Randomized trials - Ivermectin repurposing for COVID-19 treatment of outpatients with mild disease in primary health care centers
Rossana Elena Chahla, Luis Medina Ruiz, Teresa Mena, Yolanda Brepe, Paola Terranova, Eugenia Silvana Ortega, Guillermo Gabriel Barrenechea, Daniel Gustavo Goroso, María De Los Ángeles Peral De Bruno
Research, Society and Development, doi:10.33448/rsd-v11i8.30844
Randomized trials -Ivermectin repurposing for COVID-19 treatment of outpatients with mild disease in primary health care centers Ensaio aleatório -Reutilização de Ivermectina para tratamento COVID-19 de pacientes ambulatórios com doença leve em centros primários de saúde Ensayos aleatorios -Reutilización de la Ivermectina para el tratamiento con COVID-19 de pacientes ambulatorios con enfermedad leve en centros de atención primaria
Declarations Ethics approval and consent to participate The people who agreed to participate in the study gave their informed consent before starting the study. The protocol was approved by Independent Ethical Committee / Health Research Directorate, Ministry of Health, Tucumán Government, Argentina, IRB: 054/2020 in accordance with the ReNIS, National Register in Health Research, Argentina /0032.
Consent for publication "Not applicable"
Conflict of Interests The authors did not receive any monetary compensation for this work. They declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Authors' contributions ESO supervised the database. GGB, ESO and DGG contributed with the data processing and contributed to the statistical analysis. ESO, DGG and MPB were responsible for writing the manuscript. TM, YB and PT contributed to data collection. REC and LMR were the institutional managers to carry out the work. MPB supervised the project.
References
Ashburn, Thor, Drug repositioning: identifying and developing new uses for existing drugs, Nature Reviews Drug Discovery,
doi:10.1038/nrd1468Bottan, Vera-Cossio, Hoffmann, The unequal impact of the coronavirus pandemic: evidence of seventeen development countries
Bryant, Lawrie, Dowswell, Fordham, Mitchell et al., Ivermectin for prevention and treatment of COVID-19 infection: a systematic review, meta-analysis, and trial sequential analysis to inform clinical guidelines, American Journal of Therapeutics,
doi:10.1097/MJT.0000000000001442Caly, Druce, Catton, Jans, Wagsta, The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro, Antiviral Research
Caly, Wagstaff, Jans, Nuclear trafficking of proteins from RNA viruses: potential target for antivirals?, Antiviral Research
Carvallo, Hirsch, Alkis, Contreras, Study of the Efficacy and Safety of Topical Ivermectin + Iota-Carrageenan in the Prophylaxis against COVID-19 in Health Personnel, Journal of Biomedical Research and Clinical Investigation
Carvallo, Hirsch, Farinella, Safety and efficacy of the combined use of ivermectin, dexamethasone, enoxaparin and aspirin against COVID 19, medRxiv the preprint server for health sciences,
doi:10.1101/2020.09.10.20191619Cassará, Ivermectina asociada a iota-Carragenina aplicada localmente en la cavidad bucal, en la profilaxis de la enfermedad COVID-19 en el personal de salud, Estudio IVERCAR01
Chaccour, Casellas, Blanco-Di Matteo, The effect of early treatment with ivermectin on viral load, symptoms and humoral response in patients with non-severe COVID-19: A pilot, double-blind, placebo-controlled, randomized clinical trial, EClinicalMedicine
Chahla, Medina Ruiz, Mena, Cluster Randomised Trials -Ivermectin Repurposing for COVID-19 Treatment of Outpatient with Mild Disease in Primary Health Care Centers,
doi:10.1101/2021.03.29.21254554v1Chahla, Medina Ruiz, Mena, Cluster Randomised Trials -Ivermectin Repurposing for COVID-19 Treatment of Outpatient with Mild Disease in Primary Health Care Centers,
doi:10.21203/rs.3.rs-495945/v1Chahla, Medina Ruiz, Mena, Cluster Randomised Trials -Ivermectin Repurposing for COVID-19 Treatment of Outpatient with Mild Disease in Primary Health Care Centers,
doi:10.21203/rs.3.rs-495945/v2Chahla, Medina Ruiz, Ortega, Prophylaxis Covid-19 in Healthcare Agents by Intensive Treatment with Ivermectin and Iotacarrageenan (Ivercar-Tuc), American Journal of Therapeutics
Dyall, Coleman, Hart, Repurposing of clinically developed drugs for treatment of Middle East respiratory syndrome coronavirus infection, Antimicrobial Agents and Chemotherapy
Edwards, Dingsdale, Helsby, Orme, Breckenridge, The relative systemic availability of ivermectin after administration as capsule, tablet, and oral solution, European Journal of Clinical Pharmacology,
doi:10.1007/BF00637608Elgazzar, Hany, Youssef, Efficacy and Safety of Ivermectin for Treatment and prophylaxis of COVID-19 Pandemic, Research Square,
doi:10.21203/rs.3.rs-100956/v1Krolewiecki, Lifschitz, Moragas, Antiviral Effect of High-Dose Ivermectin in Adults with COVID-19: A Pilot Randomised, Controlled, Open Label, Multicentre Trial, SSRN Electronic Journal,
doi:10.2139/ssrn.3714649Lima-Morales, Méndez-Hernández, Flores, Effectiveness of a multidrug therapy consisting of ivermectin, azithromycin, montelukast and acetylsalicylic acid to prevent hospitalization and death among ambulatory COVID-19 cases in Tlaxcala, Mexico, International Journal of Infectious Diseases
Liu, Fang, Reagan, In silico drug repositioning-what we need to know, Drug Discovery Today
López-Medina, López, Hurtado, Effect of Ivermectin on Time to Resolution of Symptoms Among Adults with Mild COVID-19: A Randomized Clinical Trial, Journal American Medical Association
Marshall, Murthy, Diaz, A minimal common outcome measure set for COVID-19 clinical research, Lancet Infectious Diseases
Mayer, Krolewiecki, Ferrero, Bocchio, Barbero et al., Safety and Efficacy of a MEURI Program for the Use of High Dose Ivermectin in COVID-19 Patients, Frontiers in Public Health,
doi:.10.3389/fpubh.2022.813378Osterberg, Blaschke, Adherence to Medication, The New England Journal of Medicine
Padhi, Pati, Panda, Effect of ivermectin in the treatment of COVID-19: a trial sequential analysis highlighted the requirement of additional randomized controlled trials, Clinical Infectious Diseases,
doi:ciab692.10.1093/cid/ciab692Rodríguez Chamorro, García-Jiménez, Amariles, Rodríguez Chamorro, Faus, Revisión de tests de medición del cumplimiento terapéutico utilizados en la práctica clínica, Atención primaria,
doi:10.1157/13125407Roman, Burela, Pasupuleti, Piscoya, Vidal et al., Ivermectin for the treatment of COVID-19: a systematic review and meta-analysis of randomized controlled trials, Clinical of Infectious Diseases
Rubin, Crowe, COVID-19 Symptoms: Longitudinal Evolution and Persistence in Outpatient Settings
Wu, Mc Googan, Characteristics of and Important Lessons from the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72314 Cases from the Chinese Center for Disease Control and Prevention, Journal American Medical Association
Zhou, Yu, Du, Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study, Lancet,
doi:10.1016/S0140-6736(20)30566-3DOI record:
{
"DOI": "10.33448/rsd-v11i8.30844",
"ISSN": [
"2525-3409"
],
"URL": "http://dx.doi.org/10.33448/rsd-v11i8.30844",
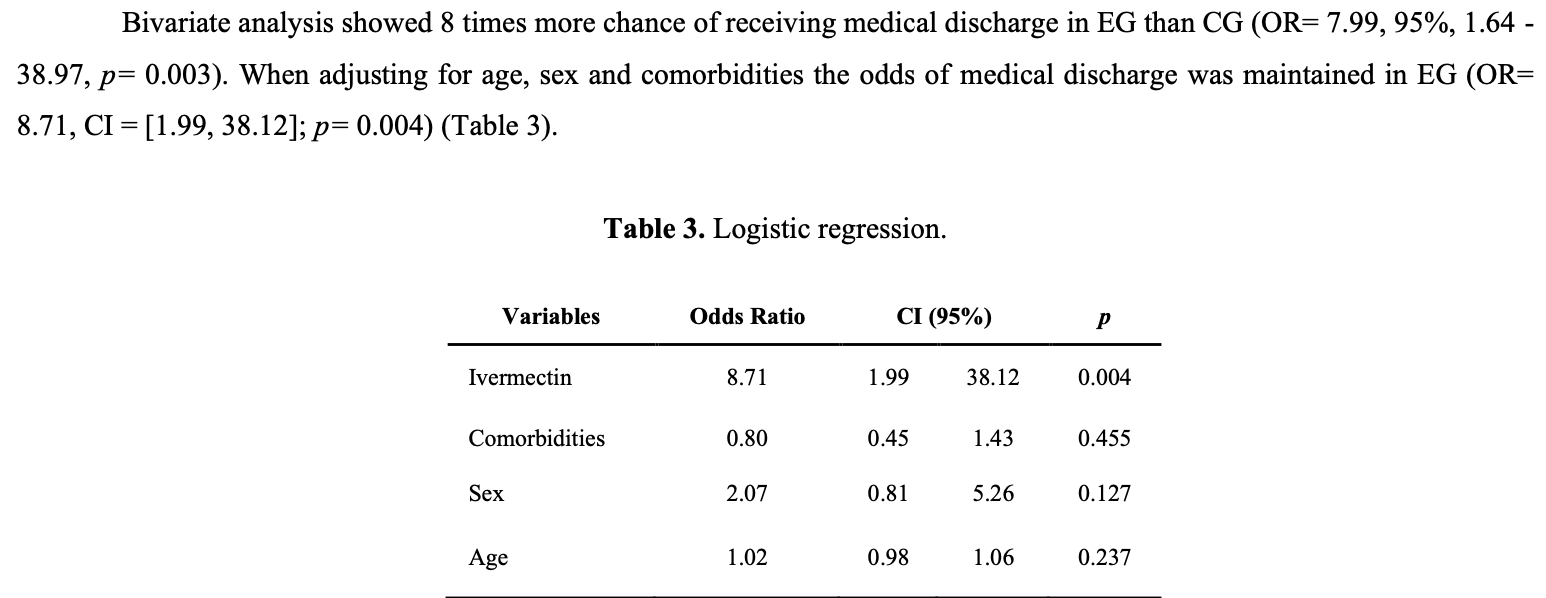
"abstract": "<jats:p>Objective: to evaluate the therapeutic intervention with Ivermectin in outpatients with COVID-19 mild disease, to increase medical discharge and prevent the progression to moderate or severe disease. Methods: Randomized Trial, n= 254. The subjects were divided into experimental (EG: n= 110) and control groups (CG: n= 144). The EG received Ivermectin orally 0.6 mg/kg weight in two doses. All participants were by physical examination COVID-19 diagnosed with negative RT-PCR at the beginning and the end of protocol. Differences between the variables were determined using the Chi-square test (p<0.05). The contagion risk (Odds Ratio) was calculated using software STATA. Results: Both groups were similar in age, sex, and comorbidities. A significant reduction in the percentage of participants with symptoms (PPS) was observed in the EG and CG when the clinical evaluation of symptoms was performed from 5th to 9th day (p= 0.0005). When the clinical evaluation was performed from 10th to 14th day there was no significant difference. A higher proportion of medical discharge was observed in EG (98.2%) vs. CG (86.1%) (p= 0.0007). EG showed 8 times more chance of receiving medical discharge than CG (OR 8.71, 95% CI: 1·99 – 38.12, p= 0.004). The treatment effect with Ivermectin to obtain medical discharge from outpatient care was analyzed by logistic regression. Then, the chance to obtain medical discharge was independent of variables sex, age, and comorbidities. Conclusion: This work supports the potential efficacy of Ivermectin in outpatient care with mild COVID-19 as a potentially useful intervention of public health consideration.</jats:p>",
"author": [
{
"ORCID": "http://orcid.org/0000-0002-3016-612X",
"affiliation": [],
"authenticated-orcid": false,
"family": "Chahla",
"given": "Rossana Elena",
"sequence": "first"
},
{
"ORCID": "http://orcid.org/0000-0002-2014-1666",
"affiliation": [],
"authenticated-orcid": false,
"family": "Ruiz",
"given": "Luis Medina",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-9838-5559",
"affiliation": [],
"authenticated-orcid": false,
"family": "Mena",
"given": "Teresa",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-4071-4927",
"affiliation": [],
"authenticated-orcid": false,
"family": "Brepe",
"given": "Yolanda",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-0370-3599",
"affiliation": [],
"authenticated-orcid": false,
"family": "Terranova",
"given": "Paola",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-8339-1160",
"affiliation": [],
"authenticated-orcid": false,
"family": "Ortega",
"given": "Eugenia Silvana",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-5781-8327",
"affiliation": [],
"authenticated-orcid": false,
"family": "Barrenechea",
"given": "Guillermo Gabriel",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-5454-9873",
"affiliation": [],
"authenticated-orcid": false,
"family": "Goroso",
"given": "Daniel Gustavo",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-0632-9427",
"affiliation": [],
"authenticated-orcid": false,
"family": "Peral de Bruno",
"given": "María de los Ángeles",
"sequence": "additional"
}
],
"container-title": "Research, Society and Development",
"container-title-short": "RSD",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2022,
6,
24
]
],
"date-time": "2022-06-24T11:54:03Z",
"timestamp": 1656071643000
},
"deposited": {
"date-parts": [
[
2022,
6,
24
]
],
"date-time": "2022-06-24T11:54:03Z",
"timestamp": 1656071643000
},
"indexed": {
"date-parts": [
[
2022,
6,
24
]
],
"date-time": "2022-06-24T12:15:58Z",
"timestamp": 1656072958816
},
"is-referenced-by-count": 0,
"issue": "8",
"issued": {
"date-parts": [
[
2022,
6,
21
]
]
},
"journal-issue": {
"issue": "8",
"published-online": {
"date-parts": [
[
2022,
6,
6
]
]
}
},
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0",
"content-version": "unspecified",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
6,
21
]
],
"date-time": "2022-06-21T00:00:00Z",
"timestamp": 1655769600000
}
}
],
"link": [
{
"URL": "https://rsdjournal.org/index.php/rsd/article/download/30844/26582",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://rsdjournal.org/index.php/rsd/article/download/30844/26582",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "19099",
"original-title": [],
"page": "e35511830844",
"prefix": "10.33448",
"published": {
"date-parts": [
[
2022,
6,
21
]
]
},
"published-online": {
"date-parts": [
[
2022,
6,
21
]
]
},
"publisher": "Research, Society and Development",
"reference-count": 0,
"references-count": 0,
"relation": {},
"resource": {
"primary": {
"URL": "https://rsdjournal.org/index.php/rsd/article/view/30844"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subtitle": [],
"title": "Randomized trials - Ivermectin repurposing for COVID-19 treatment of outpatients with mild disease in primary health care centers",
"type": "journal-article",
"volume": "11"
}