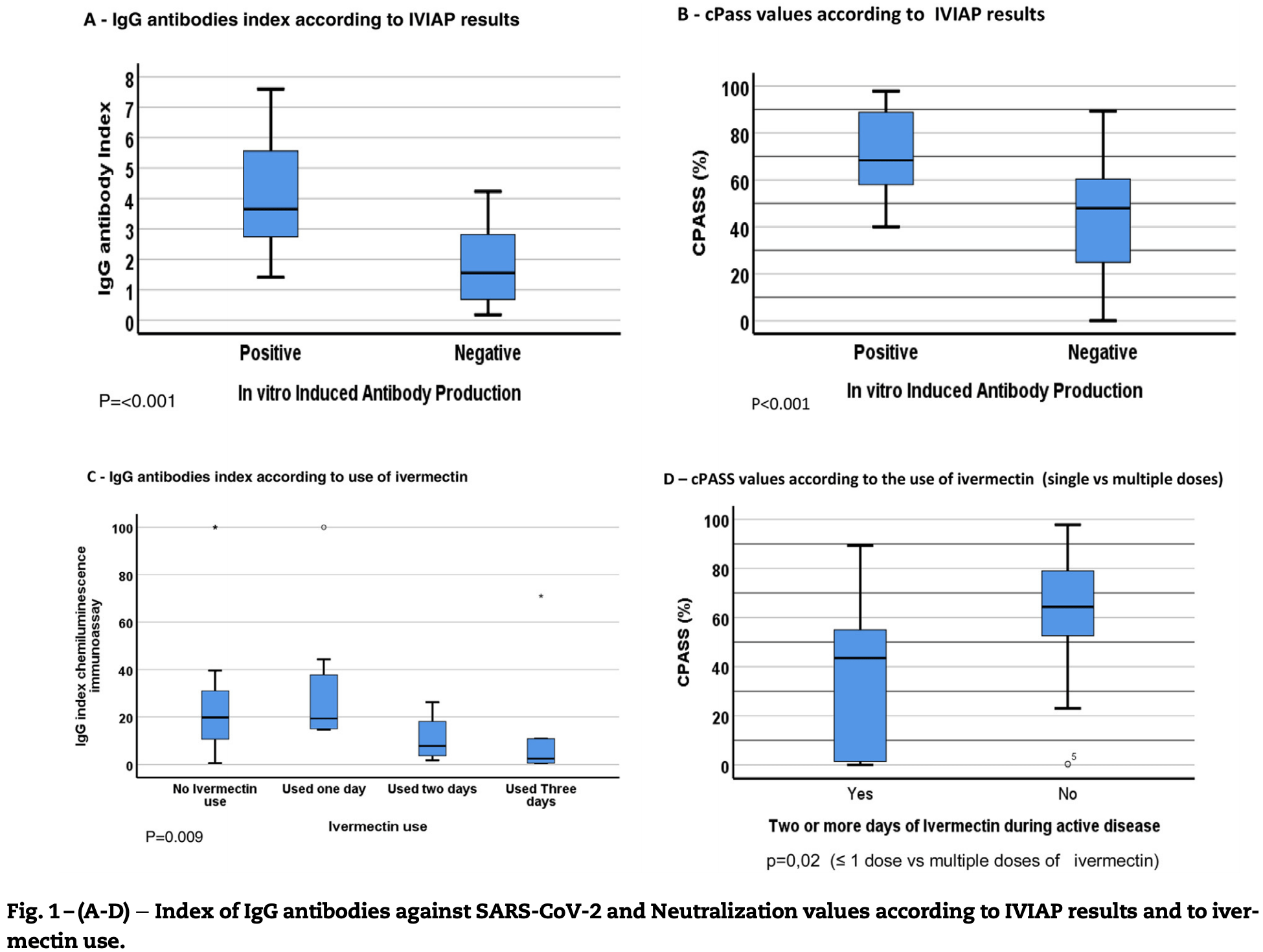
Background: Over-the-counter use of ivermectin amongst other drugs as SARS-CoV-2 treatment has been increasingly common, despite the lack of evidence on its clinical efficacy. Objective: To evaluate the effect of ivermectin use on production of antibodies against SARS-CoV-2 in health care workers (HCW) diagnosed with COVID-19 and of Th1/Th2 cytokines by stimulated peripheral blood mononuclear cells of the same cohort (PBMCs). Methods: This cross-sectional study evaluated seroconversion and neutralizing antibodies production in HCW at Complexo Hospitalar Universit ario Professor Edgard Santos (Salvador, Brazil), diagnosed with COVID-19 from May to July, 2020, as well as in vitro production of antibody against SARS-CoV-2 and Th1/Th2 cytokines. Analyses were performed between December 2020 and February 2021. Participants were stratified according to the use of ivermectin (≤ 1 dose vs. multiple doses) for treatment of COVID-19. Results: 45 HCW were included (62% women). Mean age was 39 years, and disease severity was similar across groups. Neutralizing antibodies were detected less frequently in multiple doses (70%) vs. ≤ 1 dose (97%) groups, p = 0.02). PBMCs of patients in multiple doses group also were less likely to produce antibodies against SARS-CoV-2 following in vitro stimulation with purified spike protein in comparison with patients in ≤ 1 dose group (p < 0.001). PBMCs production of Th1/Th2 cytokines levels was similar across groups. Abdominal pain (15% vs 46%, p = 0.04), diarrhea (21% vs. 55%, p = 0.05) and taste perversion (0% vs. 18%, p = 0.05) were more frequently reported by participants that used multiple doses of ivermectin. Conclusions: Although there was no evidence for differential disease severity upon ivermectin use for treatment of COVID-19 it was associated with more gastro-intestinal side-effects and impairment of anti-SARS-CoV2 antibodies production, in a dose dependent manner.
References
Amadori, De Rossi, Giaquinto, Faulkner-Valle, None
El-Tahtawy, Glue, Andrews, Mardekian, Amsden, None
Gallardo, Teiti, Rochaix, Macrocyclic lactones block 205 melanoma growth, metastases development and potentiate 206 activity of anti-BRAF V600 inhibitors, Clin Skin Cancer
Gonzalez De Castro, Clarke, Al-Lazikani, Workman, None
Guzzo, Safety, tolerability, and pharmacokinetics of 225 escalating high doses of ivermectin in healthy adult subjects
Knirsch, The effect of azithromycin on ivermectin 229 pharmacokinetics − a population pharmacokinetic model
Rusconi, Santambrogio, Marco, Lack of in vitro 201 anti-gp160 antibody production is a correlate of 202 nonprogression among HIV type 1-infected individuals, AIDS
Tang, Hu, Wang, Ivermectin, a potential 213 anticancer drug derived from an antiparasitic drug, Pharmacol
Tay, Fraser, Chan, Nuclear localization of 217 dengue virus (DENV) 1-4 non-structural protein 5; protection 218 against all 4 DENV serotypes by the inhibitor Ivermectin
Wagstaff, Sivakumaran, Heaton, Harrich, Jans, None
DOI record:
{
"DOI": "10.1016/j.bjid.2021.101603",
"ISSN": [
"1413-8670"
],
"URL": "http://dx.doi.org/10.1016/j.bjid.2021.101603",
"alternative-id": [
"S1413867021000726"
],
"article-number": "101603",
"assertion": [
{
"label": "This article is maintained by",
"name": "publisher",
"value": "Elsevier"
},
{
"label": "Article Title",
"name": "articletitle",
"value": "Self-prescribed Ivermectin use is associated with a lower rate of seroconversion in health care workers diagnosed with COVID, in a dose-dependent response"
},
{
"label": "Journal Title",
"name": "journaltitle",
"value": "The Brazilian Journal of Infectious Diseases"
},
{
"label": "CrossRef DOI link to publisher maintained version",
"name": "articlelink",
"value": "https://doi.org/10.1016/j.bjid.2021.101603"
},
{
"label": "Content Type",
"name": "content_type",
"value": "article"
},
{
"label": "Copyright",
"name": "copyright",
"value": "© 2021 Sociedade Brasileira de Infectologia. Published by Elsevier España, S.L.U."
}
],
"author": [
{
"affiliation": [],
"family": "Pedroso",
"given": "Célia",
"sequence": "first"
},
{
"ORCID": "http://orcid.org/0000-0001-7556-9670",
"affiliation": [],
"authenticated-orcid": false,
"family": "Vaz",
"given": "Sara",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-1691-6761",
"affiliation": [],
"authenticated-orcid": false,
"family": "Netto",
"given": "Eduardo Martins",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Souza",
"given": "Daniele",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Deminco",
"given": "Felice",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mayoral",
"given": "Rafaela",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-5700-4430",
"affiliation": [],
"authenticated-orcid": false,
"family": "Menezes",
"given": "Eliana",
"sequence": "additional"
},
{
"affiliation": [],
"family": "da Cunha",
"given": "Ana Patricia Amancio",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Moreira-Soto",
"given": "Andres",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Drexler",
"given": "Jan Felix",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-4673-6991",
"affiliation": [],
"authenticated-orcid": false,
"family": "Brites",
"given": "Carlos",
"sequence": "additional"
}
],
"container-title": "The Brazilian Journal of Infectious Diseases",
"container-title-short": "The Brazilian Journal of Infectious Diseases",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"clinicalkey.jp",
"clinicalkey.com",
"clinicalkey.es",
"clinicalkey.fr",
"clinicalkey.com.au",
"elsevier.com",
"sciencedirect.com"
]
},
"created": {
"date-parts": [
[
2021,
8,
12
]
],
"date-time": "2021-08-12T08:14:22Z",
"timestamp": 1628756062000
},
"deposited": {
"date-parts": [
[
2021,
9,
29
]
],
"date-time": "2021-09-29T05:09:22Z",
"timestamp": 1632892162000
},
"indexed": {
"date-parts": [
[
2024,
5,
3
]
],
"date-time": "2024-05-03T11:18:39Z",
"timestamp": 1714735119618
},
"is-referenced-by-count": 7,
"issue": "4",
"issued": {
"date-parts": [
[
2021,
7
]
]
},
"journal-issue": {
"issue": "4",
"published-print": {
"date-parts": [
[
2021,
7
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://www.elsevier.com/tdm/userlicense/1.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
7,
1
]
],
"date-time": "2021-07-01T00:00:00Z",
"timestamp": 1625097600000
}
},
{
"URL": "http://creativecommons.org/licenses/by-nc-nd/4.0/",
"content-version": "vor",
"delay-in-days": 29,
"start": {
"date-parts": [
[
2021,
7,
30
]
],
"date-time": "2021-07-30T00:00:00Z",
"timestamp": 1627603200000
}
}
],
"link": [
{
"URL": "https://api.elsevier.com/content/article/PII:S1413867021000726?httpAccept=text/xml",
"content-type": "text/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://api.elsevier.com/content/article/PII:S1413867021000726?httpAccept=text/plain",
"content-type": "text/plain",
"content-version": "vor",
"intended-application": "text-mining"
}
],
"member": "78",
"original-title": [],
"page": "101603",
"prefix": "10.1016",
"published": {
"date-parts": [
[
2021,
7
]
]
},
"published-print": {
"date-parts": [
[
2021,
7
]
]
},
"publisher": "Elsevier BV",
"reference": [
{
"DOI": "10.1016/j.antiviral.2020.104787",
"article-title": "The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro",
"author": "Caly",
"doi-asserted-by": "crossref",
"journal-title": "Antiviral Res",
"key": "10.1016/j.bjid.2021.101603_bib0001",
"volume": "178",
"year": "2020"
},
{
"DOI": "10.1016/0165-2427(94)05308-F",
"article-title": "Influence of ivermectin on cellular and humoral immune responses of lambs",
"author": "Stankiewicz",
"doi-asserted-by": "crossref",
"first-page": "347",
"issue": "3-4",
"journal-title": "Vet Immunol Immunopathol",
"key": "10.1016/j.bjid.2021.101603_bib0002",
"volume": "44",
"year": "1995"
},
{
"DOI": "10.1016/j.lfs.2007.02.025",
"article-title": "Effect of ivermectin on the cellular and humoral immune responses of rabbits",
"author": "Sajid",
"doi-asserted-by": "crossref",
"first-page": "1966",
"issue": "21",
"journal-title": "Life Sci",
"key": "10.1016/j.bjid.2021.101603_bib0003",
"volume": "80",
"year": "2007"
},
{
"DOI": "10.3201/eid2607.200841",
"article-title": "Severe acute respiratory syndrome coronavirus 2−specific antibody responses in coronavirus disease patients",
"author": "Okba",
"doi-asserted-by": "crossref",
"first-page": "1478",
"issue": "7",
"journal-title": "Emerg Infect Dis",
"key": "10.1016/j.bjid.2021.101603_bib0004",
"volume": "26",
"year": "2020"
},
{
"DOI": "10.3390/v12121357",
"doi-asserted-by": "crossref",
"key": "10.1016/j.bjid.2021.101603_bib0005",
"unstructured": "Persisting neutralizing activity to SARS-CoV-2 over months in sera of COVID-19 patients Viruses. 2020 Dec; 12(12): 1357.Published online 2020 Nov 27. doi: 10.3390/v12121357"
},
{
"article-title": "Rapid generation of neutralizing antibody responses in COVID-19 patients",
"author": "Suthar",
"issue": "3",
"journal-title": "Cell Rep Med",
"key": "10.1016/j.bjid.2021.101603_bib0006",
"volume": "1",
"year": "2020"
},
{
"DOI": "10.2807/1560-7917.ES.2020.25.3.2000045",
"article-title": "Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR",
"author": "Corman",
"doi-asserted-by": "crossref",
"first-page": "1",
"issue": "3",
"journal-title": "Eurosurveillance",
"key": "10.1016/j.bjid.2021.101603_bib0007",
"volume": "25",
"year": "2020"
},
{
"DOI": "10.1016/S0140-6736(88)91603-0",
"article-title": "In-vitro production of HIV-specific antibody in children at risk of AIDS",
"author": "Amadori",
"doi-asserted-by": "crossref",
"first-page": "852",
"issue": "8590",
"journal-title": "Lancet",
"key": "10.1016/j.bjid.2021.101603_bib0008",
"volume": "1",
"year": "1988"
},
{
"DOI": "10.1089/aid.1998.14.1341",
"article-title": "Lack of in vitro anti-gp160 antibody production is a correlate of nonprogression among HIV type 1-infected individuals",
"author": "Rusconi",
"doi-asserted-by": "crossref",
"first-page": "1341",
"issue": "15",
"journal-title": "AIDS Res Hum Retroviruses",
"key": "10.1016/j.bjid.2021.101603_bib0009",
"volume": "14",
"year": "1998"
},
{
"DOI": "10.1016/j.clsc.2016.05.001",
"article-title": "Macrocyclic lactones block melanoma growth, metastases development and potentiate activity of anti-BRAF V600 inhibitors",
"author": "Gallardo",
"doi-asserted-by": "crossref",
"first-page": "4",
"issue": "1",
"journal-title": "Clin Skin Cancer",
"key": "10.1016/j.bjid.2021.101603_bib0010",
"volume": "1",
"year": "2016"
},
{
"DOI": "10.1038/clpt.2012.237",
"article-title": "Personalized cancer medicine: molecular diagnostics, predictive biomarkers, and drug resistance",
"author": "Gonzalez de Castro",
"doi-asserted-by": "crossref",
"first-page": "252",
"issue": "3",
"journal-title": "Clin Pharmacol Ther",
"key": "10.1016/j.bjid.2021.101603_bib0011",
"volume": "93",
"year": "2013"
},
{
"DOI": "10.1016/j.phrs.2020.105207",
"article-title": "Ivermectin, a potential anticancer drug derived from an antiparasitic drug",
"author": "Tang",
"doi-asserted-by": "crossref",
"journal-title": "Pharmacol Res",
"key": "10.1016/j.bjid.2021.101603_bib0012",
"volume": "163",
"year": "2021"
},
{
"DOI": "10.1016/j.antiviral.2013.06.002",
"article-title": "Nuclear localization of dengue virus (DENV) 1-4 non-structural protein 5; protection against all 4 DENV serotypes by the inhibitor Ivermectin",
"author": "Tay",
"doi-asserted-by": "crossref",
"first-page": "301",
"journal-title": "Antiviral Res",
"key": "10.1016/j.bjid.2021.101603_bib0013",
"volume": "99",
"year": "2013"
},
{
"DOI": "10.1042/BJ20120150",
"article-title": "Ivermectin is a specific inhibitor of importin alpha/beta-mediated nuclear import able to inhibit replication of HIV-1 and dengue virus",
"author": "Wagstaff",
"doi-asserted-by": "crossref",
"first-page": "851",
"journal-title": "Biochem J",
"key": "10.1016/j.bjid.2021.101603_bib0014",
"volume": "443",
"year": "2012"
},
{
"DOI": "10.1177/009127002401382731",
"article-title": "Safety, tolerability, and pharmacokinetics of escalating high doses of ivermectin in healthy adult subjects",
"author": "Guzzo",
"doi-asserted-by": "crossref",
"first-page": "1122",
"issue": "10",
"journal-title": "J Clin Pharmacol.",
"key": "10.1016/j.bjid.2021.101603_bib0015",
"volume": "42",
"year": "2002"
},
{
"DOI": "10.1371/journal.pntd.0000236",
"article-title": "The effect of azithromycin on ivermectin pharmacokinetics – a population pharmacokinetic model analysis",
"author": "El-Tahtawy",
"doi-asserted-by": "crossref",
"first-page": "e236",
"journal-title": "PLoS Negl Trop Dis",
"key": "10.1016/j.bjid.2021.101603_bib0016",
"volume": "2",
"year": "2008"
}
],
"reference-count": 16,
"references-count": 16,
"relation": {},
"resource": {
"primary": {
"URL": "https://linkinghub.elsevier.com/retrieve/pii/S1413867021000726"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Self-prescribed Ivermectin use is associated with a lower rate of seroconversion in health care workers diagnosed with COVID, in a dose-dependent response",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1016/elsevier_cm_policy",
"volume": "25"
}