Evaluation of hydroxychloroquine or chloroquine for the prevention of COVID-19 (COPCOV): A double-blind, randomised, placebo-controlled trial
William H K Schilling, Mavuto Mukaka, James J Callery, Martin J Llewelyn, Cintia V Cruz, Mehul Dhorda, Thatsanun Ngernseng, Naomi Waithira, Maneerat Ekkapongpisit, James A Watson, Arjun Chandna, Erni J Nelwan, Raph L Hamers, Anthony Etyang, Mohammad Asim Beg, Samba Sow, William Yavo, Aurel Constant Allabi, Buddha Basnyat, Sanjib Kumar Sharma, Modupe Amofa-Sekyi, Paul Yonga, Amanda Adler, Prayoon Yuentrakul, Tanya Cope, Janjira Thaipadungpanit, Panuvit Rienpradub, Mallika Imwong, Mohammad Yazid Abdad, Stuart D Blacksell, Joel Tarning, Frejus Faustin Goudjo, Ange D Dossou, Abibatou Konaté-Touré, Serge-Brice Assi, Kra Ouffoué, Nasronudin Nasronudin, Brian Eka Rachman, Pradana Zaky Romadhon, Didi Darmahadi Dewanto, Made Oka Heryana, Theresia Novi, Ayodhia Pitaloka Pasaribu, Mutiara Mutiara, Miranda Putri Rahayu Nasution, Khairunnisa Khairunnisa, Fauzan Azima Dalimunthe, Eka Airlangga, Akmal Fahrezzy, Yanri Subronto, Nur Rahmi Ananda, Mutia Rahardjani, Atika Rimainar, Ruth Khadembu Lucinde, Molline Timbwa, Otieno Edwin Onyango, Clara Agutu, Samuel Akech, Mainga Hamaluba, Jairus Kipyego, Obadiah Ngachi, Fadima Cheick Haidara, Oumar Y Traoré, François Diarra, Basudha Khanal, Piyush Dahal, Suchita Shrestha, Samita Rijal, Youssouf Kabore, Eric Adehossi, Ousmane Guindo, Farah Naz Qamar, Abdul Momin Kazi, Charles J Woodrow, Steven Laird, Maina Cheeba, Helen Ayles, Phaik Yeong Cheah, Walter R J Taylor, Elizabeth M Batty, Kesinee Chotivanich, Sasithon Pukrittayakamee, Weerapong Phumratanaprapin, Lorenz Von Seidlein, Arjen Dondorp, Nicholas P J Day, Nicholas J White
PLOS Medicine, doi:10.1371/journal.pmed.1004428
Background Hydroxychloroquine (HCQ) has proved ineffective in treating patients hospitalised with Coronavirus Disease 2019 (COVID-19), but uncertainty remains over its safety and efficacy in chemoprevention. Previous chemoprevention randomised controlled trials (RCTs) did not individually show benefit of HCQ against COVID-19 and, although meta-analysis did suggest clinical benefit, guidelines recommend against its use.
Supporting information S1 A1 . List of COPCOV study sites. Table A2 . Baseline characteristics in the COPCOV trial (Per Protocol Analysis). Table A3 . Outcomes of Chloroquine/Hydroxychloroquine and Placebo Pre-exposure Prophylaxis against COVID-19 in the COPCOV study (Per Protocol Analysis). Table A4 . Summary of Serious Adverse Events in the COPCOV study. Table A5 . Primary and secondary outcomes of Chloroquine/Hydroxychloroquine Therapy for Pre-exposure Prophylaxis against COVID-19 (missing outcomes treated as not having had COVID-19 during the study period) ITT-Results presented as "Risk differences." Table A6 . Outcomes of Chloroquine/Hydroxychloroquine and Placebo Pre-exposure Prophylaxis against COVID-19 in the COPCOV study (removing cases for which the SEAC judged that a study endpoint could not be determined). Table A7 . Summary characteristics of previously published pre-exposure prophylaxis studies considered for meta-analysis. Table A8 . Listing of causes of PCR-confirmed respiratory illness.
Author Contributions Conceptualization: William H. K. Schilling, Arjun Chandna, Arjen Dondorp, Nicholas P. J. Day, Nicholas J. White. Data curation: William H. K. Schilling, Mavuto Mukaka, Thatsanun Ngernseng, Naomi Waithira, James A. Watson, Otieno Edwin Onyango, Elizabeth M. Batty.
References
Abella, Jolkovsky, Biney, Uspal, Hyman et al., Efficacy and Safety of Hydroxychloroquine vs Placebo for Pre-exposure SARS-CoV-2 Prophylaxis Among Health Care Workers: A Randomized Clinical Trial, JAMA Intern Med,
doi:10.1001/jamainternmed.2020.6319Boulware, Pullen, Bangdiwala, Karita, Johnston et al., A Randomized Trial of Hydroxychloroquine as Postexposure Prophylaxis for Covid-19, N Engl J Med,
doi:10.1056/NEJMoa2016638Garcia-Albeniz, Amo, Polo, Morales-Asencio, Herna ´n Ma, Systematic review and metaanalysis of randomized trials of hydroxychloroquine for the prevention of COVID-19, Eur J Epidemiol,
doi:10.1007/s10654-022-00891-4Grau-Pujol, Camprubi-Ferrer, Marti-Soler, Ferna ´ndez-Pardos, Carreras-Abad et al., Pre-exposure prophylaxis with hydroxychloroquine for COVID-19: a double-blind, placebo-controlled randomized clinical trial, Trials,
doi:10.1186/s13063-021-05758-9Krause, Fleming, Longini, Henao-Restrepo, Peto, World Health Organization Solidarity Vaccines Trial Expert Group. COVID-19 vaccine trials should seek worthwhile efficacy, Lancet,
doi:10.1016/S0140-6736(20)31821-3Liu, Cao, Xu, Wang, Zhang et al., Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro, Cell Discov,
doi:10.1038/s41421-020-0156-0Llanos-Cuentas, Schwalb, Quintana, Delfin, Alvarez et al., Hydroxychloroquine to prevent SARS-CoV-2 infection among healthcare workers: early termination of a phase 3, randomised, open-label, controlled clinical trial, BMC Res Notes,
doi:10.1186/s13104-023-06281-7Lofgren, Nicol, Bangdiwala, Pastick, Okafor et al., Safety of Hydroxychloroquine Among Outpatient Clinical Trial Participants for COVID-19, Open Forum Infect Dis,
doi:10.1093/ofid/ofaa500Lofgren, Okafor, Colette, Pastick, Skipper et al., Feasibility of SARS-CoV-2 Antibody Testing in Remote Outpatient Trials, Open Forum Infect Dis,
doi:10.1093/ofid/ofab506Macedo, Prestes, Colonetti, Candido, Uggioni et al., A systematic review and meta-analysis of the accuracy of SARS-COV-2 IGM and IGG tests in individuals with COVID-19, J Clin Virol,
doi:10.1016/j.jcv.2022.105121Mckinnon, Wang, Zervos, Saval, Marshall-Nightengale et al., Safety and tolerability of hydroxychloroquine in health care workers and first responders for the prevention of COVID-19: WHIP COVID-19 Study, Int J Infect Dis,
doi:10.1016/j.ijid.2021.12.343Mitjà, Corbacho-Monne, Ubals, Alemany, Suñer et al., A Cluster-Randomized Trial of Hydroxychloroquine for Prevention of Covid-19, N Engl J Med,
doi:10.1056/NEJMoa2021801Naggie, Milstone, Castro, Collins, Lakshmi et al., Hydroxychloroquine for pre-exposure prophylaxis of COVID-19 in health care workers: a randomized, multicenter, placebo-controlled trial Healthcare Worker Exposure Response and Outcomes of Hydroxychloroquine (HERO-HCQ), Int J Infect Dis,
doi:10.1016/j.ijid.2023.01.019Nasri, Fakhim, Salahi, Ghafel, Pourajam et al., Efficacy of Hydroxychloroquine in Pre-exposure Severe Acute Respiratory Syndrome Coronavirus 2 Prophylaxis among High-Risk HealthCare Workers: A Multicenter Study, Adv Biomed Res,
doi:10.4103/abr.abr_104_21Polo, Garcia-Albeniz, Teran, Morales, Rial-Crestelo et al., Daily tenofovir disoproxil fumarate/emtricitabine and hydroxychloroquine for pre-exposure prophylaxis of COVID-19: a double-blind placebo-controlled randomized trial in healthcare workers, Clin Microbiol Infect,
doi:10.1016/j.cmi.2022.07.006Rajasingham, Bangdiwala, Nicol, Skipper, Pastick et al., Hydroxychloroquine as Pre-exposure Prophylaxis for Coronavirus Disease 2019 (COVID-19) in Healthcare Workers: A Randomized Trial, Clin Infect Dis,
doi:10.1093/cid/ciaa1571Recovery Collaborative Group, Horby, Mafham, Linsell, Bell et al., Effect of Hydroxychloroquine in Hospitalized Patients with Covid-19, N Engl J Med,
doi:10.1056/NEJMoa2022926Rojas-Serrano, Portillo-Vasquez, Thirion-Romero, Va ´zquez-Pe ´rez, Mejı ´a-Nepomuceno et al., Hydroxychloroquine for prophylaxis of COVID-19 in health workers: A randomized clinical trial, PLoS ONE,
doi:10.1371/journal.pone.0261980Schilling, Callery, Chandna, Hamers, Watson et al., The WHO guideline on drugs to prevent COVID-19: small numbers-big conclusions, Wellcome Open Res,
doi:10.12688/wellcomeopenres.16741.2Seet, Quek, Ooi, Sengupta, Lakshminarasappa et al., Positive impact of oral hydroxychloroquine and povidone-iodine throat spray for COVID-19 prophylaxis: An open-label randomized trial, Int J Infect Dis,
doi:10.1016/j.ijid.2021.04.035Syed, Hassan, Arif, Batool, Niazi et al., Pre-exposure Prophylaxis With Various Doses of Hydroxychloroquine Among Healthcare Personnel With High-Risk Exposure to COVID-19: A Randomized Controlled Trial, Cureus,
doi:10.7759/cureus.20572Us Fda, FDA cautions against use of hydroxychloroquine or chloroquine for COVID-19 outside of the hospital setting or a clinical trial due to risk of heart rhythm problems
Vijayaraghavan, Jha, Rajbhandari, Myatra, Ghosh et al., Hydroxychloroquine plus personal protective equipment versus personal protective equipment alone for the prevention of laboratory-confirmed COVID-19 infections among healthcare workers: a multicentre, parallel-group randomised controlled trial from India, BMJ Open,
doi:10.1136/bmjopen-2021-059540Vincent, Bergeron, Benjannet, Erickson, Rollin et al., Chloroquine is a potent inhibitor of SARS coronavirus infection and spread, Virol J,
doi:10.1186/1743-422X-2-69Wang, Cao, Zhang, Yang, Liu et al., Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro, Cell Res,
doi:10.1038/s41422-020-0282-0William, Schilling, Mukaka, Watson, Chandna et al., Project administration
Yao, Ye, Zhang, Cui, Huang et al., In Vitro Antiviral Activity and Projection of Optimized Dosing Design of Hydroxychloroquine for the Treatment of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), Clin Infect Dis,
doi:10.1093/cid/ciaa237DOI record:
{
"DOI": "10.1371/journal.pmed.1004428",
"ISSN": [
"1549-1676"
],
"URL": "http://dx.doi.org/10.1371/journal.pmed.1004428",
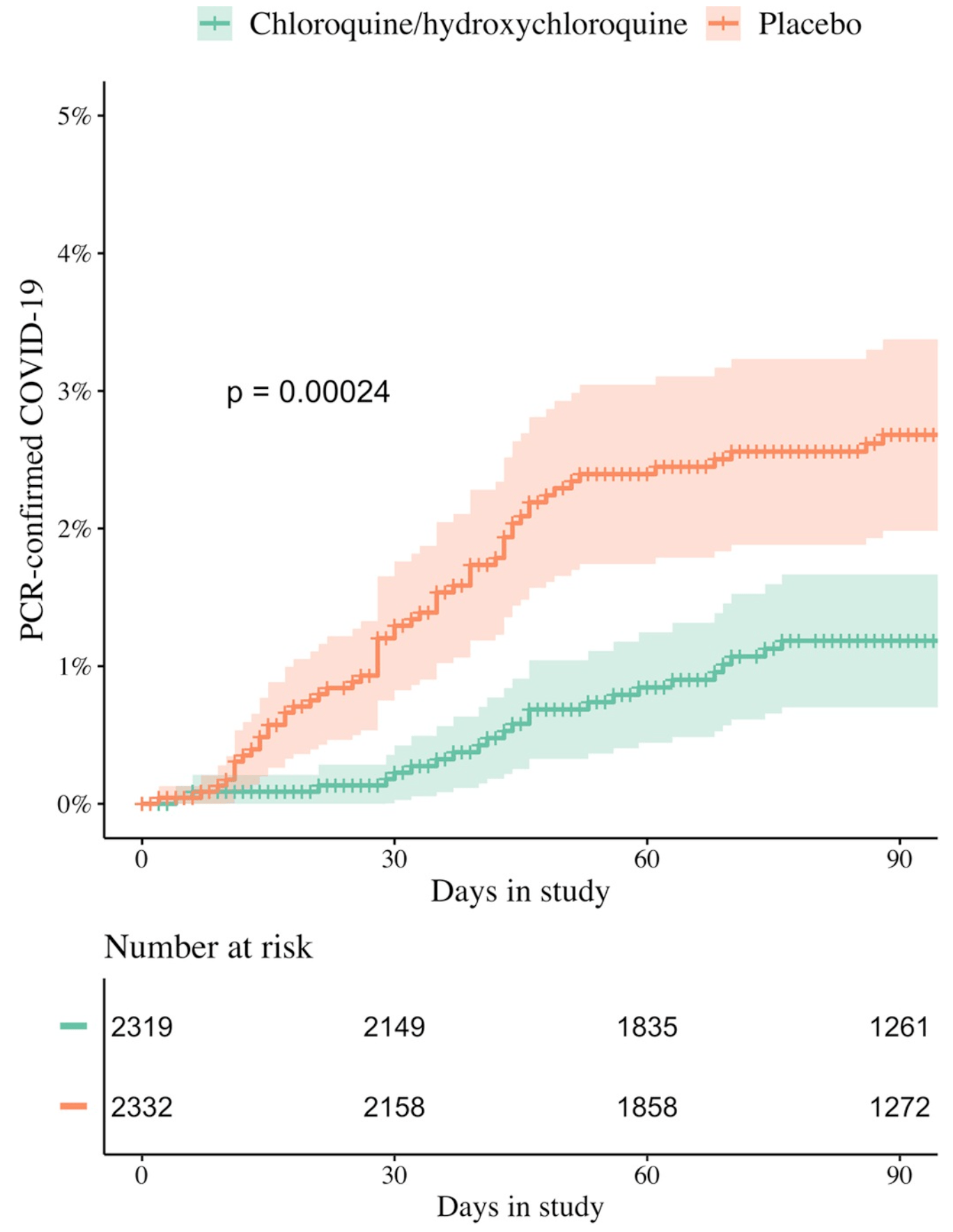
"abstract": "<jats:sec id=\"sec001\">\n<jats:title>Background</jats:title>\n<jats:p>Hydroxychloroquine (HCQ) has proved ineffective in treating patients hospitalised with Coronavirus Disease 2019 (COVID-19), but uncertainty remains over its safety and efficacy in chemoprevention. Previous chemoprevention randomised controlled trials (RCTs) did not individually show benefit of HCQ against COVID-19 and, although meta-analysis did suggest clinical benefit, guidelines recommend against its use.</jats:p>\n</jats:sec>\n<jats:sec id=\"sec002\">\n<jats:title>Methods and findings</jats:title>\n<jats:p>Healthy adult participants from the healthcare setting, and later from the community, were enrolled in 26 centres in 11 countries to a double-blind, placebo-controlled, randomised trial of COVID-19 chemoprevention. HCQ was evaluated in Europe and Africa, and chloroquine (CQ) was evaluated in Asia, (both base equivalent of 155 mg once daily). The primary endpoint was symptomatic COVID-19, confirmed by PCR or seroconversion during the 3-month follow-up period. The secondary and tertiary endpoints were: asymptomatic laboratory-confirmed Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) infection; severity of COVID-19 symptoms; all-cause PCR-confirmed symptomatic acute respiratory illness (including SARS-CoV-2 infection); participant reported number of workdays lost; genetic and baseline biochemical markers associated with symptomatic COVID-19, respiratory illness and disease severity (not reported here); and health economic analyses of HCQ and CQ prophylaxis on costs and quality of life measures (not reported here).</jats:p>\n<jats:p>The primary and safety analyses were conducted in the intention-to-treat (ITT) population. Recruitment of 40,000 (20,000 HCQ arm, 20,000 CQ arm) participants was planned but was not possible because of protracted delays resulting from controversies over efficacy and adverse events with HCQ use, vaccine rollout in some countries, and other factors. Between 29 April 2020 and 10 March 2022, 4,652 participants (46% females) were enrolled (HCQ/CQ <jats:italic>n</jats:italic> = 2,320; placebo <jats:italic>n</jats:italic> = 2,332). The median (IQR) age was 29 (23 to 39) years. SARS-CoV-2 infections (symptomatic and asymptomatic) occurred in 1,071 (23%) participants. For the primary endpoint the incidence of symptomatic COVID-19 was 240/2,320 in the HCQ/CQ versus 284/2,332 in the placebo arms (risk ratio (RR) 0.85 [95% confidence interval, 0.72 to 1.00; <jats:italic>p</jats:italic> = 0.05]).</jats:p>\n<jats:p>For the secondary and tertiary outcomes asymptomatic SARS-CoV-2 infections occurred in 11.5% of HCQ/CQ recipients and 12.0% of placebo recipients: RR: 0.96 (95% CI, 0.82 to 1.12; <jats:italic>p</jats:italic> = 0.6). There were no differences in the severity of symptoms between the groups and no severe illnesses. HCQ/CQ chemoprevention was associated with fewer PCR-confirmed all-cause respiratory infections (predominantly SARS-CoV-2): RR 0.61 (95% CI, 0.42 to 0.88; <jats:italic>p</jats:italic> = 0.009) and fewer days lost to work because of illness: 104 days per 1,000 participants over 90 days (95% CI, 12 to 199 days; <jats:italic>p</jats:italic> < 0.001). The prespecified meta-analysis of all published pre-exposure RCTs indicates that HCQ/CQ prophylaxis provided a moderate protective benefit against symptomatic COVID-19: RR 0.80 (95% CI, 0.71 to 0.91). Both drugs were well tolerated with no drug-related serious adverse events (SAEs). Study limitations include the smaller than planned study size, the relatively low number of PCR-confirmed infections, and the lower comparative accuracy of serology endpoints (in particular, the adapted dried blood spot method) compared to the PCR endpoint. The COPCOV trial was registered with <jats:ext-link xmlns:xlink=\"http://www.w3.org/1999/xlink\" ext-link-type=\"uri\" xlink:href=\"http://clinicaltrials.gov/\" xlink:type=\"simple\">ClinicalTrials.gov</jats:ext-link>; number <jats:ext-link xmlns:xlink=\"http://www.w3.org/1999/xlink\" ext-link-type=\"uri\" xlink:href=\"https://clinicaltrials.gov/ct2/show/NCT04303507\" xlink:type=\"simple\">NCT04303507</jats:ext-link>.</jats:p>\n</jats:sec>\n<jats:sec id=\"sec003\">\n<jats:title>Interpretation</jats:title>\n<jats:p>In this large placebo-controlled, double-blind randomised trial, HCQ and CQ were safe and well tolerated in COVID-19 chemoprevention, and there was evidence of moderate protective benefit in a meta-analysis including this trial and similar RCTs.</jats:p>\n</jats:sec>\n<jats:sec id=\"sec004\">\n<jats:title>Trial registration</jats:title>\n<jats:p>ClinicalTrials.gov <jats:ext-link xmlns:xlink=\"http://www.w3.org/1999/xlink\" ext-link-type=\"uri\" xlink:href=\"https://clinicaltrials.gov/ct2/show/NCT04303507\" xlink:type=\"simple\">NCT04303507</jats:ext-link>; ISRCTN Registry <jats:ext-link xmlns:xlink=\"http://www.w3.org/1999/xlink\" ext-link-type=\"uri\" xlink:href=\"https://www.isrctn.com/ISRCTN10207947\" xlink:type=\"simple\">ISRCTN10207947</jats:ext-link>.</jats:p>\n</jats:sec>",
"author": [
{
"ORCID": "http://orcid.org/0000-0002-6328-8748",
"affiliation": [],
"authenticated-orcid": true,
"family": "Schilling",
"given": "William H. K.",
"sequence": "first"
},
{
"ORCID": "http://orcid.org/0000-0002-5036-6583",
"affiliation": [],
"authenticated-orcid": true,
"family": "Mukaka",
"given": "Mavuto",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-3218-2166",
"affiliation": [],
"authenticated-orcid": true,
"family": "Callery",
"given": "James J.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-6811-1124",
"affiliation": [],
"authenticated-orcid": true,
"family": "Llewelyn",
"given": "Martin J.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cruz",
"given": "Cintia V.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-7849-3293",
"affiliation": [],
"authenticated-orcid": true,
"family": "Dhorda",
"given": "Mehul",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ngernseng",
"given": "Thatsanun",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Waithira",
"given": "Naomi",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ekkapongpisit",
"given": "Maneerat",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-5524-0325",
"affiliation": [],
"authenticated-orcid": true,
"family": "Watson",
"given": "James A.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-1313-7922",
"affiliation": [],
"authenticated-orcid": true,
"family": "Chandna",
"given": "Arjun",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-4064-5412",
"affiliation": [],
"authenticated-orcid": true,
"family": "Nelwan",
"given": "Erni J.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hamers",
"given": "Raph L.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-1267-6422",
"affiliation": [],
"authenticated-orcid": true,
"family": "Etyang",
"given": "Anthony",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-1788-5349",
"affiliation": [],
"authenticated-orcid": true,
"family": "Beg",
"given": "Mohammad Asim",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sow",
"given": "Samba",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Yavo",
"given": "William",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Allabi",
"given": "Aurel Constant",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Basnyat",
"given": "Buddha",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sharma",
"given": "Sanjib Kumar",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Amofa-Sekyi",
"given": "Modupe",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-1991-9992",
"affiliation": [],
"authenticated-orcid": true,
"family": "Yonga",
"given": "Paul",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Adler",
"given": "Amanda",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Yuentrakul",
"given": "Prayoon",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-2586-5388",
"affiliation": [],
"authenticated-orcid": true,
"family": "Cope",
"given": "Tanya",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Thaipadungpanit",
"given": "Janjira",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rienpradub",
"given": "Panuvit",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-0857-1855",
"affiliation": [],
"authenticated-orcid": true,
"family": "Imwong",
"given": "Mallika",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Abdad",
"given": "Mohammad Yazid",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-6576-726X",
"affiliation": [],
"authenticated-orcid": true,
"family": "Blacksell",
"given": "Stuart D.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-4566-4030",
"affiliation": [],
"authenticated-orcid": true,
"family": "Tarning",
"given": "Joel",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Goudjo",
"given": "Frejus Faustin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Dossou",
"given": "Ange D.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Konaté-Touré",
"given": "Abibatou",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Assi",
"given": "Serge-Brice",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ouffoué",
"given": "Kra",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Nasronudin",
"given": "Nasronudin",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-9113-1297",
"affiliation": [],
"authenticated-orcid": true,
"family": "Rachman",
"given": "Brian Eka",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Romadhon",
"given": "Pradana Zaky",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Dewanto",
"given": "Didi Darmahadi",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-7170-1750",
"affiliation": [],
"authenticated-orcid": true,
"family": "Heryana",
"given": "Made Oka",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-5912-0205",
"affiliation": [],
"authenticated-orcid": true,
"family": "Novi",
"given": "Theresia",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-3830-8073",
"affiliation": [],
"authenticated-orcid": true,
"family": "Pasaribu",
"given": "Ayodhia Pitaloka",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mutiara",
"given": "Mutiara",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-9556-572X",
"affiliation": [],
"authenticated-orcid": true,
"family": "Nasution",
"given": "Miranda Putri Rahayu",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Khairunnisa",
"given": "Khairunnisa",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Dalimunthe",
"given": "Fauzan Azima",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Airlangga",
"given": "Eka",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Fahrezzy",
"given": "Akmal",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-6367-4884",
"affiliation": [],
"authenticated-orcid": true,
"family": "Subronto",
"given": "Yanri",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ananda",
"given": "Nur Rahmi",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rahardjani",
"given": "Mutia",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-8983-747X",
"affiliation": [],
"authenticated-orcid": true,
"family": "Rimainar",
"given": "Atika",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-6926-8556",
"affiliation": [],
"authenticated-orcid": true,
"family": "Lucinde",
"given": "Ruth Khadembu",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Timbwa",
"given": "Molline",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Onyango",
"given": "Otieno Edwin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Agutu",
"given": "Clara",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Akech",
"given": "Samuel",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-0266-1414",
"affiliation": [],
"authenticated-orcid": true,
"family": "Hamaluba",
"given": "Mainga",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kipyego",
"given": "Jairus",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ngachi",
"given": "Obadiah",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Haidara",
"given": "Fadima Cheick",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Traoré",
"given": "Oumar Y.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Diarra",
"given": "François",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Khanal",
"given": "Basudha",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Dahal",
"given": "Piyush",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0009-0003-5349-5385",
"affiliation": [],
"authenticated-orcid": true,
"family": "Shrestha",
"given": "Suchita",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rijal",
"given": "Samita",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kabore",
"given": "Youssouf",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Adehossi",
"given": "Eric",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Guindo",
"given": "Ousmane",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-4173-680X",
"affiliation": [],
"authenticated-orcid": true,
"family": "Qamar",
"given": "Farah Naz",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-8253-1777",
"affiliation": [],
"authenticated-orcid": true,
"family": "Kazi",
"given": "Abdul Momin",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-5134-7165",
"affiliation": [],
"authenticated-orcid": true,
"family": "Woodrow",
"given": "Charles J.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-4216-0438",
"affiliation": [],
"authenticated-orcid": true,
"family": "Laird",
"given": "Steven",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-8392-3323",
"affiliation": [],
"authenticated-orcid": true,
"family": "Cheeba",
"given": "Maina",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-4108-2842",
"affiliation": [],
"authenticated-orcid": true,
"family": "Ayles",
"given": "Helen",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cheah",
"given": "Phaik Yeong",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-6236-0464",
"affiliation": [],
"authenticated-orcid": true,
"family": "Taylor",
"given": "Walter R. J.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-8559-452X",
"affiliation": [],
"authenticated-orcid": true,
"family": "Batty",
"given": "Elizabeth M.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Chotivanich",
"given": "Kesinee",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Pukrittayakamee",
"given": "Sasithon",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-5896-1961",
"affiliation": [],
"authenticated-orcid": true,
"family": "Phumratanaprapin",
"given": "Weerapong",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-0282-6469",
"affiliation": [],
"authenticated-orcid": true,
"family": "von Seidlein",
"given": "Lorenz",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-5190-2395",
"affiliation": [],
"authenticated-orcid": true,
"family": "Dondorp",
"given": "Arjen",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Day",
"given": "Nicholas P. J.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-1897-1978",
"affiliation": [],
"authenticated-orcid": true,
"family": "White",
"given": "Nicholas J.",
"sequence": "additional"
},
{
"affiliation": [],
"name": "on behalf of the COPCOV Collaborative Group",
"sequence": "additional"
}
],
"container-title": "PLOS Medicine",
"container-title-short": "PLoS Med",
"content-domain": {
"crossmark-restriction": false,
"domain": [
"www.plosmedicine.org"
]
},
"created": {
"date-parts": [
[
2024,
9,
12
]
],
"date-time": "2024-09-12T17:40:12Z",
"timestamp": 1726162812000
},
"deposited": {
"date-parts": [
[
2024,
9,
12
]
],
"date-time": "2024-09-12T17:40:39Z",
"timestamp": 1726162839000
},
"editor": [
{
"affiliation": [],
"family": "Jensen",
"given": "Jens-Ulrik",
"sequence": "first"
}
],
"funder": [
{
"DOI": "10.13039/100010269",
"award": [
"221307/Z/20/Z"
],
"doi-asserted-by": "publisher",
"id": [
{
"asserted-by": "publisher",
"id": "10.13039/100010269",
"id-type": "DOI"
}
],
"name": "Wellcome Trust"
}
],
"indexed": {
"date-parts": [
[
2024,
9,
13
]
],
"date-time": "2024-09-13T00:51:06Z",
"timestamp": 1726188666752
},
"is-referenced-by-count": 0,
"issue": "9",
"issued": {
"date-parts": [
[
2024,
9,
12
]
]
},
"journal-issue": {
"issue": "9",
"published-online": {
"date-parts": [
[
2024,
9,
12
]
]
}
},
"language": "en",
"license": [
{
"URL": "http://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
9,
12
]
],
"date-time": "2024-09-12T00:00:00Z",
"timestamp": 1726099200000
}
}
],
"link": [
{
"URL": "https://dx.plos.org/10.1371/journal.pmed.1004428",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "340",
"original-title": [],
"page": "e1004428",
"prefix": "10.1371",
"published": {
"date-parts": [
[
2024,
9,
12
]
]
},
"published-online": {
"date-parts": [
[
2024,
9,
12
]
]
},
"publisher": "Public Library of Science (PLoS)",
"reference": [
{
"key": "pmed.1004428.ref001",
"unstructured": "World Health Organization WHO Coronavirus (COVID-19) Dashboard. Available from: https://covid19.who.int/. Last accessed 2023 Oct 25."
},
{
"DOI": "10.1186/1743-422X-2-69",
"article-title": "Chloroquine is a potent inhibitor of SARS coronavirus infection and spread.",
"author": "MJ Vincent",
"doi-asserted-by": "crossref",
"first-page": "69",
"journal-title": "Virol J.",
"key": "pmed.1004428.ref002",
"volume": "2",
"year": "2005"
},
{
"DOI": "10.1038/s41422-020-0282-0",
"article-title": "Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro.",
"author": "M Wang",
"doi-asserted-by": "crossref",
"first-page": "269",
"journal-title": "Cell Res",
"key": "pmed.1004428.ref003",
"volume": "30",
"year": "2020"
},
{
"DOI": "10.1038/d41586-020-01695-w",
"article-title": "High-profile coronavirus retractions raise concerns about data oversight",
"author": "H Ledford",
"doi-asserted-by": "crossref",
"first-page": "160",
"journal-title": "Nature",
"key": "pmed.1004428.ref004",
"volume": "582",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2022926",
"article-title": "Effect of Hydroxychloroquine in Hospitalized Patients with Covid-19",
"author": "RECOVERY Collaborative Group",
"doi-asserted-by": "crossref",
"first-page": "2030",
"issue": "21",
"journal-title": "N Engl J Med",
"key": "pmed.1004428.ref005",
"volume": "383",
"year": "2020"
},
{
"DOI": "10.1080/14656566.2021.1898589",
"article-title": "Does hydroxychloroquine still have any role in the COVID-19 pandemic?",
"author": "WH Schilling",
"doi-asserted-by": "crossref",
"first-page": "1257",
"journal-title": "Expert Opin Pharmacother",
"key": "pmed.1004428.ref006",
"volume": "22",
"year": "2021"
},
{
"article-title": "Therapeutics and COVID-19.",
"author": "World Health Organization",
"key": "pmed.1004428.ref007"
},
{
"DOI": "10.1016/S0140-6736(21)00469-4",
"article-title": "Guidelines should not pool evidence from uncomplicated and severe COVID-19",
"author": "NJ White",
"doi-asserted-by": "crossref",
"first-page": "1262",
"issue": "10281",
"journal-title": "Lancet",
"key": "pmed.1004428.ref008",
"volume": "397",
"year": "2021"
},
{
"article-title": "India expands use of controversial drug for coronavirus despite safety concerns",
"author": "P. Pulla",
"journal-title": "Nature",
"key": "pmed.1004428.ref009",
"year": "2024"
},
{
"DOI": "10.1056/NEJMoa2016638",
"article-title": "A Randomized Trial of Hydroxychloroquine as Postexposure Prophylaxis for Covid-19",
"author": "DR Boulware",
"doi-asserted-by": "crossref",
"first-page": "517",
"journal-title": "N Engl J Med",
"key": "pmed.1004428.ref010",
"volume": "383",
"year": "2020"
},
{
"article-title": "Pre-exposure Prophylaxis With Various Doses of Hydroxychloroquine Among Healthcare Personnel With High-Risk Exposure to COVID-19: A Randomized Controlled Trial.",
"author": "F Syed",
"first-page": "e20572",
"journal-title": "Cureus",
"key": "pmed.1004428.ref011",
"volume": "13",
"year": "2021"
},
{
"DOI": "10.1001/jamainternmed.2020.6319",
"article-title": "Efficacy and Safety of Hydroxychloroquine vs Placebo for Pre-exposure SARS-CoV-2 Prophylaxis Among Health Care Workers: A Randomized Clinical Trial.",
"author": "BS Abella",
"doi-asserted-by": "crossref",
"first-page": "195",
"journal-title": "JAMA Intern Med",
"key": "pmed.1004428.ref012",
"volume": "181",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2021801",
"article-title": "A Cluster-Randomized Trial of Hydroxychloroquine for Prevention of Covid-19",
"author": "O Mitjà",
"doi-asserted-by": "crossref",
"first-page": "417",
"issue": "5",
"journal-title": "N Engl J Med",
"key": "pmed.1004428.ref013",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1186/s13063-021-05758-9",
"article-title": "Pre-exposure prophylaxis with hydroxychloroquine for COVID-19: a double-blind, placebo-controlled randomized clinical trial.",
"author": "B Grau-Pujol",
"doi-asserted-by": "crossref",
"first-page": "808",
"journal-title": "Trials",
"key": "pmed.1004428.ref014",
"volume": "22",
"year": "2021"
},
{
"DOI": "10.1093/cid/ciaa1571",
"article-title": "Hydroxychloroquine as Pre-exposure Prophylaxis for Coronavirus Disease 2019 (COVID-19) in Healthcare Workers: A Randomized Trial.",
"author": "R Rajasingham",
"doi-asserted-by": "crossref",
"first-page": "e835",
"journal-title": "Clin Infect Dis",
"key": "pmed.1004428.ref015",
"volume": "72",
"year": "2021"
},
{
"DOI": "10.1016/j.ijid.2021.04.035",
"article-title": "Positive impact of oral hydroxychloroquine and povidone-iodine throat spray for COVID-19 prophylaxis: An open-label randomized trial",
"author": "RCS Seet",
"doi-asserted-by": "crossref",
"first-page": "314",
"journal-title": "Int J Infect Dis",
"key": "pmed.1004428.ref016",
"volume": "106",
"year": "2021"
},
{
"DOI": "10.1016/j.ijid.2021.12.343",
"article-title": "Safety and tolerability of hydroxychloroquine in health care workers and first responders for the prevention of COVID-19",
"author": "JE McKinnon",
"doi-asserted-by": "crossref",
"first-page": "167",
"journal-title": "WHIP COVID-19 Study. Int J Infect Dis",
"key": "pmed.1004428.ref017",
"volume": "116",
"year": "2022"
},
{
"DOI": "10.1371/journal.pone.0261980",
"article-title": "Hydroxychloroquine for prophylaxis of COVID-19 in health workers: A randomized clinical trial.",
"author": "J Rojas-Serrano",
"doi-asserted-by": "crossref",
"first-page": "e0261980",
"journal-title": "PLoS ONE.",
"key": "pmed.1004428.ref018",
"volume": "17",
"year": "2022"
},
{
"DOI": "10.1016/j.ijid.2023.01.019",
"article-title": "Hydroxychloroquine for pre-exposure prophylaxis of COVID-19 in health care workers: a randomized, multicenter, placebo-controlled trial Healthcare Worker Exposure Response and Outcomes of Hydroxychloroquine (HERO-HCQ).",
"author": "S Naggie",
"doi-asserted-by": "crossref",
"first-page": "40",
"journal-title": "Int J Infect Dis",
"key": "pmed.1004428.ref019",
"volume": "129",
"year": "2023"
},
{
"DOI": "10.1016/j.cmi.2022.07.006",
"article-title": "Daily tenofovir disoproxil fumarate/emtricitabine and hydroxychloroquine for pre-exposure prophylaxis of COVID-19: a double-blind placebo-controlled randomized trial in healthcare workers",
"author": "R Polo",
"doi-asserted-by": "crossref",
"first-page": "85",
"issue": "1",
"journal-title": "Clin Microbiol Infect",
"key": "pmed.1004428.ref020",
"volume": "29",
"year": "2022"
},
{
"DOI": "10.1136/bmjopen-2021-059540",
"article-title": "Hydroxychloroquine plus personal protective equipment versus personal protective equipment alone for the prevention of laboratory-confirmed COVID-19 infections among healthcare workers: a multicentre, parallel-group randomised controlled trial from India",
"author": "BK Tirupakuzhi Vijayaraghavan",
"doi-asserted-by": "crossref",
"first-page": "e059540",
"issue": "6",
"journal-title": "BMJ Open",
"key": "pmed.1004428.ref021",
"volume": "12",
"year": "2022"
},
{
"DOI": "10.1186/s13104-023-06281-7",
"article-title": "Hydroxychloroquine to prevent SARS-CoV-2 infection among healthcare workers: early termination of a phase 3, randomised, open-label, controlled clinical trial.",
"author": "A Llanos-Cuentas",
"doi-asserted-by": "crossref",
"first-page": "22",
"issue": "1",
"journal-title": "BMC Res Notes",
"key": "pmed.1004428.ref022",
"volume": "16",
"year": "2023"
},
{
"article-title": "Efficacy of Hydroxychloroquine in Pre-exposure Severe Acute Respiratory Syndrome Coronavirus 2 Prophylaxis among High-Risk HealthCare Workers",
"author": "E Nasri",
"first-page": "3",
"journal-title": "A Multicenter Study. Adv Biomed Res",
"key": "pmed.1004428.ref023",
"volume": "12",
"year": "2023"
},
{
"DOI": "10.1007/s10654-022-00891-4",
"article-title": "Systematic review and meta-analysis of randomized trials of hydroxychloroquine for the prevention of COVID-19",
"author": "X Garcia-Albeniz",
"doi-asserted-by": "crossref",
"first-page": "789",
"journal-title": "Eur J Epidemiol",
"key": "pmed.1004428.ref024",
"volume": "37",
"year": "2022"
},
{
"article-title": "WHO Living guideline: Drugs to prevent COVID-19.",
"author": "World Health Organization",
"key": "pmed.1004428.ref025",
"year": "2023"
},
{
"DOI": "10.1038/s41421-020-0156-0",
"article-title": "Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro",
"author": "J Liu",
"doi-asserted-by": "crossref",
"first-page": "16",
"journal-title": "Cell Discov",
"key": "pmed.1004428.ref026",
"volume": "6",
"year": "2020"
},
{
"DOI": "10.1093/cid/ciaa237",
"article-title": "In Vitro Antiviral Activity and Projection of Optimized Dosing Design of Hydroxychloroquine for the Treatment of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2).",
"author": "X Yao",
"doi-asserted-by": "crossref",
"first-page": "732",
"journal-title": "Clin Infect Dis",
"key": "pmed.1004428.ref027",
"volume": "71",
"year": "2020"
},
{
"DOI": "10.1016/j.jcv.2022.105121",
"article-title": "A systematic review and meta-analysis of the accuracy of SARS-COV-2 IGM and IGG tests in individuals with COVID-19.",
"author": "ACL Macedo",
"doi-asserted-by": "crossref",
"first-page": "105121",
"journal-title": "J Clin Virol",
"key": "pmed.1004428.ref028",
"volume": "148",
"year": "2022"
},
{
"article-title": "FDA cautions against use of hydroxychloroquine or chloroquine for COVID-19 outside of the hospital setting or a clinical trial due to risk of heart rhythm problems.",
"author": "FDA US",
"key": "pmed.1004428.ref029",
"year": "2023"
},
{
"article-title": "Feasibility of SARS-CoV-2 Antibody Testing in Remote Outpatient Trials.",
"author": "SM Lofgren",
"issue": "11",
"journal-title": "Open Forum Infect Dis.",
"key": "pmed.1004428.ref030",
"volume": "8",
"year": "2021"
},
{
"DOI": "10.1093/ofid/ofaa500",
"article-title": "Safety of Hydroxychloroquine Among Outpatient Clinical Trial Participants for COVID-19.",
"author": "SM Lofgren",
"doi-asserted-by": "crossref",
"issue": "11",
"journal-title": "Open Forum Infect Dis.",
"key": "pmed.1004428.ref031",
"volume": "7",
"year": "2020"
},
{
"DOI": "10.12688/wellcomeopenres.16741.1",
"article-title": "The WHO guideline on drugs to prevent COVID-19: small numbers- big conclusions.",
"author": "WH Schilling",
"doi-asserted-by": "crossref",
"first-page": "e71",
"journal-title": "Wellcome Open Res",
"key": "pmed.1004428.ref032",
"volume": "6",
"year": "2021"
},
{
"DOI": "10.1016/S0140-6736(20)31821-3",
"article-title": "World Health Organization Solidarity Vaccines Trial Expert Group. COVID-19 vaccine trials should seek worthwhile efficacy",
"author": "P Krause",
"doi-asserted-by": "crossref",
"first-page": "741",
"journal-title": "Lancet",
"key": "pmed.1004428.ref033",
"volume": "396",
"year": "2020"
}
],
"reference-count": 33,
"references-count": 33,
"relation": {},
"resource": {
"primary": {
"URL": "https://dx.plos.org/10.1371/journal.pmed.1004428"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Evaluation of hydroxychloroquine or chloroquine for the prevention of COVID-19 (COPCOV): A double-blind, randomised, placebo-controlled trial",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1371/journal.pmed.corrections_policy",
"volume": "21"
}