Abstract: Hindawi
Advances in Virology
Volume 2022, Article ID 3014686, 6 pages
https://doi.org/10.1155/2022/3014686
Research Article
The Use of Mebendazole in COVID-19 Patients: An Observational
Retrospective Single Center Study
Mostafa W. Galal,1 Mahmoud Ahmed,2 Yanqiu Shao,3,4 Chao Xing,4,5 Wael Ali,6
Abd Elhamid Baly,7 Abdallah Elfky,8 Khaled Amer,9 John Schoggins,10
Hesham A. Sadek ,11,2 and Zeinab N. Gobara 7
1
School of Medicine, Aim Shams University, Cairo, Egypt
Department of Internal Medicine Cardiology, University of Texas Southwestern Medical Center, Dallas, TX 75390, USA
3
Department of Statistical Science, Southern Methodist University, Dallas, TX 75275, USA
4
Department of Population and Data Sciences, University of Texas Southwestern Medical Center, Dallas, TX 75390, USA
5
McDermott Center for Human Growth and Development and Department of Bioinformatics,
University of Texas Southwestern Medical Center, Dallas, TX 75390, USA
6
Egyptian Center for Research in Regenerative Medicine, Cairo, Egypt
7
Department of Clinical Pathology, Cura El-Nasr Hospitals, Cairo, Egypt
8
Department of Radiology, Cura El-Nasr Hospitals, Helwan university, Cairo, Egypt
9
Department of Biochemistry, University of Texas Southwestern Medical Center, Dallas, TX 75390, USA
10
Epartments Microbiology, University of Texas Southwestern Medical Center, Dallas, TX 75390, USA
11
Departments Biophysics and Molecular Biology and Center for Regenerative Science and Medicine,
University of Texas Southwestern Medical Center, Dallas, TX 75390, USA
2
Correspondence should be addressed to Zeinab N. Gobara; zgobaramd@gmail.com
Received 5 February 2022; Revised 16 May 2022; Accepted 26 August 2022; Published 10 December 2022
Academic Editor: Shih-Chao Lin
Copyright © 2022 Mostafa W. Galal et al. Tis is an open access article distributed under the Creative Commons Attribution
License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is
properly cited.
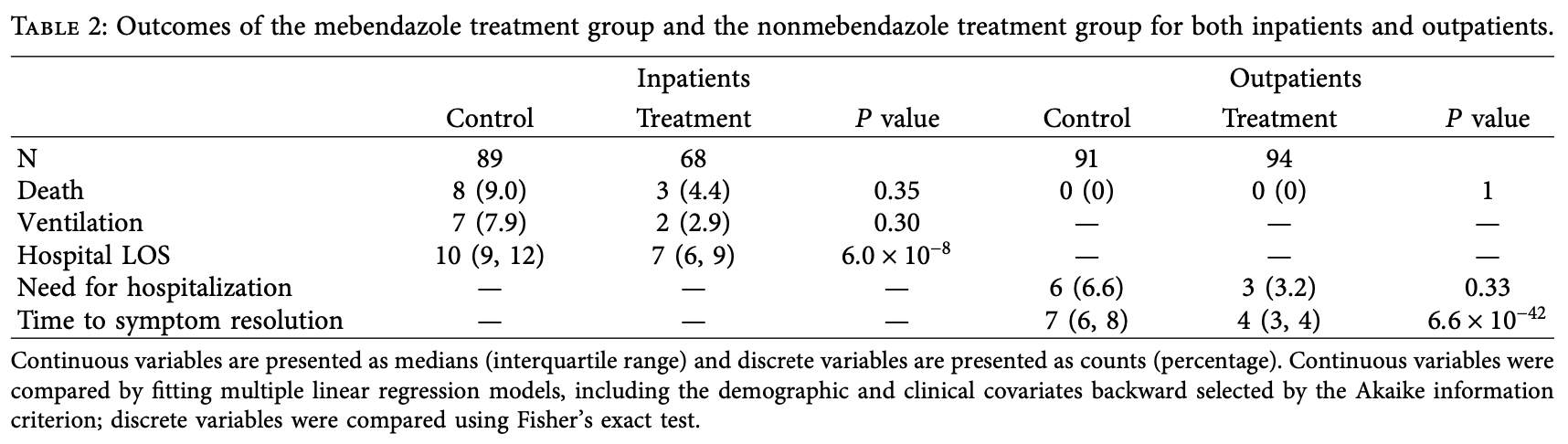
Background. An in-silico screen identifed mebendazole with potential antiviral activity that could be a repurposed drug against SARSCoV-2. Mebendazole is a well-tolerated and cheap antihelminthic agent that is readily available worldwide and thus could be a
therapeutic tool in the fght against COVID-19. Methods. Tis is an observational retrospective study of PCR-confrmed COVID-19
patients who received mebendazole with the intention-to-treat. Te study included an inpatient cohort (157 inpatients) and an outpatient
cohort (185 outpatients). Of the 157 inpatients and 185 outpatients, 68 (43.3%) and 94 (50.8%) received mebendazole, respectively.
Patients who presented within the same timeframe but did not receive mebendazole were used as controls. Patients received standard-ofcare treatment including remdesivir, dexamethasone, and anticoagulants as deemed necessary by the treating physician. Te following
clinical outcomes were evaluated: for the inpatient cohort, length of stay (LOS) at the hospital, need for ventilation (combined invasive
and noninvasive), and mortality; for the outpatient cohort, time to symptom resolution, need for hospitalization, and mortality. Results.
For the inpatient cohort, the median age did not difer between the treatment and control groups; 62 (56, 67) vs. 62 (56, 68), P, and there
was a comparable proportion of males in both groups; 43 (63%) vs. 55 (62%), P � 0.85. Te hospital LOS was 3.5 days shorter in the
treatment group compared to the..
DOI record:
{
"DOI": "10.1155/2022/3014686",
"ISSN": [
"1687-8647",
"1687-8639"
],
"URL": "http://dx.doi.org/10.1155/2022/3014686",
"abstract": "<jats:p>Background. An in-silico screen identified mebendazole with potential antiviral activity that could be a repurposed drug against SARS-CoV-2. Mebendazole is a well-tolerated and cheap antihelminthic agent that is readily available worldwide and thus could be a therapeutic tool in the fight against COVID-19. Methods. This is an observational retrospective study of PCR-confirmed COVID-19 patients who received mebendazole with the intention-to-treat. The study included an inpatient cohort (157 inpatients) and an outpatient cohort (185 outpatients). Of the 157 inpatients and 185 outpatients, 68 (43.3%) and 94 (50.8%) received mebendazole, respectively. Patients who presented within the same timeframe but did not receive mebendazole were used as controls. Patients received standard-of-care treatment including remdesivir, dexamethasone, and anticoagulants as deemed necessary by the treating physician. The following clinical outcomes were evaluated: for the inpatient cohort, length of stay (LOS) at the hospital, need for ventilation (combined invasive and noninvasive), and mortality; for the outpatient cohort, time to symptom resolution, need for hospitalization, and mortality. Results. For the inpatient cohort, the median age did not differ between the treatment and control groups; 62 (56, 67) vs. 62 (56, 68), <jats:inline-formula>\n <math xmlns=\"http://www.w3.org/1998/Math/MathML\" id=\"M1\">\n <mi>P</mi>\n </math>\n </jats:inline-formula>, and there was a comparable proportion of males in both groups; 43 (63%) vs. 55 (62%), <jats:inline-formula>\n <math xmlns=\"http://www.w3.org/1998/Math/MathML\" id=\"M2\">\n <mi>P</mi>\n <mo>=</mo>\n <mn>0.85</mn>\n </math>\n </jats:inline-formula>. The hospital LOS was 3.5 days shorter in the treatment group compared to the control group (<jats:inline-formula>\n <math xmlns=\"http://www.w3.org/1998/Math/MathML\" id=\"M3\">\n <mi>P</mi>\n <mo><</mo>\n <mn>0.001</mn>\n </math>\n </jats:inline-formula>). There were fewer patients who required invasive or noninvasive ventilation in the treatment group, 2 (2.9%) vs. 7 (7.9%), and the mortality rate is lower in the treatment group, 3 (4.4%) vs. 8 (9.0%), though the differences did not reach statistical significance. For the outpatient cohort, the median age was lower in the treatment group compared with the control group; 40 (34, 48) vs. 48 (41, 54), <jats:inline-formula>\n <math xmlns=\"http://www.w3.org/1998/Math/MathML\" id=\"M4\">\n <mi>P</mi>\n <mo><</mo>\n <mn>0.001</mn>\n </math>\n </jats:inline-formula>. There was a comparable proportion of males between both groups; 50 (53%) vs. 52 (57%), <jats:inline-formula>\n <math xmlns=\"http://www.w3.org/1998/Math/MathML\" id=\"M5\">\n <mi>P</mi>\n <mo>=</mo>\n <mn>0.59</mn>\n </math>\n </jats:inline-formula>. Patients in the treatment group were 3.3 days closer to symptom resolution (<jats:inline-formula>\n <math xmlns=\"http://www.w3.org/1998/Math/MathML\" id=\"M6\">\n <mi>P</mi>\n <mo><</mo>\n <mn>0.001</mn>\n </math>\n </jats:inline-formula>). There were numerically fewer patients requiring hospitalization in the treatment group compared with the control group, 3 (3.2%) vs. 6 (6.6%), though this did not reach statistical significance (<jats:inline-formula>\n <math xmlns=\"http://www.w3.org/1998/Math/MathML\" id=\"M7\">\n <mi>P</mi>\n <mo>=</mo>\n <mn>0.33</mn>\n </math>\n </jats:inline-formula>). Conclusion. In this retrospective observational study, the use of mebendazole in COVID-19 patients was associated with shorter hospitalizations in the inpatient cohort and shorter durations of symptom resolution in the outpatient cohort. The findings from this small observational study are hypothesis-generating and preclude drawing conclusions about clinical efficacy. Further studies are needed to examine the role of mebendazole in the treatment of COVID-19 patients.</jats:p>",
"alternative-id": [
"3014686",
"3014686"
],
"author": [
{
"affiliation": [
{
"name": "School of Medicine, Aim Shams University, Cairo, Egypt"
}
],
"family": "Galal",
"given": "Mostafa W.",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Department of Internal Medicine Cardiology, University of Texas Southwestern Medical Center, Dallas, TX 75390, USA"
}
],
"family": "Ahmed",
"given": "Mahmoud",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Statistical Science, Southern Methodist University, Dallas, TX 75275, USA"
},
{
"name": "Department of Population and Data Sciences, University of Texas Southwestern Medical Center, Dallas, TX 75390, USA"
}
],
"family": "Shao",
"given": "Yanqiu",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Population and Data Sciences, University of Texas Southwestern Medical Center, Dallas, TX 75390, USA"
},
{
"name": "McDermott Center for Human Growth and Development and Department of Bioinformatics, University of Texas Southwestern Medical Center, Dallas, TX 75390, USA"
}
],
"family": "Xing",
"given": "Chao",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Egyptian Center for Research in Regenerative Medicine, Cairo, Egypt"
}
],
"family": "Ali",
"given": "Wael",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Clinical Pathology, Cura El-Nasr Hospitals, Cairo, Egypt"
}
],
"family": "Baly",
"given": "Abd Elhamid",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Radiology, Cura El-Nasr Hospitals, Helwan university, Cairo, Egypt"
}
],
"family": "Elfiky",
"given": "Abdallah",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Biochemistry, University of Texas Southwestern Medical Center, Dallas, TX 75390, USA"
}
],
"family": "Amer",
"given": "Khaled",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Epartments Microbiology, University of Texas Southwestern Medical Center, Dallas, TX 75390, USA"
}
],
"family": "Schoggins",
"given": "John",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-4745-366X",
"affiliation": [
{
"name": "Departments Biophysics and Molecular Biology and Center for Regenerative Science and Medicine, University of Texas Southwestern Medical Center, Dallas, TX 75390, USA"
},
{
"name": "Department of Internal Medicine Cardiology, University of Texas Southwestern Medical Center, Dallas, TX 75390, USA"
}
],
"authenticated-orcid": true,
"family": "Sadek",
"given": "Hesham A.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-8921-259X",
"affiliation": [
{
"name": "Department of Clinical Pathology, Cura El-Nasr Hospitals, Cairo, Egypt"
}
],
"authenticated-orcid": true,
"family": "Gobara",
"given": "Zeinab N.",
"sequence": "additional"
}
],
"container-title": "Advances in Virology",
"container-title-short": "Advances in Virology",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2022,
12,
10
]
],
"date-time": "2022-12-10T19:35:09Z",
"timestamp": 1670700909000
},
"deposited": {
"date-parts": [
[
2022,
12,
10
]
],
"date-time": "2022-12-10T19:35:12Z",
"timestamp": 1670700912000
},
"editor": [
{
"affiliation": [],
"family": "Lin",
"given": "Shih-Chao",
"sequence": "additional"
}
],
"indexed": {
"date-parts": [
[
2022,
12,
11
]
],
"date-time": "2022-12-11T05:27:10Z",
"timestamp": 1670736430808
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2022,
12,
10
]
]
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "unspecified",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
12,
10
]
],
"date-time": "2022-12-10T00:00:00Z",
"timestamp": 1670630400000
}
}
],
"link": [
{
"URL": "http://downloads.hindawi.com/journals/av/2022/3014686.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "http://downloads.hindawi.com/journals/av/2022/3014686.xml",
"content-type": "application/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "http://downloads.hindawi.com/journals/av/2022/3014686.pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "98",
"original-title": [],
"page": "1-6",
"prefix": "10.1155",
"published": {
"date-parts": [
[
2022,
12,
10
]
]
},
"published-print": {
"date-parts": [
[
2022,
12,
10
]
]
},
"publisher": "Hindawi Limited",
"reference": [
{
"DOI": "10.1080/01652176.2020.1727993",
"doi-asserted-by": "publisher",
"key": "1"
},
{
"DOI": "10.1038/s41586-020-2008-3",
"doi-asserted-by": "publisher",
"key": "2"
},
{
"DOI": "10.1038/s41586-020-2012-7",
"doi-asserted-by": "publisher",
"key": "3"
},
{
"DOI": "10.1038/s41467-021-22580-8",
"doi-asserted-by": "publisher",
"key": "4"
},
{
"DOI": "10.1038/s41564-020-00835-2",
"doi-asserted-by": "publisher",
"key": "5"
},
{
"DOI": "10.1101/2021.06.17.21258639",
"doi-asserted-by": "publisher",
"key": "6"
},
{
"DOI": "10.1126/science.abl4784",
"doi-asserted-by": "publisher",
"key": "7"
},
{
"DOI": "10.1136/bmj.n2713",
"doi-asserted-by": "publisher",
"key": "8"
},
{
"article-title": "Identification of atovaquone as and mebendazole as repurposed drugs with antiviral activity against SARS-CoV-2 chemRxiv",
"author": "A. F. Mahmoud Ahmed",
"key": "9",
"year": "2021"
},
{
"DOI": "10.1001/jama.1974.03240100030022",
"doi-asserted-by": "publisher",
"key": "10"
},
{
"DOI": "10.1128/CMR.14.1.114-128.2001",
"doi-asserted-by": "publisher",
"key": "11"
},
{
"DOI": "10.1136/bmj.l4257",
"doi-asserted-by": "publisher",
"key": "12"
},
{
"DOI": "10.3347/kjp.2021.59.3.189",
"doi-asserted-by": "publisher",
"key": "13"
},
{
"DOI": "10.1016/0020-7519(88)90175-0",
"doi-asserted-by": "publisher",
"key": "14"
},
{
"DOI": "10.3390/cancers11091284",
"doi-asserted-by": "publisher",
"key": "15"
},
{
"article-title": "Management protocol for COVID-19 patients version 1.4/30th may 2020 ministry of health and population (MOHP), Egypt",
"author": "H. H. Masoud",
"key": "16",
"year": "2020"
},
{
"DOI": "10.1186/s12879-018-3201-y",
"doi-asserted-by": "publisher",
"key": "17"
},
{
"author": "LiverTox",
"key": "18",
"volume-title": "Clinical and Research Information on Drug-Induced Liver Injury",
"year": "2012"
},
{
"DOI": "10.1080/00034983.1982.11687523",
"doi-asserted-by": "publisher",
"key": "19"
},
{
"DOI": "10.1186/s13643-021-01636-2",
"doi-asserted-by": "publisher",
"key": "20"
},
{
"DOI": "10.1371/journal.pone.0252411",
"doi-asserted-by": "publisher",
"key": "21"
},
{
"DOI": "10.1007/s40620-020-00790-5",
"doi-asserted-by": "publisher",
"key": "22"
},
{
"DOI": "10.1016/j.antiviral.2020.104787",
"doi-asserted-by": "publisher",
"key": "23"
},
{
"DOI": "10.1038/s41421-020-0156-0",
"doi-asserted-by": "publisher",
"key": "24"
},
{
"article-title": "Toxicology rounds: ivermectin not the crisis it’s claimed to Be",
"author": "L. Gussow",
"journal-title": "Emergency Medicine News",
"key": "25",
"volume": "43",
"year": "2021"
},
{
"article-title": "Identification of atovaquone and mebendazole as repurposed drugs with antiviral activity against SARS-CoV-2 (version 5)",
"author": "M. Ahmed",
"key": "26",
"year": "2021"
}
],
"reference-count": 26,
"references-count": 26,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.hindawi.com/journals/av/2022/3014686/"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Infectious Diseases",
"Virology"
],
"subtitle": [],
"title": "The Use of Mebendazole in COVID-19 Patients: An Observational Retrospective Single Center Study",
"type": "journal-article",
"volume": "2022"
}