Colchicine and high-intensity rosuvastatin in the treatment of non-critically ill patients hospitalised with COVID-19: a randomised clinical trial
Tayyab Shah, Marianne Mccarthy, Irem Nasir, Herb Archer, Elio Ragheb, Jonathan Kluger, Nitu Kashyap, Carlos Paredes, Prashant Patel, Jing Lu, Prakash Kandel, Christopher Song, Mustafa Khan, Haocheng Huang, Faheem Ul Haq, Rami Ahmad, Christopher Howes, Brian Cambi, Gilead Lancaster, Michael Cleman, Charles Dela Cruz, Helen Parise, Dr Alexandra Lansky
BMJ Open, doi:10.1136/bmjopen-2022-067910
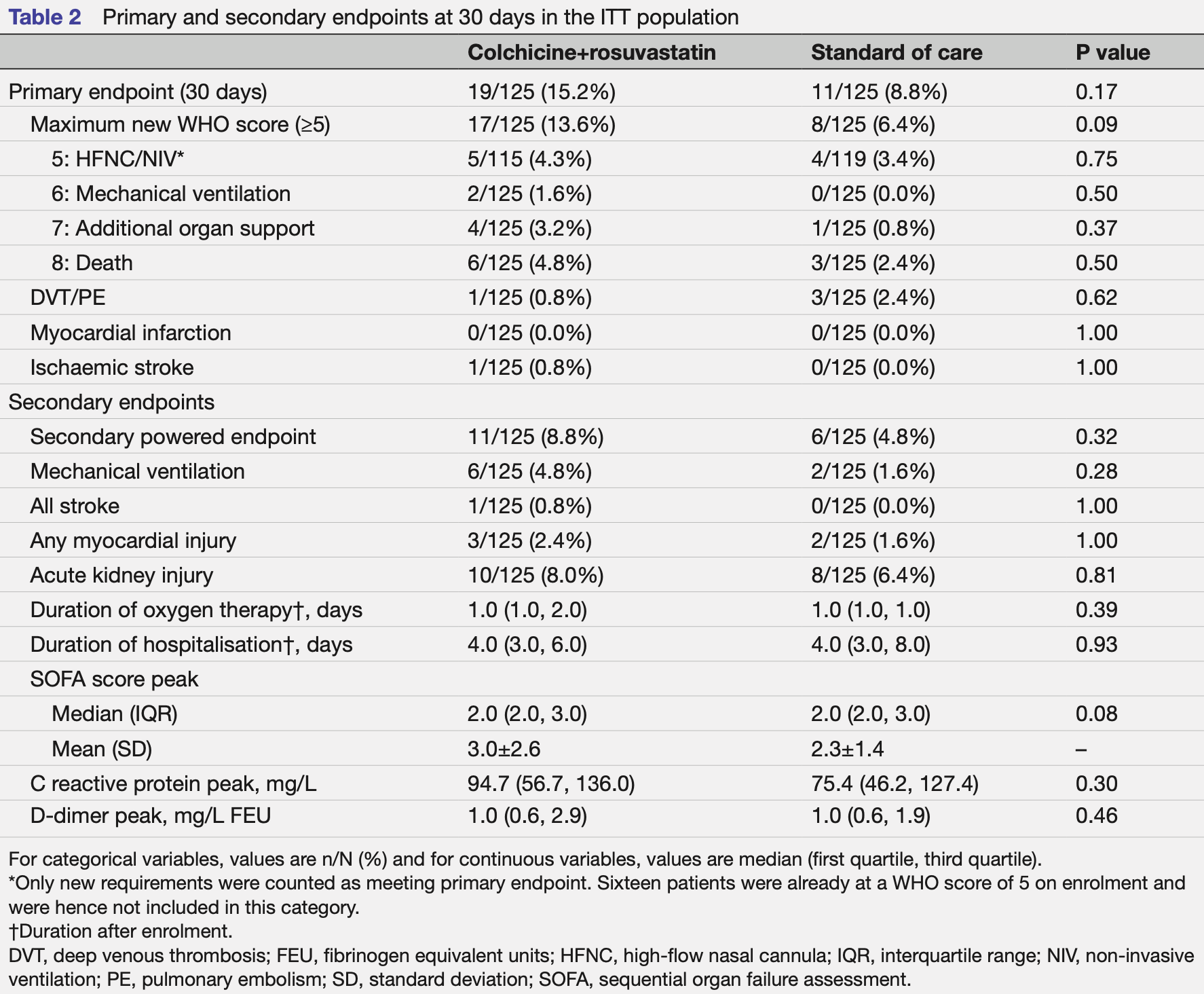
Objective To evaluate the effect of colchicine and highintensity rosuvastatin in addition to standard of care on the progression of COVID-19 disease in hospitalised patients. Design A pragmatic, open-label, multicentre, randomised controlled trial conducted from October 2020 to September 2021. Follow-up was conducted at 30 and 60 days. The electronic medical record was used at all stages of the trial including screening, enrolment, randomisation, event ascertainment and follow-up. Setting Four centres in the Yale New Haven Health System. Participants Non-critically ill hospitalised patients with COVID-19. Interventions Patients were randomised 1:1 to either colchicine plus high-intensity rosuvastatin in addition to standard of care versus standard of care alone. Assigned treatment was continued for the duration of index hospitalisation or 30 days, whichever was shorter. Primary and secondary outcome measures The prespecified primary endpoint was progression to severe COVID-19 disease (new high-flow or non-invasive ventilation, mechanical ventilation, need for vasopressors, renal replacement therapy or extracorporeal membrane oxygenation, or death) or arterial/venous thromboembolic events (ischaemic stroke, myocardial infarction, deep venous thrombosis or pulmonary embolism) evaluated at 30 days. Results Among the 250 patients randomised in this trial (125 to each arm), the median age was 61 years, 44% were women, 15% were Black and 26% were Hispanic/ Latino. As part of the standard of care, patients received remdesivir (87%), dexamethasone (92%), tocilizumab (18%), baricitinib (2%), prophylactic/therapeutic anticoagulation (98%) and aspirin (91%). The trial was terminated early by the data and safety monitoring board for futility. No patients were lost to follow-up due to electronic medical record follow-up. There was no significant difference in the primary endpoint at 30 days between the active arm and standard of care arm (15.2% vs 8.8%, respectively, p=0.17).
Conclusions In this small, open-label, randomised trial of non-critically ill hospitalised patients with COVID-19, the combination of colchicine and rosuvastatin in addition to standard of care did not appear to reduce the risk of progression of COVID-19 disease or thromboembolic events, although the trial was underpowered due to a lower-than-expected event rate. The trial leveraged the power of electronic medical records for efficiency and improved follow-up and demonstrates the utility of incorporating electronic medical records into future trials. Trial registration NCT04472611.
Ethics approval This study involved human participants and was approved by Yale Institutional Review Board (IRB Protocol ID: 2000027950). Participants gave informed consent to participate in the study before taking part. Provenance and peer review Not commissioned; externally peer reviewed.
Data availability statement The data contains protected patient information and at this time are not available to be shared publicly. *This analysis excluded all patients in active arm who did not receive both colchicine and rosuvastatin for at least 1 dose in hospital and all patients in standard of care arm who received a single dose of colchicine or any statin in hospital. BMJ Publishing Group Limited (BMJ) disclaims all liability and responsibility arising from any reliance Supplemental material placed on this supplemental material which has been supplied by the author(s)
References
Ackermann, Verleden, Kuehnel, Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in COVID-19, N Engl J Med,
doi:10.1056/NEJMoa2015432Bikdeli, Madhavan, Jimenez, COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up: JACC state-of-the-art review, J Am Coll Cardiol,
doi:10.1016/j.jacc.2020.04.031Caricchio, Abbate, Gordeev, Effect of canakinumab vs placebo on survival without invasive mechanical ventilation in patients hospitalized with severe COVID-19: a randomized clinical trial, JAMA,
doi:10.1001/jama.2021.9508Cariou, Goronflot, Rimbert, Routine use of statins and increased COVID-19 related mortality in inpatients with type 2 diabetes: results from the CORONADO study, Diabetes Metab,
doi:10.1016/j.diabet.2020.10.001Deftereos, Giannopoulos, Vrachatis, Effect of colchicine vs standard care on cardiac and inflammatory biomarkers and clinical outcomes in patients hospitalized with coronavirus disease 2019: the GRECCO-19 randomized clinical trial, JAMA Netw Open,
doi:10.1001/jamanetworkopen.2020.13136Fedson, Treating the host response to emerging virus diseases: lessons learned from sepsis, pneumonia, influenza and ebola, Ann Transl Med,
doi:10.21037/atm.2016.11.03Gaitán-Duarte, Álvarez-Moreno, Cj, Effectiveness of rosuvastatin plus colchicine, emtricitabine/tenofovir and combinations thereof in hospitalized patients with COVID-19: a pragmatic, open-label randomized trial, EClinicalMedicine,
doi:10.1016/j.eclinm.2021.101242Group, Colchicine in patients admitted to hospital with COVID-19 (recovery): a randomised, controlled, open-label, platform trial, Lancet Respir Med,
doi:10.1016/S2213-2600(21)00435-5Group, Tocilizumab in patients admitted to hospital with COVID-19 (recovery): a randomised, controlled, open-label, platform trial, Lancet,
doi:10.1016/S0140-6736(21)00676-0Gupta, Madhavan, Poterucha, Association between antecedent statin use and decreased mortality in hospitalized patients with COVID-19, Nat Commun,
doi:10.1038/s41467-021-21553-1Huang, Cen, Wang, Synergistic effects of colchicine combined with atorvastatin in rats with hyperlipidemia, Lipids Health Dis,
doi:10.1186/1476-511X-13-67Investigators, Atorvastatin versus placebo in patients with COVID-19 in intensive care: randomized controlled trial, BMJ,
doi:10.1136/bmj-2021-068407Kruger, Bailey, Bellomo, A multicenter randomized trial of atorvastatin therapy in intensive care patients with severe sepsis, Am J Respir Crit Care Med,
doi:10.1164/rccm.201209-1718OCLansky, Messé, Brickman, Proposed standardized neurological endpoints for cardiovascular clinical trials: an academic research consortium initiative, J Am Coll Cardiol,
doi:10.1016/j.jacc.2016.11.045Lawler, Goligher, Berger, Therapeutic anticoagulation with heparin in noncritically ill patients with COVID-19, N Engl J Med,
doi:10.1056/NEJMoa2105911Lopes, Bonjorno, Giannini, Beneficial effects of colchicine for moderate to severe COVID-19: a randomised, doubleblinded, placebo-controlled clinical trial, RMD Open,
doi:10.1136/rmdopen-2020-001455Marconi, Ramanan, De Bono, Efficacy and safety of baricitinib for the treatment of hospitalised adults with COVID-19 (COV-BARRIER): a randomised, double-blind, parallel-group, placebo-controlled phase 3 trial, Lancet Respir Med,
doi:10.1016/S2213-2600(21)00331-3Oldenburg, Pinsky, Brogdon, Effect of oral azithromycin vs placebo on COVID-19 symptoms in outpatients with SARS-cov-2 infection: a randomized clinical trial, JAMA,
doi:10.1001/jama.2021.11517Patel, Snaith, Thickett, Randomized double-blind placebo-controlled trial of 40 mg/day of atorvastatin in reducing the severity of sepsis in ward patients (asepsis trial), Crit Care,
doi:10.1186/cc11895Perico, Ostermann, Bontempeill, Colchicine interferes with L-selectin and leukocyte function-associated antigen-1 expression on human T lymphocytes and inhibits T cell activation, J Am Soc Nephrol,
doi:10.1681/ASN.V74594Pope, Tschopp, The role of interleukin-1 and the inflammasome in gout: implications for therapy, Arthritis Rheum,
doi:10.1002/art.22938Pourdowlat, Saghafi, Mozafari, Efficacy and safety of colchicine treatment in patients with COVID-19: a prospective, multicenter, randomized clinical trial, Phytother Res,
doi:10.1002/ptr.7319Reis, Silva, Silva, Effect of early treatment with hydroxychloroquine or lopinavir and ritonavir on risk of hospitalization among patients with COVID-19: the together randomized clinical trial, JAMA Netw Open,
doi:10.1001/jamanetworkopen.2021.6468Shah, Mccarthy, Nasir, Design and rationale of the colchicine/statin for the prevention of COVID-19 complications (COLSTAT) trial, Contemp Clin Trials,
doi:10.1016/j.cct.2021.106547Talasaz, Sadeghipour, Aghakouchakzadeh, Investigating lipid-modulating agents for prevention or treatment of COVID-19: JACC state-of-the-art review, J Am Coll Cardiol,
doi:10.1016/j.jacc.2021.08.021Tang, Li, Wang, Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia, J Thromb Haemost,
doi:10.1111/jth.14768Tardif, Bouabdallaoui, Allier, Colchicine for community-treated patients with COVID-19 (COLCORONA): a phase 3, randomised, double-blinded, adaptive, placebo-controlled, multicentre trial, Lancet Respir Med,
doi:10.1016/S2213-2600(21)00222-8Tardif, Kouz, Waters, Efficacy and safety of low-dose colchicine after myocardial infarction, N Engl J Med,
doi:10.1056/NEJMoa1912388Tikoo, Patel, Kumar, Tissue specific up regulation of ACE2 in rabbit model of atherosclerosis by atorvastatin: role of epigenetic histone modifications, Biochem Pharmacol,
doi:10.1016/j.bcp.2014.11.013Vaduganathan, Vardeny, Michel, Renin-angiotensinaldosterone system inhibitors in patients with COVID-19, N Engl J Med,
doi:10.1056/NEJMsr2005760Viveiros, Rasmuson, Vu, Sex differences in COVID-19: candidate pathways, genetics of ACE2, and sex hormones, Am J Physiol Heart Circ Physiol,
doi:10.1152/ajpheart.00755.2020Wang, Chen, Yang, Inflammation-associated factors for predicting in-hospital mortality in patients with COVID-19, J Med Virol,
doi:10.1002/jmv.26771DOI record:
{
"DOI": "10.1136/bmjopen-2022-067910",
"ISSN": [
"2044-6055",
"2044-6055"
],
"URL": "http://dx.doi.org/10.1136/bmjopen-2022-067910",
"abstract": "<jats:sec><jats:title>Objective</jats:title><jats:p>To evaluate the effect of colchicine and high-intensity rosuvastatin in addition to standard of care on the progression of COVID-19 disease in hospitalised patients.</jats:p></jats:sec><jats:sec><jats:title>Design</jats:title><jats:p>A pragmatic, open-label, multicentre, randomised controlled trial conducted from October 2020 to September 2021. Follow-up was conducted at 30 and 60 days. The electronic medical record was used at all stages of the trial including screening, enrolment, randomisation, event ascertainment and follow-up.</jats:p></jats:sec><jats:sec><jats:title>Setting</jats:title><jats:p>Four centres in the Yale New Haven Health System.</jats:p></jats:sec><jats:sec><jats:title>Participants</jats:title><jats:p>Non-critically ill hospitalised patients with COVID-19.</jats:p></jats:sec><jats:sec><jats:title>Interventions</jats:title><jats:p>Patients were randomised 1:1 to either colchicine plus high-intensity rosuvastatin in addition to standard of care versus standard of care alone. Assigned treatment was continued for the duration of index hospitalisation or 30 days, whichever was shorter.</jats:p></jats:sec><jats:sec><jats:title>Primary and secondary outcome measures</jats:title><jats:p>The prespecified primary endpoint was progression to severe COVID-19 disease (new high-flow or non-invasive ventilation, mechanical ventilation, need for vasopressors, renal replacement therapy or extracorporeal membrane oxygenation, or death) or arterial/venous thromboembolic events (ischaemic stroke, myocardial infarction, deep venous thrombosis or pulmonary embolism) evaluated at 30 days.</jats:p></jats:sec><jats:sec><jats:title>Results</jats:title><jats:p>Among the 250 patients randomised in this trial (125 to each arm), the median age was 61 years, 44% were women, 15% were Black and 26% were Hispanic/Latino. As part of the standard of care, patients received remdesivir (87%), dexamethasone (92%), tocilizumab (18%), baricitinib (2%), prophylactic/therapeutic anticoagulation (98%) and aspirin (91%). The trial was terminated early by the data and safety monitoring board for futility. No patients were lost to follow-up due to electronic medical record follow-up. There was no significant difference in the primary endpoint at 30 days between the active arm and standard of care arm (15.2% vs 8.8%, respectively, p=0.17).</jats:p></jats:sec><jats:sec><jats:title>Conclusions</jats:title><jats:p>In this small, open-label, randomised trial of non-critically ill hospitalised patients with COVID-19, the combination of colchicine and rosuvastatin in addition to standard of care did not appear to reduce the risk of progression of COVID-19 disease or thromboembolic events, although the trial was underpowered due to a lower-than-expected event rate. The trial leveraged the power of electronic medical records for efficiency and improved follow-up and demonstrates the utility of incorporating electronic medical records into future trials.</jats:p></jats:sec><jats:sec><jats:title>Trial registration</jats:title><jats:p><jats:ext-link xmlns:xlink=\"http://www.w3.org/1999/xlink\" ext-link-type=\"clintrialgov\" xlink:href=\"NCT04472611\">NCT04472611</jats:ext-link>.</jats:p></jats:sec>",
"alternative-id": [
"10.1136/bmjopen-2022-067910"
],
"author": [
{
"affiliation": [],
"family": "Shah",
"given": "Tayyab",
"sequence": "first"
},
{
"affiliation": [],
"family": "McCarthy",
"given": "Marianne",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Nasir",
"given": "Irem",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Archer",
"given": "Herb",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ragheb",
"given": "Elio",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kluger",
"given": "Jonathan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kashyap",
"given": "Nitu",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Paredes",
"given": "Carlos",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Patel",
"given": "Prashant",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lu",
"given": "Jing",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kandel",
"given": "Prakash",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Song",
"given": "Christopher",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Khan",
"given": "Mustafa",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Huang",
"given": "Haocheng",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ul Haq",
"given": "Faheem",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ahmad",
"given": "Rami",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Howes",
"given": "Christopher",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cambi",
"given": "Brian",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lancaster",
"given": "Gilead",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cleman",
"given": "Michael",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Dela Cruz",
"given": "Charles",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Parise",
"given": "Helen",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-8002-7497",
"affiliation": [],
"authenticated-orcid": false,
"family": "Lansky",
"given": "Alexandra",
"sequence": "additional"
}
],
"clinical-trial-number": [
{
"clinical-trial-number": "nct04472611",
"registry": "10.18810/clinical-trials-gov"
},
{
"clinical-trial-number": "nct04472611",
"registry": "10.18810/clinical-trials-gov"
}
],
"container-title": "BMJ Open",
"container-title-short": "BMJ Open",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"bmj.com"
]
},
"created": {
"date-parts": [
[
2023,
2,
25
]
],
"date-time": "2023-02-25T02:16:32Z",
"timestamp": 1677291392000
},
"deposited": {
"date-parts": [
[
2023,
2,
25
]
],
"date-time": "2023-02-25T02:16:56Z",
"timestamp": 1677291416000
},
"funder": [
{
"DOI": "10.13039/100000041",
"award": [
"7-20-COVID-162"
],
"doi-asserted-by": "publisher",
"name": "American Diabetes Association"
}
],
"indexed": {
"date-parts": [
[
2023,
2,
25
]
],
"date-time": "2023-02-25T05:34:24Z",
"timestamp": 1677303264715
},
"is-referenced-by-count": 0,
"issue": "2",
"issued": {
"date-parts": [
[
2023,
2
]
]
},
"journal-issue": {
"issue": "2",
"published-online": {
"date-parts": [
[
2023,
2,
24
]
]
},
"published-print": {
"date-parts": [
[
2023,
2
]
]
}
},
"language": "en",
"license": [
{
"URL": "http://creativecommons.org/licenses/by-nc/4.0/",
"content-version": "unspecified",
"delay-in-days": 23,
"start": {
"date-parts": [
[
2023,
2,
24
]
],
"date-time": "2023-02-24T00:00:00Z",
"timestamp": 1677196800000
}
}
],
"link": [
{
"URL": "https://syndication.highwire.org/content/doi/10.1136/bmjopen-2022-067910",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "239",
"original-title": [],
"page": "e067910",
"prefix": "10.1136",
"published": {
"date-parts": [
[
2023,
2
]
]
},
"published-online": {
"date-parts": [
[
2023,
2,
24
]
]
},
"published-print": {
"date-parts": [
[
2023,
2
]
]
},
"publisher": "BMJ",
"reference": [
{
"DOI": "10.1056/NEJMoa2007764",
"doi-asserted-by": "publisher",
"key": "2023022418152730000_13.2.e067910.1"
},
{
"DOI": "10.1056/NEJMoa2021436",
"doi-asserted-by": "publisher",
"key": "2023022418152730000_13.2.e067910.2"
},
{
"DOI": "10.1016/S0140-6736(21)00676-0",
"doi-asserted-by": "publisher",
"key": "2023022418152730000_13.2.e067910.3"
},
{
"DOI": "10.1016/S2213-2600(21)00331-3",
"article-title": "Efficacy and safety of baricitinib for the treatment of hospitalised adults with COVID-19 (COV-BARRIER): a randomised, double-blind, parallel-group, placebo-controlled phase 3 trial",
"author": "Marconi",
"doi-asserted-by": "crossref",
"first-page": "1407",
"journal-title": "Lancet Respir Med",
"key": "2023022418152730000_13.2.e067910.4",
"volume": "9",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2105911",
"doi-asserted-by": "publisher",
"key": "2023022418152730000_13.2.e067910.5"
},
{
"DOI": "10.1002/jmv.26771",
"article-title": "Inflammation-associated factors for predicting in-hospital mortality in patients with COVID-19",
"author": "Wang",
"doi-asserted-by": "crossref",
"first-page": "2908",
"journal-title": "J Med Virol",
"key": "2023022418152730000_13.2.e067910.6",
"volume": "93",
"year": "2021"
},
{
"DOI": "10.1038/s41577-021-00588-x",
"article-title": "Inflammasome activation at the crux of severe COVID-19",
"author": "Vora",
"doi-asserted-by": "crossref",
"first-page": "694",
"journal-title": "Nat Rev Immunol",
"key": "2023022418152730000_13.2.e067910.7",
"volume": "21",
"year": "2021"
},
{
"DOI": "10.4049/jimmunol.1400368",
"doi-asserted-by": "publisher",
"key": "2023022418152730000_13.2.e067910.8"
},
{
"DOI": "10.1056/nejmsr2005760",
"doi-asserted-by": "publisher",
"key": "2023022418152730000_13.2.e067910.9"
},
{
"DOI": "10.1111/jth.14768",
"doi-asserted-by": "publisher",
"key": "2023022418152730000_13.2.e067910.10"
},
{
"DOI": "10.1016/j.jacc.2020.04.031",
"doi-asserted-by": "publisher",
"key": "2023022418152730000_13.2.e067910.11"
},
{
"DOI": "10.1056/NEJMoa2015432",
"doi-asserted-by": "publisher",
"key": "2023022418152730000_13.2.e067910.12"
},
{
"DOI": "10.1016/S0140-6736(20)30937-5",
"doi-asserted-by": "publisher",
"key": "2023022418152730000_13.2.e067910.13"
},
{
"DOI": "10.1681/ASN.V74594",
"doi-asserted-by": "publisher",
"key": "2023022418152730000_13.2.e067910.14"
},
{
"DOI": "10.1016/j.semarthrit.2015.06.013",
"doi-asserted-by": "publisher",
"key": "2023022418152730000_13.2.e067910.15"
},
{
"DOI": "10.1002/art.22938",
"doi-asserted-by": "publisher",
"key": "2023022418152730000_13.2.e067910.16"
},
{
"DOI": "10.1038/nrcardio.2017.161",
"doi-asserted-by": "publisher",
"key": "2023022418152730000_13.2.e067910.17"
},
{
"DOI": "10.1056/NEJMoa1912388",
"doi-asserted-by": "publisher",
"key": "2023022418152730000_13.2.e067910.18"
},
{
"DOI": "10.1056/NEJMoa2021372",
"doi-asserted-by": "publisher",
"key": "2023022418152730000_13.2.e067910.19"
},
{
"DOI": "10.21037/atm.2016.11.03",
"article-title": "Treating the host response to emerging virus diseases: lessons learned from sepsis, pneumonia, influenza and ebola",
"author": "Fedson",
"doi-asserted-by": "crossref",
"first-page": "421",
"journal-title": "Ann Transl Med",
"key": "2023022418152730000_13.2.e067910.20",
"volume": "4",
"year": "2016"
},
{
"article-title": "Effects of rosuvastatin on expression of angiotensin-converting enzyme 2 after vascular balloon injury in rats",
"author": "Li",
"first-page": "151",
"journal-title": "J Geriatr Cardiol",
"key": "2023022418152730000_13.2.e067910.21",
"volume": "10",
"year": "2013"
},
{
"DOI": "10.1016/j.bcp.2014.11.013",
"doi-asserted-by": "publisher",
"key": "2023022418152730000_13.2.e067910.22"
},
{
"DOI": "10.1164/rccm.201209-1718OC",
"doi-asserted-by": "publisher",
"key": "2023022418152730000_13.2.e067910.23"
},
{
"DOI": "10.1186/cc11895",
"doi-asserted-by": "crossref",
"key": "2023022418152730000_13.2.e067910.24",
"unstructured": "Patel JM , Snaith C , Thickett DR , et al . Randomized double-blind placebo-controlled trial of 40 mg/day of atorvastatin in reducing the severity of sepsis in ward patients (asepsis trial). Crit Care 2012;16:R231. doi:10.1186/cc11895"
},
{
"DOI": "10.1016/j.jacc.2021.08.021",
"doi-asserted-by": "publisher",
"key": "2023022418152730000_13.2.e067910.25"
},
{
"DOI": "10.1001/jamanetworkopen.2020.13136",
"doi-asserted-by": "crossref",
"key": "2023022418152730000_13.2.e067910.26",
"unstructured": "Deftereos SG , Giannopoulos G , Vrachatis DA , et al . Effect of colchicine vs standard care on cardiac and inflammatory biomarkers and clinical outcomes in patients hospitalized with coronavirus disease 2019: the GRECCO-19 randomized clinical trial. JAMA Netw Open 2020;3:e2013136. doi:10.1001/jamanetworkopen.2020.13136"
},
{
"DOI": "10.1136/rmdopen-2020-001455",
"doi-asserted-by": "crossref",
"key": "2023022418152730000_13.2.e067910.27",
"unstructured": "Lopes MI , Bonjorno LP , Giannini MC , et al . Beneficial effects of colchicine for moderate to severe COVID-19: a randomised, double-blinded, placebo-controlled clinical trial. RMD Open 2021;7:e001455. doi:10.1136/rmdopen-2020-001455"
},
{
"DOI": "10.1016/S2213-2600(21)00222-8",
"article-title": "Colchicine for community-treated patients with COVID-19 (COLCORONA): a phase 3, randomised, double-blinded, adaptive, placebo-controlled, multicentre trial",
"author": "Tardif",
"doi-asserted-by": "crossref",
"first-page": "924",
"journal-title": "Lancet Respir Med",
"key": "2023022418152730000_13.2.e067910.28",
"volume": "9",
"year": "2021"
},
{
"DOI": "10.1016/S2213-2600(21)00435-5",
"article-title": "Colchicine in patients admitted to hospital with COVID-19 (recovery): a randomised, controlled, open-label, platform trial",
"author": "Group",
"doi-asserted-by": "crossref",
"first-page": "1419",
"journal-title": "Lancet Respir Med",
"key": "2023022418152730000_13.2.e067910.29",
"volume": "9",
"year": "2021"
},
{
"DOI": "10.1002/ptr.7319",
"article-title": "Efficacy and safety of colchicine treatment in patients with COVID-19: a prospective, multicenter, randomized clinical trial",
"author": "Pourdowlat",
"doi-asserted-by": "crossref",
"first-page": "891",
"journal-title": "Phytother Res",
"key": "2023022418152730000_13.2.e067910.30",
"volume": "36",
"year": "2022"
},
{
"DOI": "10.1016/j.eclinm.2021.101242",
"doi-asserted-by": "crossref",
"key": "2023022418152730000_13.2.e067910.31",
"unstructured": "Gaitán-Duarte HG , Álvarez-Moreno C , Rincón-Rodríguez CJ , et al . Effectiveness of rosuvastatin plus colchicine, emtricitabine/tenofovir and combinations thereof in hospitalized patients with COVID-19: a pragmatic, open-label randomized trial. EClinicalMedicine 2022;43:101242. doi:10.1016/j.eclinm.2021.101242"
},
{
"DOI": "10.1038/s41467-021-21553-1",
"doi-asserted-by": "crossref",
"key": "2023022418152730000_13.2.e067910.32",
"unstructured": "Gupta A , Madhavan MV , Poterucha TJ , et al . Association between antecedent statin use and decreased mortality in hospitalized patients with COVID-19. Nat Commun 2021;12:1325. doi:10.1038/s41467-021-21553-1"
},
{
"DOI": "10.1016/amjcard.202008.004",
"doi-asserted-by": "publisher",
"key": "2023022418152730000_13.2.e067910.33"
},
{
"DOI": "10.1136/bmj-2021-068407",
"doi-asserted-by": "crossref",
"key": "2023022418152730000_13.2.e067910.34",
"unstructured": "INSPIRATION-S Investigators . Atorvastatin versus placebo in patients with COVID-19 in intensive care: randomized controlled trial. BMJ 2022;376:e068407. doi:10.1136/bmj-2021-068407"
},
{
"DOI": "10.1016/j.diabet.2020.10.001",
"article-title": "Routine use of statins and increased COVID-19 related mortality in inpatients with type 2 diabetes: results from the CORONADO study",
"author": "Cariou",
"doi-asserted-by": "crossref",
"first-page": "101202",
"journal-title": "Diabetes Metab",
"key": "2023022418152730000_13.2.e067910.35",
"volume": "47",
"year": "2021"
},
{
"DOI": "10.1371/journal.pone.0256899",
"doi-asserted-by": "crossref",
"key": "2023022418152730000_13.2.e067910.36",
"unstructured": "Ayeh SK , Abbey EJ , Khalifa BAA , et al . Statins use and COVID-19 outcomes in hospitalized patients. PLoS One 2021;16:e0256899. doi:10.1371/journal.pone.0256899"
},
{
"DOI": "10.1186/1476-511X-13-67",
"doi-asserted-by": "crossref",
"key": "2023022418152730000_13.2.e067910.37",
"unstructured": "Huang C , Cen C , Wang C , et al . Synergistic effects of colchicine combined with atorvastatin in rats with hyperlipidemia. Lipids Health Dis 2014;13:67. doi:10.1186/1476-511X-13-67"
},
{
"DOI": "10.1016/j.cct.2021.106547",
"article-title": "Design and rationale of the colchicine/statin for the prevention of COVID-19 complications (COLSTAT) trial",
"author": "Shah",
"doi-asserted-by": "crossref",
"first-page": "106547",
"journal-title": "Contemp Clin Trials",
"key": "2023022418152730000_13.2.e067910.38",
"volume": "110",
"year": "2021"
},
{
"DOI": "10.1016/j.jacc.2018.08.1038",
"doi-asserted-by": "publisher",
"key": "2023022418152730000_13.2.e067910.39"
},
{
"DOI": "10.1016/j.jacc.2016.11.045",
"doi-asserted-by": "publisher",
"key": "2023022418152730000_13.2.e067910.40"
},
{
"DOI": "10.1152/ajpheart.00755.2020",
"article-title": "Sex differences in COVID-19: candidate pathways, genetics of ACE2, and sex hormones",
"author": "Viveiros",
"doi-asserted-by": "crossref",
"first-page": "H296",
"journal-title": "Am J Physiol Heart Circ Physiol",
"key": "2023022418152730000_13.2.e067910.41",
"volume": "320",
"year": "2021"
},
{
"DOI": "10.1001/jama.2021.11517",
"article-title": "Effect of oral azithromycin vs placebo on COVID-19 symptoms in outpatients with SARS-cov-2 infection: a randomized clinical trial",
"author": "Oldenburg",
"doi-asserted-by": "crossref",
"first-page": "490",
"journal-title": "JAMA",
"key": "2023022418152730000_13.2.e067910.42",
"volume": "326",
"year": "2021"
},
{
"DOI": "10.1001/jama.2021.9508",
"article-title": "Effect of canakinumab vs placebo on survival without invasive mechanical ventilation in patients hospitalized with severe COVID-19: a randomized clinical trial",
"author": "Caricchio",
"doi-asserted-by": "crossref",
"first-page": "230",
"journal-title": "JAMA",
"key": "2023022418152730000_13.2.e067910.43",
"volume": "326",
"year": "2021"
},
{
"DOI": "10.1001/jamanetworkopen.2021.6468",
"doi-asserted-by": "crossref",
"key": "2023022418152730000_13.2.e067910.44",
"unstructured": "Reis G , Moreira Silva E , Medeiros Silva DC , et al . Effect of early treatment with hydroxychloroquine or lopinavir and ritonavir on risk of hospitalization among patients with COVID-19: the together randomized clinical trial. JAMA Netw Open 2021;4:e216468. doi:10.1001/jamanetworkopen.2021.6468"
}
],
"reference-count": 44,
"references-count": 44,
"relation": {},
"resource": {
"primary": {
"URL": "https://bmjopen.bmj.com/lookup/doi/10.1136/bmjopen-2022-067910"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"General Medicine"
],
"subtitle": [],
"title": "Colchicine and high-intensity rosuvastatin in the treatment of non-critically ill patients hospitalised with COVID-19: a randomised clinical trial",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1136/crossmarkpolicy",
"volume": "13"
}