Effectiveness of a multidrug therapy consisting of Ivermectin, Azithromycin, Montelukast, and Acetylsalicylic acid to prevent hospitalization and death among ambulatory COVID-19 cases in Tlaxcala, Mexico
René Lima-Morales, Pablo Méndez-Hernández, Yvonne N Flores, Patricia Osorno-Romero, Christian Ronal Sancho-Hernández, Elizabeth Cuecuecha-Rugerio, Adrián Nava-Zamora, Diego Rolando Hernández-Galdamez, Daniela Karola Romo-Dueñas, Jorge Salmerón
International Journal of Infectious Diseases, doi:10.1016/j.ijid.2021.02.014
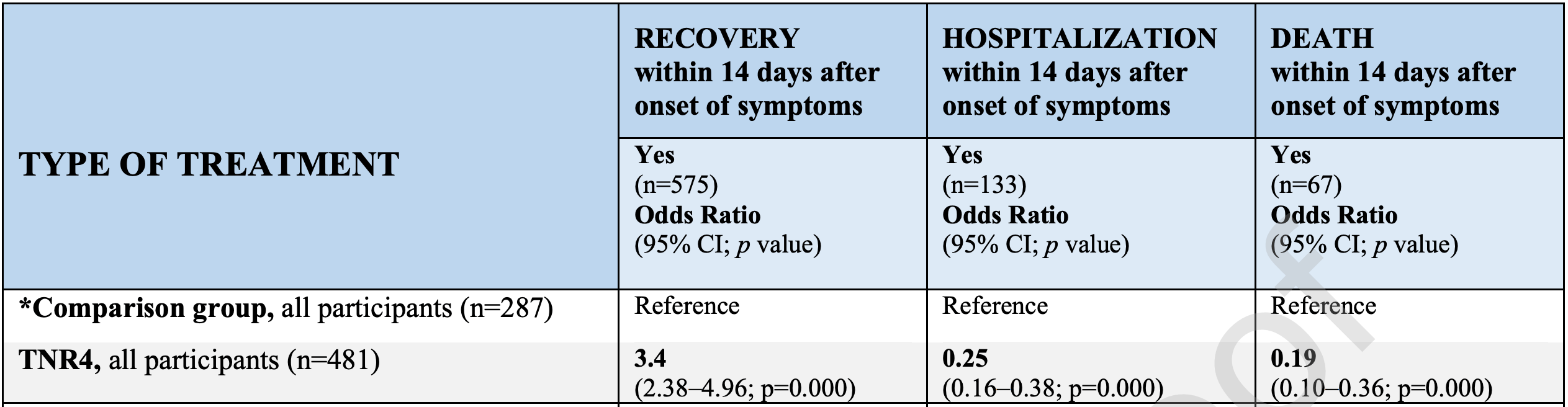
There is an urgent need for effective treatments to prevent or attenuate lung and systemic inflammation, endotheliitis, and thrombosis related to COVID-19. This study aimed to assess the effectiveness of a multidrug-therapy consisting of Ivermectin, Azithromycin, Montelukast, and Acetylsalicylic acid ("TNR4" therapy) to prevent hospitalization and death among ambulatory COVID-19 cases in Tlaxcala, Mexico. Design and methods: A comparative effectiveness study was performed among 768 confirmed SARS-CoV-2 cases aged 18-80 years, who received ambulatory care at the Ministry of Health of Tlaxcala. A total of 481 cases received the TNR4 therapy, while 287 received another treatment (comparison group). All participants received home visits and/or phone calls for clinical evaluation during the 14 days after enrollment. Results: Nearly 85% of cases who received the TNR4 recovered within 14 days compared to 59% in the comparison group. The likelihood of recovery within 14 days was 3.4 times greater among the TNR4 group than in the comparison group. Patients treated with TNR4 had a 75% and 81% lower risk of being hospitalized or death, respectively, than the comparison group. Conclusions: TNR4 therapy improved recovery and prevented the risk of hospitalization and death among ambulatory COVID-19 cases.
Differences between means were estimated from linear regression, and differences between proportions were evaluated using the chi2 test. A p-value 0.05 was considered to be significant. Proportions and means were adjusted by age, sex, comorbidities, and occupation. A p-value 0Á05 was considered significant. a Comparison group: participants who did not accept the TNR4 treatment because they were asymptomatic, were already taking another treatment, or they had self-medicated for cold and flu. However, they agreed to take part in the follow-up portion of the study.
Conflict of interest The authors declare no conflict of interest in this article.
Ethical approval Approval for this study was granted by the Ministry of Health of the Tlaxcala state, Mexico (#CEI02092020).
References
Almerie, Kerrigan, The association between obesity and poor outcome after COVID-19 indicates a potential therapeutic role for montelukast, Med Hypotheses
Anderson, Meredith, Yeung, Frei, Selwyn et al., The effect of cholesterol-lowering and antioxidant therapy on endothelium-dependent coronary vasomotion, N Engl J Med
Arshad, Kilgore, Chaudhry, Jacobsen, Wang et al., Treatment with Hydroxychloroquine, Azithromycin, and combination in patients hospitalized with COVID-19, Int J Infect Dis
Awortwe, Cascorbi, Meta-analysis on outcome-worsening comorbidities of COVID-19 and related potential drug-drug interactions, Pharmacol Res
Back, Marzolini, Hodge, Marra, Boyle et al., COVID-19 treatment in patients with comorbidities: Awareness of drug-drug interactions, Br J Clin Pharmacol
Baud, Qi, Nielsen-Saines, Musso, Pomar et al., Real estimates of mortality following COVID-19 infection, Lancet Infect Dis
Beigel, Tomashek, Dodd, Mehta, Zingman et al., Remdesivir for the treatment of Covid-19-Preliminary report, N Engl J Med
Bianconi, Violi, Fallarino, Pignatelli, Sahebkar et al., Is acetylsalicylic acid a safe and potentially useful choice for adult patients with cOVID-19?, Drugs
Bleyzac, Goutelle, Bourguignon, Tod, Azithromycin for COVID-19: More than just an antimicrobial?, Clin Drug Investig
Bozek, Winterstein, Montelukast's ability to fight COVID-19 infection, J Asthma
Cai, Yang, Liu, Chen, Shu et al., Experimental treatment with favipiravir for COVID-19: An open-label control study
Caly, Druce, Catton, Jans, Wagstaff, The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro, Antiviral Res
Cao, Wang, Wen, Liu, Wang et al., A trial of lopinavir-ritonavir in adults hospitalized with severe covid-19, N Engl J Med
Carvallo, Hirsch, Farinella, Safety and efficacy of the combined use of Ivermectin, dexamethasone, enoxaparin and aspirin against COVID-19, medRxiv
Cavalcanti, Zampieri, Rosa, Azevedo, Veiga et al., Hydroxychloroquine with or without azithromycin in mild-to-moderate Covid-19,
doi:10.1056/NEJMoa2019014Damle, Vourvahis, Wang, Leaney, Corrigan, Clinical pharmacology perspectives on the antiviral activity of azithromycin and use in COVID-19, Clin Pharmacol Ther
David, Frank, Rémy, Oliver, Anti-tumor necrosis factor-α treatment improves endothelial function in patients with rheumatoid arthritis, Circulation
El-Aziz, Stockand, Recent progress and challenges in drug development against COVID-19 coronavirus (SARS-CoV-2) -An update on the status, Infect Genet Evol
Fang, Karakiulakis, Roth, Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection?, Lancet Resp Med
Feldmann, Maini, Woody, Holgate, Winter et al., Trials of anti-tumor necrosis factor therapy for COVID-19 are urgently needed, Lancet
Fidan, Aydo, As a potential treatment of COVID-19: Montelukast, Med Hypotheses
Flammer, Sudano, Hermann, Gay, Forster et al., Angiotensinconverting enzyme inhibition improves vascular function in rheumatoid arthritis, Circulation,
doi:10.1161/CIRCULATIONAHA.107.734384Gao, Tian, Yang, Breakthrough: Chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies, Biosci Trends
Guan, Ni, Hu, Liang, Ou et al., Clinical characteristics of coronavirus disease 2019 in China, N Engl J Med
Gupta, Sahoo, Singh, Ivermectin: Potential candidate for the treatment of Covid 19, Braz J Infect Dis
Huang, Wang, Li, Ren, Zhao et al., Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China, Lancet
Hung, Lung, Tso, Liu, Chung et al., Triple combination of interferon beta-1b, lopinavir-2013; ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: An open-label, randomised, phase 2 trial, Lancet,
doi:10.1016/S0140-6736(20)31042-4Jabeen, Khader, Jabeen, A review on the antiparasitic drug ivermectin for various viral infections and possibilities of using it for novel Severe Acute Respiratory Syndrome Coronavirus 2: New hope to treat coronavirus disease
Janiaud, Axfors, Van't Hooft, Saccilotto, Agarwal et al., The worldwide clinical trial research response to the COVID-19 pandemic -The first 100 days
Jean, Lee, Hsueh, Treatment options for COVID-19: The reality and challenges, J Microbiol Immunol Infect
Li, Hao, Zhao, Du, Zhou, SARS-CoV-2 infection-induced immune responses: Friends or foes?, Scand J Immunol
Ortega, Zambrano, Jastrzebska, Liprandi, Rangel et al., Understanding severe acute respiratory syndrome coronavirus 2 replication to design efficient drug combination therapies, Intervirology
Pani, Lauriola, Romandini, Scaglione, Macrolides and viral infections: Focus on Azithromycin in COVID-19 pathology, Int J Antimicrob Agents
Poduri, Joshi, Jagadeesh, Drugs targeting various stages of the SARS-CoV-2 life cycle: Exploring promising drugs for the treatment of Covid-19, Cell Signal
Portmann-Baracco, Alberti, Accinelli, Antiviral and anti-inflammatory of Ivermectin and its potential use in COVID-19, Arch Bronchomeunol,
doi:10.1016/j.arbres.2020.06.011Rajter, Sherman, Fatteh, Vogel, Sacks et al., ICON (Ivermectin in COvid Nineteen) study: Use of Ivermectin is associated with lower mortality in hospitalized patients with COVID19, medRxiv
Rosenberg, Dufort, Udo, Wilberschied, Kumar et al., Association of treatment with Hydroxychloroquine or Azithromycin with inhospital mortality in patients with COVID-19 in New York State, JAMA
Sanders, Monogue, Jodlowski, Cutrell, Pharmacologic treatments for coronavirus disease 2019 (COVID-19): A review, JAMA
Taddei, Virdis, Ghiadoni, Mattei, Salvetti, Effects of angiotensin converting enzyme inhibition on endothelium-dependent vasodilatation in essential hypertensive patients, J Hypertens
Tang, Li, Wang, Sun, Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia, J Thromb Haemost
Tsang, Chan, Cho, Yu, Yim et al., An update on COVID-19 pandemic: The epidemiology, pathogenesis, prevention and treatment strategies, Expert Rev Anti Infect Ther
Varga, Flammer, Steiger, Haberecker, Andermatt et al., Endothelial cell infection and endotheliitis in COVID-19, Lancet
Viecca, Radovanovic, Forleo, Santus, Enhanced platelet inhibition treatment improves hypoxemia in patients with severe Covid-19 and hypercoagulability. A case control, proof of concept study, Pharmacol Res
Wang, Zhang, Du, Du, Zhao et al., Remdesivir in adults with severe COVID-19: A randomised, double-blind, placebo-controlled, multicentre trial, Lancet
DOI record:
{
"DOI": "10.1016/j.ijid.2021.02.014",
"ISSN": [
"1201-9712"
],
"URL": "http://dx.doi.org/10.1016/j.ijid.2021.02.014",
"alternative-id": [
"S1201971221001004"
],
"assertion": [
{
"label": "This article is maintained by",
"name": "publisher",
"value": "Elsevier"
},
{
"label": "Article Title",
"name": "articletitle",
"value": "Effectiveness of a multidrug therapy consisting of Ivermectin, Azithromycin, Montelukast, and Acetylsalicylic acid to prevent hospitalization and death among ambulatory COVID-19 cases in Tlaxcala, Mexico"
},
{
"label": "Journal Title",
"name": "journaltitle",
"value": "International Journal of Infectious Diseases"
},
{
"label": "CrossRef DOI link to publisher maintained version",
"name": "articlelink",
"value": "https://doi.org/10.1016/j.ijid.2021.02.014"
},
{
"label": "Content Type",
"name": "content_type",
"value": "article"
},
{
"label": "Copyright",
"name": "copyright",
"value": "© 2021 The Authors. Published by Elsevier Ltd on behalf of International Society for Infectious Diseases."
}
],
"author": [
{
"affiliation": [],
"family": "Lima-Morales",
"given": "René",
"sequence": "first"
},
{
"ORCID": "http://orcid.org/0000-0002-5598-5439",
"affiliation": [],
"authenticated-orcid": false,
"family": "Méndez-Hernández",
"given": "Pablo",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Flores",
"given": "Yvonne N.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Osorno-Romero",
"given": "Patricia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sancho-Hernández",
"given": "Christian Ronal",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cuecuecha-Rugerio",
"given": "Elizabeth",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Nava-Zamora",
"given": "Adrián",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-4140-455X",
"affiliation": [],
"authenticated-orcid": false,
"family": "Hernández-Galdamez",
"given": "Diego Rolando",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Romo-Dueñas",
"given": "Daniela Karola",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Salmerón",
"given": "Jorge",
"sequence": "additional"
}
],
"container-title": "International Journal of Infectious Diseases",
"container-title-short": "International Journal of Infectious Diseases",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"ijidonline.com",
"clinicalkey.jp",
"clinicalkey.com",
"clinicalkey.es",
"clinicalkey.fr",
"clinicalkey.com.au",
"elsevier.com",
"sciencedirect.com"
]
},
"created": {
"date-parts": [
[
2021,
2,
10
]
],
"date-time": "2021-02-10T06:58:26Z",
"timestamp": 1612940306000
},
"deposited": {
"date-parts": [
[
2021,
8,
4
]
],
"date-time": "2021-08-04T16:42:00Z",
"timestamp": 1628095320000
},
"indexed": {
"date-parts": [
[
2024,
3,
29
]
],
"date-time": "2024-03-29T11:53:54Z",
"timestamp": 1711713234852
},
"is-referenced-by-count": 26,
"issued": {
"date-parts": [
[
2021,
4
]
]
},
"language": "en",
"license": [
{
"URL": "https://www.elsevier.com/tdm/userlicense/1.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
4,
1
]
],
"date-time": "2021-04-01T00:00:00Z",
"timestamp": 1617235200000
}
},
{
"URL": "http://creativecommons.org/licenses/by-nc-nd/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
2,
4
]
],
"date-time": "2021-02-04T00:00:00Z",
"timestamp": 1612396800000
}
}
],
"link": [
{
"URL": "https://api.elsevier.com/content/article/PII:S1201971221001004?httpAccept=text/xml",
"content-type": "text/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://api.elsevier.com/content/article/PII:S1201971221001004?httpAccept=text/plain",
"content-type": "text/plain",
"content-version": "vor",
"intended-application": "text-mining"
}
],
"member": "78",
"original-title": [],
"page": "598-605",
"prefix": "10.1016",
"published": {
"date-parts": [
[
2021,
4
]
]
},
"published-print": {
"date-parts": [
[
2021,
4
]
]
},
"publisher": "Elsevier BV",
"reference": [
{
"DOI": "10.1016/j.meegid.2020.104327",
"article-title": "Recent progress and challenges in drug development against COVID-19 coronavirus (SARS-CoV-2) — An update on the status",
"author": "Abd El-Aziz",
"doi-asserted-by": "crossref",
"first-page": "104327",
"issue": "September",
"journal-title": "Infect Genet Evol",
"key": "10.1016/j.ijid.2021.02.014_bib0005",
"volume": "83",
"year": "2020"
},
{
"DOI": "10.1016/j.mehy.2020.109883",
"article-title": "The association between obesity and poor outcome after COVID-19 indicates a potential therapeutic role for montelukast",
"author": "Almerie",
"doi-asserted-by": "crossref",
"first-page": "109883",
"issue": "October",
"journal-title": "Med Hypotheses",
"key": "10.1016/j.ijid.2021.02.014_bib0010",
"volume": "143",
"year": "2020"
},
{
"DOI": "10.1056/NEJM199502233320802",
"article-title": "The effect of cholesterol-lowering and antioxidant therapy on endothelium-dependent coronary vasomotion",
"author": "Anderson",
"doi-asserted-by": "crossref",
"first-page": "488",
"issue": "February (8)",
"journal-title": "N Engl J Med",
"key": "10.1016/j.ijid.2021.02.014_bib0015",
"volume": "332",
"year": "1995"
},
{
"DOI": "10.1016/j.ijid.2020.06.099",
"article-title": "Treatment with Hydroxychloroquine, Azithromycin, and combination in patients hospitalized with COVID-19",
"author": "Arshad",
"doi-asserted-by": "crossref",
"first-page": "396",
"issue": "August",
"journal-title": "Int J Infect Dis",
"key": "10.1016/j.ijid.2021.02.014_bib0020",
"volume": "97",
"year": "2020"
},
{
"DOI": "10.1016/j.phrs.2020.105250",
"article-title": "Meta-analysis on outcome-worsening comorbidities of COVID-19 and related potential drug-drug interactions",
"author": "Awortwe",
"doi-asserted-by": "crossref",
"journal-title": "Pharmacol Res",
"key": "10.1016/j.ijid.2021.02.014_bib0025",
"year": "2020"
},
{
"article-title": "COVID-19 treatment in patients with comorbidities: Awareness of drug–drug interactions",
"author": "Back",
"issue": "May",
"journal-title": "Br J Clin Pharmacol",
"key": "10.1016/j.ijid.2021.02.014_bib0030",
"year": "2020"
},
{
"DOI": "10.1016/S1473-3099(20)30195-X",
"article-title": "Real estimates of mortality following COVID-19 infection",
"author": "Baud",
"doi-asserted-by": "crossref",
"first-page": "773",
"issue": "July (7)",
"journal-title": "Lancet Infect Dis",
"key": "10.1016/j.ijid.2021.02.014_bib0035",
"volume": "20",
"year": "2020"
},
{
"article-title": "Remdesivir for the treatment of Covid-19—Preliminary report",
"author": "Beigel",
"issue": "May",
"journal-title": "N Engl J Med",
"key": "10.1016/j.ijid.2021.02.014_bib0040",
"year": "2020"
},
{
"article-title": "Is acetylsalicylic acid a safe and potentially useful choice for adult patients with cOVID-19?",
"author": "Bianconi",
"first-page": "1",
"issue": "July",
"journal-title": "Drugs",
"key": "10.1016/j.ijid.2021.02.014_bib0045",
"year": "2020"
},
{
"DOI": "10.1007/s40261-020-00933-3",
"article-title": "Azithromycin for COVID-19: More than just an antimicrobial?",
"author": "Bleyzac",
"doi-asserted-by": "crossref",
"first-page": "683",
"issue": "August (8)",
"journal-title": "Clin Drug Investig",
"key": "10.1016/j.ijid.2021.02.014_bib0050",
"volume": "40",
"year": "2020"
},
{
"DOI": "10.1080/02770903.2020.1786112",
"article-title": "Montelukast’s ability to fight COVID-19 infection",
"author": "Bozek",
"doi-asserted-by": "crossref",
"first-page": "1",
"issue": "June",
"journal-title": "J Asthma",
"key": "10.1016/j.ijid.2021.02.014_bib0055",
"year": "2020"
},
{
"article-title": "Experimental treatment with favipiravir for COVID-19: An open-label control study",
"author": "Cai",
"first-page": "1",
"issue": "March",
"journal-title": "Engineering (Beijing, China)",
"key": "10.1016/j.ijid.2021.02.014_bib0060",
"year": "2020"
},
{
"DOI": "10.1016/j.antiviral.2020.104787",
"article-title": "The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro",
"author": "Caly",
"doi-asserted-by": "crossref",
"first-page": "104787",
"journal-title": "Antiviral Res",
"key": "10.1016/j.ijid.2021.02.014_bib0065",
"volume": "178",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2001282",
"article-title": "A trial of lopinavir-ritonavir in adults hospitalized with severe covid-19",
"author": "Cao",
"doi-asserted-by": "crossref",
"first-page": "1787",
"issue": "19",
"journal-title": "N Engl J Med",
"key": "10.1016/j.ijid.2021.02.014_bib0070",
"volume": "382",
"year": "2020"
},
{
"article-title": "Safety and efficacy of the combined use of Ivermectin, dexamethasone, enoxaparin and aspirin against COVID-19",
"author": "Carvallo",
"first-page": "1",
"journal-title": "medRxiv",
"key": "10.1016/j.ijid.2021.02.014_bib0075",
"year": "2020"
},
{
"article-title": "Hydroxychloroquine with or without azithromycin in mild-to-moderate Covid-19",
"author": "Cavalcanti",
"issue": "July",
"journal-title": "N Engl J Med",
"key": "10.1016/j.ijid.2021.02.014_bib0080",
"year": "2020"
},
{
"author": "Comisión Federal para la Protección contra Riesgos Sanitarios",
"key": "10.1016/j.ijid.2021.02.014_bib0085",
"series-title": "Bases de Datos de Licencias Sanitarias de Insumos para la Salud [Conjunto de Datos]. Documentos",
"year": "2016"
},
{
"DOI": "10.1002/cpt.1857",
"article-title": "Clinical pharmacology perspectives on the antiviral activity of azithromycin and use in COVID-19",
"author": "Damle",
"doi-asserted-by": "crossref",
"first-page": "201",
"issue": "August (2)",
"journal-title": "Clin Pharmacol Ther",
"key": "10.1016/j.ijid.2021.02.014_bib0090",
"volume": "108",
"year": "2020"
},
{
"article-title": "Anti–tumor necrosis factor-α treatment improves endothelial function in patients with rheumatoid arthritis",
"author": "David",
"first-page": "2184",
"issue": "October (17)",
"journal-title": "Circulation",
"key": "10.1016/j.ijid.2021.02.014_bib0095",
"volume": "106",
"year": "2002"
},
{
"DOI": "10.1016/S2213-2600(20)30116-8",
"article-title": "Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection?",
"author": "Fang",
"doi-asserted-by": "crossref",
"first-page": "e21",
"journal-title": "Lancet Resp Med",
"key": "10.1016/j.ijid.2021.02.014_bib0100",
"volume": "8",
"year": "2020"
},
{
"DOI": "10.1016/S0140-6736(20)30858-8",
"article-title": "Trials of anti-tumor necrosis factor therapy for COVID-19 are urgently needed",
"author": "Feldmann",
"doi-asserted-by": "crossref",
"first-page": "1407",
"issue": "May (10234)",
"journal-title": "Lancet (London, England)",
"key": "10.1016/j.ijid.2021.02.014_bib0105",
"volume": "395",
"year": "2020"
},
{
"DOI": "10.1016/j.mehy.2020.109828",
"article-title": "As a potential treatment of COVID-19: Montelukast",
"author": "Fidan",
"doi-asserted-by": "crossref",
"first-page": "109828",
"issue": "May",
"journal-title": "Med Hypotheses",
"key": "10.1016/j.ijid.2021.02.014_bib0110",
"volume": "142",
"year": "2020"
},
{
"DOI": "10.1161/CIRCULATIONAHA.107.734384",
"article-title": "Angiotensin-converting enzyme inhibition improves vascular function in rheumatoid arthritis",
"author": "Flammer",
"doi-asserted-by": "crossref",
"first-page": "2262",
"issue": "17",
"journal-title": "Circulation",
"key": "10.1016/j.ijid.2021.02.014_bib0115",
"volume": "117",
"year": "2008"
},
{
"DOI": "10.5582/bst.2020.01047",
"article-title": "Breakthrough: Chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies",
"author": "Gao",
"doi-asserted-by": "crossref",
"first-page": "72",
"issue": "March (1)",
"journal-title": "Biosci Trends",
"key": "10.1016/j.ijid.2021.02.014_bib0120",
"volume": "14",
"year": "2020"
},
{
"author": "Gobierno de México",
"first-page": "1",
"key": "10.1016/j.ijid.2021.02.014_bib0125",
"series-title": "Datos Abiertos de México — Información Referente a Casos COVID-19 en México – Bases de Datos COVID-19",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2002032",
"article-title": "Clinical characteristics of coronavirus disease 2019 in China",
"author": "Guan",
"doi-asserted-by": "crossref",
"first-page": "1708",
"issue": "February (18)",
"journal-title": "N Engl J Med",
"key": "10.1016/j.ijid.2021.02.014_bib0130",
"volume": "382",
"year": "2020"
},
{
"DOI": "10.1016/j.bjid.2020.06.002",
"article-title": "Ivermectin: Potential candidate for the treatment of Covid 19",
"author": "Gupta",
"doi-asserted-by": "crossref",
"journal-title": "Braz J Infect Dis",
"key": "10.1016/j.ijid.2021.02.014_bib0135",
"year": "2020"
},
{
"DOI": "10.1016/S0140-6736(20)30183-5",
"article-title": "Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China",
"author": "Huang",
"doi-asserted-by": "crossref",
"first-page": "497",
"issue": "February (10223)",
"journal-title": "Lancet",
"key": "10.1016/j.ijid.2021.02.014_bib0140",
"volume": "395",
"year": "2020"
},
{
"DOI": "10.1016/S0140-6736(20)31042-4",
"article-title": "Triple combination of interferon beta-1b, lopinavir–2013; ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: An open-label, randomised, phase 2 trial",
"author": "Hung",
"doi-asserted-by": "crossref",
"first-page": "1695",
"issue": "May (10238)",
"journal-title": "Lancet",
"key": "10.1016/j.ijid.2021.02.014_bib0145",
"volume": "395",
"year": "2020"
},
{
"DOI": "10.22159/ajpcr.2020.v13i8.38357",
"article-title": "A review on the antiparasitic drug ivermectin for various viral infections and possibilities of using it for novel Severe Acute Respiratory Syndrome Coronavirus 2: New hope to treat coronavirus disease-2019",
"author": "Jabeen",
"doi-asserted-by": "crossref",
"first-page": "21",
"issue": "June",
"journal-title": "Asian J Pharm Clin Res",
"key": "10.1016/j.ijid.2021.02.014_bib0150",
"volume": "13",
"year": "2020"
},
{
"DOI": "10.12688/f1000research.26707.1",
"article-title": "The worldwide clinical trial research response to the COVID-19 pandemic — The first 100 days",
"author": "Janiaud",
"doi-asserted-by": "crossref",
"first-page": "1193",
"journal-title": "F1000Research",
"key": "10.1016/j.ijid.2021.02.014_bib0155",
"volume": "9",
"year": "2020"
},
{
"DOI": "10.1016/j.jmii.2020.03.034",
"article-title": "Treatment options for COVID-19: The reality and challenges",
"author": "Jean",
"doi-asserted-by": "crossref",
"first-page": "436",
"issue": "June (3)",
"journal-title": "J Microbiol Immunol Infect",
"key": "10.1016/j.ijid.2021.02.014_bib0160",
"volume": "53",
"year": "2020"
},
{
"article-title": "SARS-CoV-2 infection-induced immune responses: Friends or foes?",
"author": "Li",
"issue": "August (2)",
"journal-title": "Scand J Immunol",
"key": "10.1016/j.ijid.2021.02.014_bib0165",
"volume": "92",
"year": "2020"
},
{
"article-title": "Understanding severe acute respiratory syndrome coronavirus 2 replication to design efficient drug combination therapies",
"author": "Ortega",
"first-page": "1",
"issue": "October",
"journal-title": "Intervirology",
"key": "10.1016/j.ijid.2021.02.014_bib0170",
"year": "2020"
},
{
"DOI": "10.1016/j.ijantimicag.2020.106053",
"article-title": "Macrolides and viral infections: Focus on Azithromycin in COVID-19 pathology",
"author": "Pani",
"doi-asserted-by": "crossref",
"first-page": "106053",
"journal-title": "Int J Antimicrob Agents",
"key": "10.1016/j.ijid.2021.02.014_bib0175",
"volume": "56",
"year": "2020"
},
{
"DOI": "10.1016/j.cellsig.2020.109721",
"article-title": "Drugs targeting various stages of the SARS-CoV-2 life cycle: Exploring promising drugs for the treatment of Covid-19",
"author": "Poduri",
"doi-asserted-by": "crossref",
"first-page": "109721",
"issue": "October",
"journal-title": "Cell Signal",
"key": "10.1016/j.ijid.2021.02.014_bib0180",
"volume": "74",
"year": "2020"
},
{
"article-title": "Antiviral and anti-inflammatory of Ivermectin and its potential use in COVID-19",
"author": "Portmann-Baracco",
"journal-title": "Arch Bronchomeunol",
"key": "10.1016/j.ijid.2021.02.014_bib0185",
"year": "2020"
},
{
"article-title": "ICON (Ivermectin in COvid Nineteen) study: Use of Ivermectin is associated with lower mortality in hospitalized patients with COVID19",
"author": "Rajter",
"issue": "January",
"journal-title": "medRxiv",
"key": "10.1016/j.ijid.2021.02.014_bib0190",
"year": "2020"
},
{
"DOI": "10.1001/jama.2020.8630",
"article-title": "Association of treatment with Hydroxychloroquine or Azithromycin with in-hospital mortality in patients with COVID-19 in New York State",
"author": "Rosenberg",
"doi-asserted-by": "crossref",
"first-page": "2493",
"issue": "June (24)",
"journal-title": "JAMA",
"key": "10.1016/j.ijid.2021.02.014_bib0195",
"volume": "323",
"year": "2020"
},
{
"article-title": "Pharmacologic treatments for coronavirus disease 2019 (COVID-19): A review",
"author": "Sanders",
"issue": "April",
"journal-title": "JAMA",
"key": "10.1016/j.ijid.2021.02.014_bib0200",
"year": "2020"
},
{
"author": "Secretaría de Salud",
"key": "10.1016/j.ijid.2021.02.014_bib0205",
"series-title": "NORMA Oficial Mexicana NOM-017-SSA2-2012",
"year": "2013"
},
{
"author": "Secretaría de Salud-Gobierno de México",
"key": "10.1016/j.ijid.2021.02.014_bib0210",
"series-title": "Lineamiento Estandarizado para la Vigilancia Epidemiológica y por Laboratorio de la Enfermedad Respiratoria Viral",
"year": "2020"
},
{
"DOI": "10.1097/00004872-199816040-00006",
"article-title": "Effects of angiotensin converting enzyme inhibition on endothelium-dependent vasodilatation in essential hypertensive patients",
"author": "Taddei",
"doi-asserted-by": "crossref",
"first-page": "447",
"issue": "April (4)",
"journal-title": "J Hypertens",
"key": "10.1016/j.ijid.2021.02.014_bib0215",
"volume": "16",
"year": "1998"
},
{
"DOI": "10.1111/jth.14768",
"article-title": "Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia",
"author": "Tang",
"doi-asserted-by": "crossref",
"first-page": "844",
"issue": "April (4)",
"journal-title": "J Thromb Haemost",
"key": "10.1016/j.ijid.2021.02.014_bib0220",
"volume": "18",
"year": "2020"
},
{
"article-title": "An update on COVID-19 pandemic: The epidemiology, pathogenesis, prevention and treatment strategies",
"author": "Tsang",
"first-page": "1",
"issue": "December",
"journal-title": "Expert Rev Anti Infect Ther",
"key": "10.1016/j.ijid.2021.02.014_bib0225",
"year": "2020"
},
{
"DOI": "10.1016/S0140-6736(20)30937-5",
"article-title": "Endothelial cell infection and endotheliitis in COVID-19",
"author": "Varga",
"doi-asserted-by": "crossref",
"first-page": "1417",
"issue": "May (10234)",
"journal-title": "Lancet (London, England)",
"key": "10.1016/j.ijid.2021.02.014_bib0230",
"volume": "395",
"year": "2020"
},
{
"DOI": "10.1016/j.phrs.2020.104950",
"article-title": "Enhanced platelet inhibition treatment improves hypoxemia in patients with severe Covid-19 and hypercoagulability. A case control, proof of concept study",
"author": "Viecca",
"doi-asserted-by": "crossref",
"first-page": "104950",
"journal-title": "Pharmacol Res",
"key": "10.1016/j.ijid.2021.02.014_bib0235",
"volume": "158",
"year": "2020"
},
{
"DOI": "10.1016/S0140-6736(20)31022-9",
"article-title": "Remdesivir in adults with severe COVID-19: A randomised, double-blind, placebo-controlled, multicentre trial",
"author": "Wang",
"doi-asserted-by": "crossref",
"first-page": "1569",
"issue": "May (10236)",
"journal-title": "Lancet (London, England).",
"key": "10.1016/j.ijid.2021.02.014_bib0240",
"volume": "395",
"year": "2020"
},
{
"author": "World Health Organization",
"first-page": "1",
"key": "10.1016/j.ijid.2021.02.014_bib0245",
"series-title": "Contact Tracing in the Context of COVID-19. WHO Guide",
"year": "2020"
}
],
"reference-count": 49,
"references-count": 49,
"relation": {
"has-review": [
{
"asserted-by": "object",
"id": "10.32388/27SABJ",
"id-type": "doi"
}
]
},
"resource": {
"primary": {
"URL": "https://linkinghub.elsevier.com/retrieve/pii/S1201971221001004"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Infectious Diseases",
"Microbiology (medical)",
"General Medicine"
],
"subtitle": [],
"title": "Effectiveness of a multidrug therapy consisting of Ivermectin, Azithromycin, Montelukast, and Acetylsalicylic acid to prevent hospitalization and death among ambulatory COVID-19 cases in Tlaxcala, Mexico",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1016/elsevier_cm_policy",
"volume": "105"
}