Clinical Variants, Characteristics, and Outcomes Among COVID-19 Patients: A Case Series Analysis at a Tertiary Care Hospital in Karachi, Pakistan
Tasnim Ahsan, Bharta Rani, Glenis Roomana Siddiqui, Glenis D‘souza, Razzaq Memon, Irfan Lutfi, Omer I. Hasan, Rushma Javed, Farhan Khan, Muhammad Hassan
Cureus, doi:10.7759/cureus.14761
Introduction Coronavirus disease 2019 has become a global threat to public health. The current study investigates alterations in the biological estimates concerning the severity, recovery, mortality, and assessment of treatment-based outcomes.
Methods A case series of 165 COVID-19 patients admitted to OMI Institute (a tertiary care hospital) was conducted between May and August 2020. The data regarding demographic characteristics, comorbid conditions, radiographic abnormalities, biological estimations, symptoms, treatment, disease progression, complications, and outcomes were recorded using a structured questionnaire. Laboratory estimations included complete blood count (CBC), renal and electrolyte profile, liver function tests (LFTs), hematological indices, and inflammatory markers. Chest X-ray, electrocardiogram (ECG), and a high-resolution computed tomography (HRCT) scan were also performed, and data were extracted from the medical records. Analysis was done using the Statistical Package for the Social Sciences (SPSS) version 22.0.
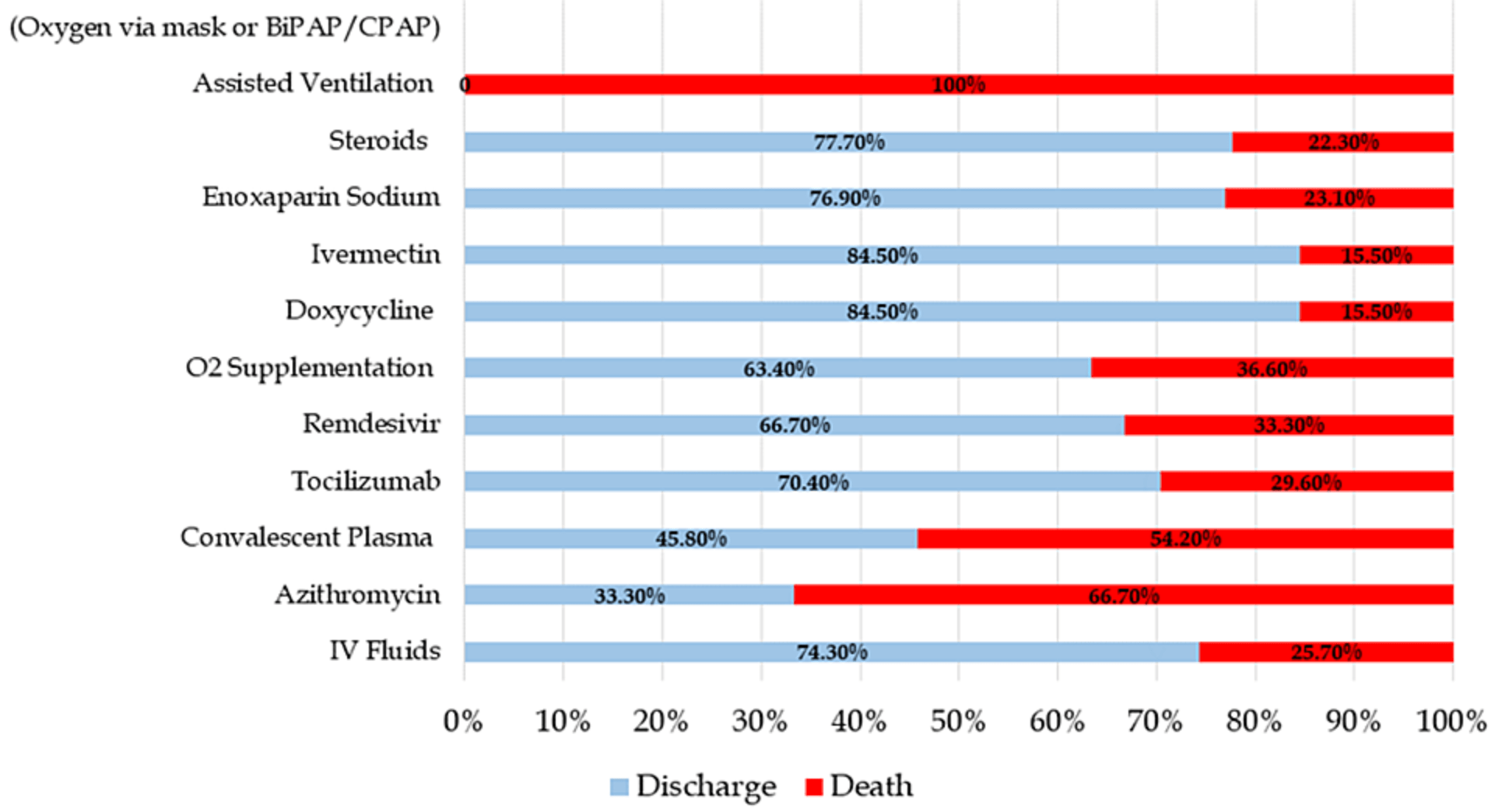
Results Out of the 165 COVID-19 patients, 79.4% recovered and were successfully discharged, while 20.6% of inpatient died. The patients' mean age was 56.03 ± 15.96 years, with a male majority (55.1%). The most common comorbid conditions were diabetes and hypertension; fever and dry cough were among the most frequently reported symptoms. The chest imaging findings among the severe/critical COVID-19 patients showed extensive bilateral patchy opacities. The median laboratory investigations, including neutrophil-tolymphocyte ratio (NLR) (14.83), C-reactive protein (CRP) (7.4 mg/dl), lactate dehydrogenase (LDH) (786 IU/L), ferritin (1401.15 mcg/ml), and mean oxygen saturation (88.25%), were significantly altered among cases with increased disease severity and those who expired (p<0.05). The proportion of acute respiratory distress syndrome (ARDS) and sepsis development was significantly high among severe/critical COVID-19 patients (p<0.05). Treatment with tocilizumab, remdesivir, doxycycline, ivermectin, enoxaparin sodium, and steroids was deemed to be potentially effective treatment options in terms of reducing COVID-19 severity and chances of recovery. Furthermore, age (OR 1.05; p=0.047), presence of comorbidity (OR 8.471; p=0.004), high NLR, LDH (final outcome) (OR 1.361 and 1.018; p<0.05), and CRP levels (midpoint) (OR 1.631; p=0.05) were identified as the strong predictors of death among COVID-19 patients.
Conclusion The study identified several alterations in the clinical profile of the COVID-19 patients concerning severity during the hospital stay, affecting prognosis. Clinically, tocilizumab, remdesivir, doxycycline, ivermectin, enoxaparin sodium, and steroids were identified as potential therapeutic options for COVID-19 due to their ability to alter disease-associated severity and recovery rate.
Additional Information Disclosures Human subjects: Consent was obtained or waived by all participants in this study. Medicell Institute of Diabetes, Endocrinology & Metabolism (MIDEM) issued approval IRB-005/MHS/20. The Independent Ethical Review Committee of Medicell Institute of Diabetes, Endocrinology & Metabolism (MIDEM) approved the study protocol (Reference no. IRB-005/MHS/20; Dated: 27th April 2020). Written informed consent was obtained from each patient or the next of kin before inclusion in the study. Animal subjects: All authors have confirmed that this study did not involve animal subjects or tissue. Conflicts of interest: In compliance with the ICMJE uniform disclosure form, all authors declare the following: Payment/services info: All authors have declared that no financial support was received from any organization for the submitted work. Financial relationships: All authors have declared that they have no financial relationships at present or within the previous three years with any organizations that might have an interest in the submitted work. Other relationships: All authors have declared that there are no other relationships or activities that could appear to have influenced the submitted work.
References
Adhikari, Meng, Wu, Epidemiology, causes, clinical manifestation and diagnosis, prevention and control of coronavirus disease (COVID-19) during the early outbreak period: a scoping review, Infect Dis Poverty,
doi:10.1186/s40249-020-00646-xAsghar, Kazmi, Khan, Clinical profiles, characteristics, and outcomes of the first 100 admitted COVID-19 patients in Pakistan: a single-center retrospective study in a tertiary care hospital of Karachi, Cureus,
doi:10.7759/cureus.c34Ciccullo, Borghetti, Verme, Neutrophil-to-lymphocyte ratio and clinical outcome in COVID-19: a report from the Italian front line, Int J Antimicrob Agents
Duan, Liu, Li, Effectiveness of convalescent plasma therapy in severe COVID-19 patients, Proc Natl Acad Sci U S A,
doi:10.1073/pnas.2004168117Falasca, Nardacci, Colombo, Postmortem findings in Italian patients with COVID-19: a descriptive full autopsy study of cases with and without comorbidities, J Infect Dis,
doi:10.1093/infdis/jiaa578Francone, Iafrate, Masci, Chest CT score in COVID-19 patients: correlation with disease severity and short-term prognosis, Eur Radiol,
doi:10.1007/s00330-020-07033-yHorby, Campbell, Staplin, Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): preliminary results of a randomised, controlled, open-label,
doi:10.1101/2021.02.11.21249258Jeronimo, Farias, Val, Methylprednisolone as adjunctive therapy for patients hospitalized with COVID-19 (Metcovid): a randomised, double-blind, phase IIb, placebo-controlled trial, Clin Infect Dis,
doi:10.1093/cid/ciaa1177Khan Chachar, Khan, Khan, Clinical and demographic characteristics including comorbidities and their outcomes among patients hospitalized with COVID-19 in four tertiary care hospitals across Lahore, Cureus,
doi:10.7759/cureus.12663Liu, Li, Liu, Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS-CoV-2 infected patients, EBioMedicine,
doi:10.1016/j.ebiom.2020.102763Mejía, Medina, Cornejo, Oxygen saturation as a predictor of mortality in hospitalized adult patients with COVID-19 in a public hospital in Lima, Peru, PLoS One,
doi:10.1371/journal.pone.0244171Mushtaq, Zafar Mahmood, Jamil, Aziz, Ali, Outcome of COVID-19 patients with use of Tocilizumab: a single center experience, Int Immunopharmacol,
doi:10.1016/j.intimp.2020.106926Riaz, Saleem, Hazrat, Ahmed, Sajid et al., Mental health outcomes and coping strategies among health care workers exposed to coronavirus disease 2019 (COVID-19), IJEHSR
Simonovich, Pratx, Scibona, A randomized trial of convalescent plasma in Covid-19 severe pneumonia, N Engl J Med,
doi:10.1056/NEJMoa2031304Syed, Shamim, Zaidi, Past and current coronavirus outbreaks; focusing on coronavirus disease 2019 in comparison with severe acute respiratory syndrome and middle east respiratory syndrome, Int J Endorsing Health Sci Res,
doi:10.29052/IJEHSR.v8.i3.2020.159-170Wang, Zhang, Du, Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial, Lancet,
doi:10.1016/S0140-6736(20)31022-9Wu, Chen, Cai, Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China, JAMA Int Med,
doi:10.1001/jamainternmed.2020.0994Wu, Chen, Cai, Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China, JAMA Intern Med,
doi:10.1001/jamainternmed.2020.0994Wu, Mcgoogan, Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China. Summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention, JAMA,
doi:10.1001/jama.2020.2648Zhou, Chen, Wang, Zhao, Wei et al., Low serum calcium: a new, important indicator of COVID-19 patients from mild/moderate to severe/critical, Biosci Rep,
doi:10.1042/BSR20202690Zhou, Yu, Du, Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study, Lancet,
doi:10.1016/S0140-6736(20)30566-3DOI record:
{
"DOI": "10.7759/cureus.14761",
"ISSN": [
"2168-8184"
],
"URL": "http://dx.doi.org/10.7759/cureus.14761",
"author": [
{
"affiliation": [],
"family": "Ahsan",
"given": "Tasnim",
"sequence": "first"
},
{
"affiliation": [],
"family": "Rani",
"given": "Bharta",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Siddiqui",
"given": "Roomana",
"sequence": "additional"
},
{
"affiliation": [],
"family": "D‘Souza",
"given": "Glenis",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Memon",
"given": "Razzaq",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lutfi",
"given": "Irfan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "I. Hasan",
"given": "Omer",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Javed",
"given": "Rushma",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Khan",
"given": "Farhan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hassan",
"given": "Muhammad",
"sequence": "additional"
}
],
"container-title": "Cureus",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2021,
4,
29
]
],
"date-time": "2021-04-29T17:03:19Z",
"timestamp": 1619715799000
},
"deposited": {
"date-parts": [
[
2024,
2,
9
]
],
"date-time": "2024-02-09T19:27:06Z",
"timestamp": 1707506826000
},
"indexed": {
"date-parts": [
[
2024,
3,
28
]
],
"date-time": "2024-03-28T16:38:34Z",
"timestamp": 1711643914789
},
"is-referenced-by-count": 5,
"issued": {
"date-parts": [
[
2021,
4,
29
]
]
},
"language": "en",
"link": [
{
"URL": "https://www.cureus.com/articles/56545-clinical-variants-characteristics-and-outcomes-among-covid-19-patients-a-case-series-analysis-at-a-tertiary-care-hospital-in-karachi-pakistan",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "297",
"original-title": [],
"prefix": "10.7759",
"published": {
"date-parts": [
[
2021,
4,
29
]
]
},
"published-print": {
"date-parts": [
[
2021,
4,
29
]
]
},
"publisher": "Springer Science and Business Media LLC",
"reference": [
{
"DOI": "10.29052/IJEHSR.v8.i3.2020.159-170",
"article-title": "Past and current coronavirus outbreaks; focusing on coronavirus disease 2019 in comparison with severe acute respiratory syndrome and middle east respiratory syndrome",
"author": "Syed I",
"doi-asserted-by": "publisher",
"journal-title": "Int J Endorsing Health Sci Res",
"key": "ref1",
"unstructured": "Syed I, Shamim N, Zaidi S. Past and current coronavirus outbreaks; focusing on coronavirus disease 2019 in comparison with severe acute respiratory syndrome and middle east respiratory syndrome. Int J Endorsing Health Sci Res. 2020, 8:159-170. 10.29052/IJEHSR.v8.i3.2020.159-170",
"volume": "8",
"year": "2020"
},
{
"DOI": "10.1016/j.tim.2016.03.003",
"article-title": "Epidemiology, genetic recombination, and pathogenesis of coronaviruses",
"author": "Su S",
"doi-asserted-by": "publisher",
"journal-title": "Trends Microbiol",
"key": "ref2",
"unstructured": "Su S, Wong G, Shi W, et al.. Epidemiology, genetic recombination, and pathogenesis of coronaviruses. Trends Microbiol. 2016, 24:490-502. 10.1016/j.tim.2016.03.003",
"volume": "24",
"year": "2016"
},
{
"key": "ref3",
"unstructured": "World Health Organization (WHO). Coronavirus disease (COVID-19) pandemic. (2020). Accessed. March 18, 2021: https://covid19.who.int/."
},
{
"DOI": "10.29052/2412-3188.v7.i1.2020.9-18",
"article-title": "Psychological response & perceived risk associated with coronavirus disease",
"author": "Khan S",
"doi-asserted-by": "publisher",
"journal-title": "Ann Psychophysiol",
"key": "ref4",
"unstructured": "Khan S, Saleem Y, Aziz S. Psychological response & perceived risk associated with coronavirus disease. Ann Psychophysiol. 2020, 7:9-18. 10.29052/2412-3188.v7.i1.2020.9-18",
"volume": "7",
"year": "2020"
},
{
"DOI": "10.29052/JEHSR.v8.i2.2020.56-66",
"article-title": "Mental health outcomes and coping strategies among health care workers exposed to coronavirus disease 2019 (COVID-19)",
"author": "Riaz S",
"doi-asserted-by": "crossref",
"journal-title": "IJEHSR",
"key": "ref5",
"unstructured": "Riaz S, Saleem Y, Hazrat H, Ahmed F, Sajid U, Qadri SF, Rufan S. Mental health outcomes and coping strategies among health care workers exposed to coronavirus disease 2019 (COVID-19). IJEHSR. 2020, 8:56-66.",
"volume": "8",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2007764",
"article-title": "Remdesivir for the treatment of Covid-19 - final report",
"author": "Beigel JH",
"doi-asserted-by": "publisher",
"journal-title": "N Engl J Med",
"key": "ref6",
"unstructured": "Beigel JH, Tomashek KM, Dodd LE, et al.. Remdesivir for the treatment of Covid-19 - final report. N Engl J Med. 2020, 383:1813-26. 10.1056/NEJMoa2007764",
"volume": "383",
"year": "2020"
},
{
"DOI": "10.1016/S0140-6736(20)31022-9",
"article-title": "Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial",
"author": "Wang Y",
"doi-asserted-by": "publisher",
"journal-title": "Lancet",
"key": "ref7",
"unstructured": "Wang Y, Zhang D, Du G, et al.. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020, 395:1569-78. 10.1016/S0140-6736(20)31022-9",
"volume": "395",
"year": "2020"
},
{
"DOI": "10.1056%2FNEJMoa2021436",
"article-title": "Dexamethasone in hospitalized patients with COVID-19 - preliminary report",
"author": "The Recovery Collaborative Group",
"doi-asserted-by": "publisher",
"journal-title": "N Engl J Med",
"key": "ref8",
"unstructured": "The Recovery Collaborative Group. Dexamethasone in hospitalized patients with COVID-19 - preliminary report. N Engl J Med. 2021, 384:693-704. 10.1056%2FNEJMoa2021436",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1093/cid/ciaa1177",
"article-title": "Methylprednisolone as adjunctive therapy for patients hospitalized with COVID-19 (Metcovid): a randomised, double-blind, phase IIb, placebo-controlled trial",
"author": "Jeronimo CMP",
"doi-asserted-by": "publisher",
"journal-title": "Clin Infect Dis",
"key": "ref9",
"unstructured": "Jeronimo CMP, Farias MEL, Val FFA, et al.. Methylprednisolone as adjunctive therapy for patients hospitalized with COVID-19 (Metcovid): a randomised, double-blind, phase IIb, placebo-controlled trial. Clin Infect Dis. 2020, ciaa1177. 10.1093/cid/ciaa1177",
"year": "2020"
},
{
"key": "ref10",
"unstructured": "FDA letter of authorization- reissuance of convalescent plasma. (2020). Accessed. November 30, 2020: https://healthcapusa.com/blog/fda-letter-of-authorization-reissuance-of-convalescent-plasma/."
},
{
"DOI": "10.1073/pnas.2004168117",
"article-title": "Effectiveness of convalescent plasma therapy in severe COVID-19 patients",
"author": "Duan K",
"doi-asserted-by": "publisher",
"journal-title": "Proc Natl Acad Sci U S A",
"key": "ref11",
"unstructured": "Duan K, Liu B, Li C, et al.. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proc Natl Acad Sci U S A. 2020, 117:9490-6. 10.1073/pnas.2004168117",
"volume": "117",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2031304",
"article-title": "A randomized trial of convalescent plasma in Covid-19 severe pneumonia",
"author": "Simonovich VA",
"doi-asserted-by": "publisher",
"journal-title": "N Engl J Med",
"key": "ref12",
"unstructured": "Simonovich VA, Burgos Pratx LD, Scibona P, et al.. A randomized trial of convalescent plasma in Covid-19 severe pneumonia. N Engl J Med. 2021, 384:619-2. 10.1056/NEJMoa2031304",
"volume": "384",
"year": "2021"
},
{
"key": "ref13",
"unstructured": "Plasma therapy no cure for virus. ministry. (2020). Accessed: June 17, 2020: https://www.dawn.com/news/1564044."
},
{
"DOI": "10.20944/preprints202004.0189.v1",
"article-title": "Coronavirus disease 2019 (COVID- 19): current literature and status in India [Preprint]",
"author": "Jamwal A",
"doi-asserted-by": "publisher",
"journal-title": "Preprints",
"key": "ref14",
"unstructured": "Jamwal A, Bhatnagar S, Sharma P. Coronavirus disease 2019 (COVID- 19): current literature and status in India [Preprint]. Preprints. 2020, 10.20944/preprints202004.0189.v1",
"year": "2020"
},
{
"DOI": "10.1001/jama.2020.2648",
"article-title": "Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China. Summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention",
"author": "Wu Z",
"doi-asserted-by": "publisher",
"journal-title": "JAMA",
"key": "ref15",
"unstructured": "Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China. Summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020, 323:1239-42. 10.1001/jama.2020.2648",
"volume": "323",
"year": "2020"
},
{
"DOI": "10.1186/s40249-020-00646-x",
"article-title": "Epidemiology, causes, clinical manifestation and diagnosis, prevention and control of coronavirus disease (COVID-19) during the early outbreak period: a scoping review",
"author": "Adhikari SP",
"doi-asserted-by": "publisher",
"journal-title": "Infect Dis Poverty",
"key": "ref16",
"unstructured": "Adhikari SP, Meng S, Wu YJ, et al.. Epidemiology, causes, clinical manifestation and diagnosis, prevention and control of coronavirus disease (COVID-19) during the early outbreak period: a scoping review. Infect Dis Poverty. 2020, 9:29. 10.1186/s40249-020-00646-x",
"volume": "9",
"year": "2020"
},
{
"DOI": "10.1093/infdis/jiaa578",
"article-title": "Postmortem findings in Italian patients with COVID-19: a descriptive full autopsy study of cases with and without comorbidities",
"author": "Falasca L",
"doi-asserted-by": "publisher",
"journal-title": "J Infect Dis",
"key": "ref17",
"unstructured": "Falasca L, Nardacci R, Colombo D, et al.. Postmortem findings in Italian patients with COVID-19: a descriptive full autopsy study of cases with and without comorbidities. J Infect Dis. 2020, 222:1807-15. 10.1093/infdis/jiaa578",
"volume": "222",
"year": "2020"
},
{
"DOI": "10.1007/s00330-020-07033-y",
"article-title": "Chest CT score in COVID-19 patients: correlation with disease severity and short-term prognosis",
"author": "Francone M",
"doi-asserted-by": "publisher",
"journal-title": "Eur Radiol",
"key": "ref18",
"unstructured": "Francone M, Iafrate F, Masci GM, et al.. Chest CT score in COVID-19 patients: correlation with disease severity and short-term prognosis. Eur Radiol. 2020, 30:6808-17. 10.1007/s00330-020-07033-y",
"volume": "30",
"year": "2020"
},
{
"DOI": "10.1042/BSR20202690",
"article-title": "Low serum calcium: a new, important indicator of COVID-19 patients from mild/moderate to severe/critical",
"author": "Zhou X",
"doi-asserted-by": "publisher",
"journal-title": "Biosci Rep",
"key": "ref19",
"unstructured": "Zhou X, Chen D, Wang L, Zhao Y, Wei L, Chen Z, Yang B. Low serum calcium: a new, important indicator of COVID-19 patients from mild/moderate to severe/critical. Biosci Rep. 2020, 40:BSR20202690. 10.1042/BSR20202690",
"volume": "40",
"year": "2020"
},
{
"DOI": "10.1016/j.ebiom.2020.102763",
"article-title": "Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS-CoV-2 infected patients",
"author": "Liu J",
"doi-asserted-by": "publisher",
"journal-title": "EBioMedicine",
"key": "ref20",
"unstructured": "Liu J, Li S, Liu J, et al.. Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS-CoV-2 infected patients. EBioMedicine. 2020, 55:102763. 10.1016/j.ebiom.2020.102763",
"volume": "55",
"year": "2020"
},
{
"DOI": "10.1016%2Fj.ijantimicag.2020.106017",
"article-title": "Neutrophil-to-lymphocyte ratio and clinical outcome in COVID-19: a report from the Italian front line",
"author": "Ciccullo A",
"doi-asserted-by": "publisher",
"journal-title": "Int J Antimicrob Agents",
"key": "ref21",
"unstructured": "Ciccullo A, Borghetti A, Dal Verme LZ, et al.. Neutrophil-to-lymphocyte ratio and clinical outcome in COVID-19: a report from the Italian front line. Int J Antimicrob Agents. 2020, 56:106017. 10.1016%2Fj.ijantimicag.2020.106017",
"volume": "56",
"year": "2020"
},
{
"DOI": "10.7759/cureus.c34",
"article-title": "Clinical profiles, characteristics, and outcomes of the first 100 admitted COVID-19 patients in Pakistan: a single-center retrospective study in a tertiary care hospital of Karachi",
"author": "Asghar MS",
"doi-asserted-by": "publisher",
"journal-title": "Cureus",
"key": "ref22",
"unstructured": "Asghar MS, Haider Kazmi SJ, Ahmed Khan N, et al.. Clinical profiles, characteristics, and outcomes of the first 100 admitted COVID-19 patients in Pakistan: a single-center retrospective study in a tertiary care hospital of Karachi. Cureus. 2020, 12:c34. 10.7759/cureus.c34",
"volume": "12",
"year": "2020"
},
{
"DOI": "10.1001/jamainternmed.2020.0994",
"article-title": "Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China",
"author": "Wu C",
"doi-asserted-by": "publisher",
"journal-title": "JAMA Int Med",
"key": "ref23",
"unstructured": "Wu C, Chen X, Cai Y, et al.. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Int Med. 2020, 180:934-943. 10.1001/jamainternmed.2020.0994",
"volume": "180",
"year": "2020"
},
{
"DOI": "10.1016/j.intimp.2020.106926",
"article-title": "Outcome of COVID-19 patients with use of Tocilizumab: a single center experience",
"author": "Zain Mushtaq M",
"doi-asserted-by": "publisher",
"journal-title": "Int Immunopharmacol",
"key": "ref24",
"unstructured": "Zain Mushtaq M, Bin Zafar Mahmood S, Jamil B, Aziz A, Ali SA. Outcome of COVID-19 patients with use of Tocilizumab: a single center experience. Int Immunopharmacol. 2020, 88:106926. 10.1016/j.intimp.2020.106926",
"volume": "88",
"year": "2020"
},
{
"key": "ref25",
"unstructured": "World Health Organization. COVID-19 weekly epidemiological update. (2020). Accessed. February 14, 2021: https://www.who.int/publications/m/item/weekly-epidemiological-update---16-february-2021."
},
{
"DOI": "10.1101/2021.02.11.21249258",
"article-title": "Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): preliminary results of a randomised, controlled, open-label, platform trial [Preprint]",
"author": "Horby PW",
"doi-asserted-by": "publisher",
"journal-title": "MedRxiv",
"key": "ref26",
"unstructured": "Horby PW, Campbell M, Staplin N, et al.. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): preliminary results of a randomised, controlled, open-label, platform trial [Preprint]. MedRxiv. 2021, 10.1101/2021.02.11.21249258",
"year": "2021"
},
{
"DOI": "10.1001/jamainternmed.2020.0994",
"article-title": "Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China",
"author": "Wu C",
"doi-asserted-by": "publisher",
"journal-title": "JAMA Intern Med",
"key": "ref27",
"unstructured": "Wu C, Chen X, Cai Y, et al.. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020, 180:934-43. 10.1001/jamainternmed.2020.0994",
"volume": "180",
"year": "2020"
},
{
"DOI": "10.1016/S0140-6736(20)30566-3",
"article-title": "Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study",
"author": "Zhou F",
"doi-asserted-by": "publisher",
"journal-title": "Lancet",
"key": "ref28",
"unstructured": "Zhou F, Yu T, Du R, et al.. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020, 395:1054-62. 10.1016/S0140-6736(20)30566-3",
"volume": "395",
"year": "2020"
},
{
"DOI": "10.7759/cureus.12663",
"article-title": "Clinical and demographic characteristics including comorbidities and their outcomes among patients hospitalized with COVID-19 in four tertiary care hospitals across Lahore",
"author": "Khan Chachar AZ",
"doi-asserted-by": "publisher",
"journal-title": "Cureus",
"key": "ref29",
"unstructured": "Khan Chachar AZ, Khan K, Khan AA, et al.. Clinical and demographic characteristics including comorbidities and their outcomes among patients hospitalized with COVID-19 in four tertiary care hospitals across Lahore. Cureus. 2021, 13:e12663. 10.7759/cureus.12663",
"volume": "13",
"year": "2021"
},
{
"DOI": "10.1371/journal.pone.0244171",
"article-title": "Oxygen saturation as a predictor of mortality in hospitalized adult patients with COVID-19 in a public hospital in Lima, Peru",
"author": "Mejía F",
"doi-asserted-by": "publisher",
"journal-title": "PLoS One",
"key": "ref30",
"unstructured": "Mejía F, Medina C, Cornejo E, et al.. Oxygen saturation as a predictor of mortality in hospitalized adult patients with COVID-19 in a public hospital in Lima, Peru. PLoS One. 2020, 15:e0244171. 10.1371/journal.pone.0244171",
"volume": "15",
"year": "2020"
}
],
"reference-count": 30,
"references-count": 30,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.cureus.com/articles/56545-clinical-variants-characteristics-and-outcomes-among-covid-19-patients-a-case-series-analysis-at-a-tertiary-care-hospital-in-karachi-pakistan"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Aerospace Engineering"
],
"subtitle": [],
"title": "Clinical Variants, Characteristics, and Outcomes Among COVID-19 Patients: A Case Series Analysis at a Tertiary Care Hospital in Karachi, Pakistan",
"type": "journal-article"
}