Ongoing use of SSRIs and the hospital course of COVID-19 patients: a retrospective outcome analysis
Steven H Rauchman, Sherri G Mendelson, Courtney Rauchman, Lora J Kasselman, Aaron Pinkhasov, Allison B Reiss
doi:10.1101/2021.10.25.21265218
Background: The SARS-CoV2 virus continues to have devastating consequences worldwide. Though vaccinations have helped to reduce the impact of the virus, new strains still pose a threat to unvaccinated, and to a lesser extent vaccinated, individuals. Therefore, it is imperative to identify treatments that can prevent the development of severe COVID-19. Recently, acute use of SSRI antidepressants in COVID+ patients has been shown to reduce the severity of symptoms compared to placebo. Since SSRIs are a widely used anti-depressant, the aim of this study was to determine if COVID+ patients already on SSRI treatment upon admission to the hospital had reduced mortality compared to COVID+ patients not on chronic SSRI treatment. Methods: A retrospective observational study design was used. Electronic medical records of 9,043 patients with a laboratory-confirmed diagnosis of Covid-19 from 03/2020 to 03/2021from six hospitals were queried for demographic and clinical information. Using R, a logistic regression model was run with mortality as the outcome and SSRI status as the exposure. An adjusted logistic regression model was run to account for age category, gender, and race. All tests were considered significant at p of 0.05 or less.
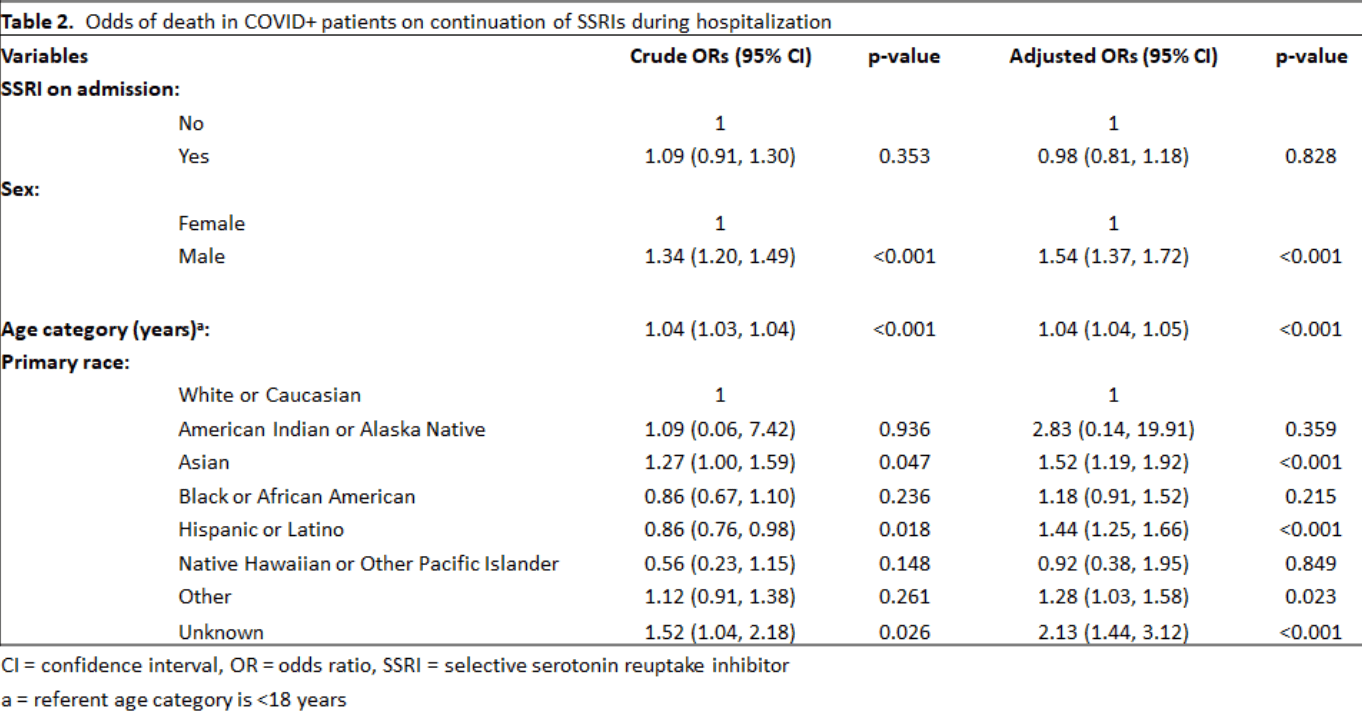
Results: In this sample, no patients admitted on SSRIs had them discontinued. This is consistent with current recommendations. There was no significant difference in the odds of dying between COVID+ patients on chronic SSRIs vs COVID+ patients not taking SSRIs, after controlling for age category, gender, and race. The odds of COVID+ patients on SSRIs dying was 0.98 (95%CI: 0.81, 1.18) compared to COVID+ patients not on SSRIs (p=0.83).
Conclusion: In times of pandemics due to novel infectious agents it is difficult, but critical to evaluate safety and efficacy of drugs that might be repurposed for treatment. This large sample size of 9,043 patients suggests that there will be no significant benefit to use of SSRIs to .
Competing interests The authors declare that they have no competing interests.
References
Anagha, Shihabudheen, Uvais, Side Effect Profiles of Selective Serotonin Reuptake Inhibitors: A Cross-Sectional Study in a Naturalistic Setting. Primary Care Companion for CNS Disorders,
doi:10.4088/PCC.20m02747Bainum, Fike, Mechelay, Effect of Abrupt Discontinuation of Antidepressants in Critically Ill Hospitalized Adults, Pharmacotherapy,
doi:10.1002/phar.1992Brimson, Prasanth, Malar, Drugs that offer the potential to reduce hospitalization and mortality from SARS-CoV-2 infection: The possible role of the sigma-1 receptor and autophagy, Expert Opinion on Therapeutic Targets,
doi:10.1080/14728222.2021.1952987Brody, Gu, Antidepressant Use Among Adults: United States, 2015-2018, NCHS Data Brief
Chiou, Hsu, Chen, Repurposing existing drugs: identification of SARS-CoV-2 3C-like protease inhibitors, Journal of Enzyme Inhibition and Medicinal Chemistry,
doi:10.1080/14756366.2020.1850710Ghareghani, Zibara, Sadeghi, Fluvoxamine stimulates oligodendrogenesis of cultured neural stem cells and attenuates inflammation and demyelination in an animal model of multiple sclerosis, Scientific Reports,
doi:10.1038/s41598-017-04968-zGu, Tyagi, Jain, Thrombocytopathy and endotheliopathy: crucial contributors to COVID-19 thromboinflammation, Nature Reviews Cardiology,
doi:10.1038/s41569-020-00469-1Hashimoto, Repurposing of CNS drugs to treat COVID-19 infection: targeting the sigma-1 receptor, European Archives of Psychiatry and Clinical Neuroscience,
doi:10.1007/s00406-020-01231-xHoertel, Sánchez-Rico, Vernet, Association between antidepressant use and 426 reduced risk of intubation or death in hospitalized patients with COVID-19: results . from an 427 observational study, Molecular Psychiatry,
doi:10.1038/s41380-021-01021-4Ishima, Fujita, Hashimoto, Interaction of new antidepressants with sigma-1 receptor chaperones and their potentiation of neurite outgrowth in PC12 cells, European Journal of Pharmacology,
doi:10.1016/j.ejphar.2014.01.064Kelly, Rubenfeld, Masson, Using Selective Serotonin Reuptake Inhibitors and Serotonin-Norepinephrine Reuptake Inhibitors in Critical Care: A Systematic Review of the Evidence for Benefit or Harm, Critical Care Medicine,
doi:10.1097/CCM.0000000000002308Kuriakose, Singh, Pau, Developing Treatment Guidelines During a Pandemic Health Crisis: Lessons Learned From COVID-19, Annals of Internal Medicine,
doi:10.7326/M21-1647Lenze, Mattar, Zorumski, Fluvoxamine vs Placebo and Clinical 429 Deterioration in Outpatients With Symptomatic COVID-19: A Randomized Clinical Trial, JAMA,
doi:10.1001/jama.2020.22760Lohia, Kapur, Benjaram, Statins and clinical outcomes in hospitalized COVID-19 patients with and without Diabetes Mellitus: a retrospective cohort study with propensity score matching, Cardiovascular Diabetology,
doi:10.1186/s12933-021-01336-0Lopes, Macedo, De, Silva, Effect of Discontinuing vs Continuing Angiotensin-Converting Enzyme Inhibitors and Angiotensin II Receptor Blockers on Days Alive and Out of the Hospital in Patients Admitted With COVID-19: A Randomized Clinical Trial, JAMA,
doi:10.1001/jama.2020.25864Lopez, Can a common antidepressant help in the fight against COVID-19? LA Times
Meikle, Creeden, Mccullumsmith, SSRIs: Applications in inflammatory lung disease and implications for COVID-19, Neuropsychopharmacology Reports,
doi:10.1002/npr2.12194Pashaei, Drug repurposing of selective serotonin reuptake inhibitors: Could these drugs help fight COVID-19 and save lives?, Journal of Clinical Neuroscience,
doi:10.1016/j.jocn.2021.03.010Pigott, Pato, Heureux, A controlled comparison of adjuvant lithium carbonate or thyroid hormone in clomipramine-treated patients with obsessive-compulsive disorder, Journal of Clinical Psychopharmacology
Reis, Silva, Silva, Effect of Early Treatment with Fluvoxamine on Risk of Emergency Care and Hospitalization Among Patients with COVID-19: The TOGETHER Randomized Platform Clinical Trial, medRxiv,
doi:10.1101/2021.08.19.21262323Seftel, Boulware, Prospective Cohort of Fluvoxamine for Early Treatment of Coronavirus Disease 19, Open Forum Infectious Diseases,
doi:10.1093/ofid/ofab050Sukhatme, Reiersen, Vayttaden, Fluvoxamine: A Review of Its Mechanism of Action and Its Role in COVID-19, Frontiers in Pharmacology,
doi:10.3389/fphar.2021.652688Szabo, Kovacs, Frecska, Rajnavolgyi, Psychedelic N,N-dimethyltryptamine and 5-methoxy-N,N-dimethyltryptamine modulate innate and adaptive inflammatory responses through the sigma-1 receptor of human monocyte-derived dendritic cells, PLoS One,
doi:10.1371/journal.pone.0106533Xu, Chen, Pan, Repurposing clinically approved drugs for COVID-19 treatment targeting SARS-CoV-2 papain-like protease, International Journal of Biological Macromolecules,
doi:10.1016/j.ijbiomac.2021.07.184{ 'institution': [{'name': 'medRxiv'}],
'indexed': {'date-parts': [[2024, 2, 15]], 'date-time': '2024-02-15T16:49:42Z', 'timestamp': 1708015782368},
'posted': {'date-parts': [[2021, 10, 26]]},
'group-title': 'Infectious Diseases (except HIV/AIDS)',
'reference-count': 33,
'publisher': 'Cold Spring Harbor Laboratory',
'content-domain': {'domain': [], 'crossmark-restriction': False},
'accepted': {'date-parts': [[2021, 10, 26]]},
'abstract': '<jats:title>Abstract</jats:title><jats:sec><jats:title>Background</jats:title><jats:p>The '
'SARS-CoV2 virus continues to have devastating consequences worldwide. Though vaccinations '
'have helped to reduce the impact of the virus, new strains still pose a threat to '
'unvaccinated, and to a lesser extent vaccinated, individuals. Therefore, it is imperative to '
'identify treatments that can prevent the development of severe COVID-19. Recently, acute use '
'of SSRI antidepressants in COVID+ patients has been shown to reduce the severity of symptoms '
'compared to placebo. Since SSRIs are a widely used anti-depressant, the aim of this study was '
'to determine if COVID+ patients already on SSRI treatment upon admission to the hospital had '
'reduced mortality compared to COVID+ patients not on chronic SSRI '
'treatment.</jats:p></jats:sec><jats:sec><jats:title>Methods</jats:title><jats:p>A '
'retrospective observational study design was used. Electronic medical records of 9,043 '
'patients with a laboratory-confirmed diagnosis of Covid-19 from 03/2020 to 03/2021from six '
'hospitals were queried for demographic and clinical information. Using R, a logistic '
'regression model was run with mortality as the outcome and SSRI status as the exposure. An '
'adjusted logistic regression model was run to account for age category, gender, and race. All '
'tests were considered significant at p of 0.05 or '
'less.</jats:p></jats:sec><jats:sec><jats:title>Results</jats:title><jats:p>In this sample, no '
'patients admitted on SSRIs had them discontinued. This is consistent with current '
'recommendations. There was no significant difference in the odds of dying between COVID+ '
'patients on chronic SSRIs vs COVID+ patients not taking SSRIs, after controlling for age '
'category, gender, and race. The odds of COVID+ patients on SSRIs dying was 0.98 (95%CI: 0.81, '
'1.18) compared to COVID+ patients not on SSRIs '
'(p=0.83).</jats:p></jats:sec><jats:sec><jats:title>Conclusion</jats:title><jats:p>In times of '
'pandemics due to novel infectious agents it is difficult, but critical to evaluate safety and '
'efficacy of drugs that might be repurposed for treatment. This large sample size of 9,043 '
'patients suggests that there will be no significant benefit to use of SSRIs to decrease '
'mortality rates for hospitalized patients with Covid-19 who are not currently on SSRI '
'medications. This study shows the utility of large clinical databases in addressing the '
'urgent issue of determining what commonly prescribed drugs might be useful in treating '
'COVID-19.</jats:p></jats:sec>',
'DOI': '10.1101/2021.10.25.21265218',
'type': 'posted-content',
'created': { 'date-parts': [[2021, 10, 26]],
'date-time': '2021-10-26T23:25:37Z',
'timestamp': 1635290737000},
'source': 'Crossref',
'is-referenced-by-count': 1,
'title': 'Ongoing use of SSRIs and the hospital course of COVID-19 patients: a retrospective outcome '
'analysis',
'prefix': '10.1101',
'author': [ {'given': 'Steven H.', 'family': 'Rauchman', 'sequence': 'first', 'affiliation': []},
{'given': 'Sherri G.', 'family': 'Mendelson', 'sequence': 'additional', 'affiliation': []},
{'given': 'Courtney', 'family': 'Rauchman', 'sequence': 'additional', 'affiliation': []},
{'given': 'Lora J.', 'family': 'Kasselman', 'sequence': 'additional', 'affiliation': []},
{'given': 'Aaron', 'family': 'Pinkhasov', 'sequence': 'additional', 'affiliation': []},
{ 'ORCID': 'http://orcid.org/0000-0002-4478-2441',
'authenticated-orcid': False,
'given': 'Allison B.',
'family': 'Reiss',
'sequence': 'additional',
'affiliation': []}],
'member': '246',
'reference': [ { 'key': '2021102804150300000_2021.10.25.21265218v1.1',
'doi-asserted-by': 'publisher',
'DOI': '10.7326/M21-1647'},
{ 'key': '2021102804150300000_2021.10.25.21265218v1.2',
'doi-asserted-by': 'publisher',
'DOI': '10.1038/s41586-020-2577-1'},
{ 'key': '2021102804150300000_2021.10.25.21265218v1.3',
'doi-asserted-by': 'publisher',
'DOI': '10.1016/j.ijbiomac.2021.07.184'},
{ 'key': '2021102804150300000_2021.10.25.21265218v1.4',
'doi-asserted-by': 'publisher',
'DOI': '10.1080/14756366.2020.1850710'},
{ 'key': '2021102804150300000_2021.10.25.21265218v1.5',
'doi-asserted-by': 'publisher',
'DOI': '10.1371/journal.ppat.1009840'},
{ 'key': '2021102804150300000_2021.10.25.21265218v1.6',
'doi-asserted-by': 'publisher',
'DOI': '10.1002/14651858.CD013587'},
{ 'key': '2021102804150300000_2021.10.25.21265218v1.7',
'doi-asserted-by': 'publisher',
'DOI': '10.1038/s41380-021-01021-4'},
{ 'key': '2021102804150300000_2021.10.25.21265218v1.8',
'unstructured': 'Lopez S. Can a common antidepressant help in the fight against COVID-19? '
'LA Times. 2021. '
'https://www.latimes.com/california/story/2021-02-03/can-common-antidepressant-help-covid-19-fight'},
{ 'key': '2021102804150300000_2021.10.25.21265218v1.9',
'doi-asserted-by': 'publisher',
'DOI': '10.1002/npr2.12194'},
{ 'key': '2021102804150300000_2021.10.25.21265218v1.10',
'doi-asserted-by': 'publisher',
'DOI': '10.1016/j.jocn.2021.03.010'},
{ 'key': '2021102804150300000_2021.10.25.21265218v1.11',
'doi-asserted-by': 'publisher',
'DOI': '10.1016/j.biopha.2021.111437'},
{ 'key': '2021102804150300000_2021.10.25.21265218v1.12',
'doi-asserted-by': 'publisher',
'DOI': '10.3389/fphar.2021.652688'},
{ 'key': '2021102804150300000_2021.10.25.21265218v1.13',
'doi-asserted-by': 'publisher',
'DOI': '10.1016/S0140-6736(20)30183-5'},
{ 'key': '2021102804150300000_2021.10.25.21265218v1.14',
'doi-asserted-by': 'publisher',
'DOI': '10.1038/d41586-021-01470-5'},
{ 'key': '2021102804150300000_2021.10.25.21265218v1.15',
'doi-asserted-by': 'publisher',
'DOI': '10.1001/jama.2020.22760'},
{ 'key': '2021102804150300000_2021.10.25.21265218v1.16',
'doi-asserted-by': 'publisher',
'DOI': '10.1101/2021.08.19.21262323'},
{ 'key': '2021102804150300000_2021.10.25.21265218v1.17',
'doi-asserted-by': 'publisher',
'DOI': '10.1093/ofid/ofab050'},
{ 'key': '2021102804150300000_2021.10.25.21265218v1.18',
'unstructured': 'Brody DJ , Gu Q. Antidepressant Use Among Adults: United States, '
'2015-2018. NCHS Data Brief. 2020;(377):1-8.'},
{ 'key': '2021102804150300000_2021.10.25.21265218v1.19',
'doi-asserted-by': 'publisher',
'DOI': '10.4088/PCC.20m02747'},
{ 'key': '2021102804150300000_2021.10.25.21265218v1.20',
'doi-asserted-by': 'publisher',
'DOI': '10.1016/j.ejphar.2014.01.064'},
{ 'key': '2021102804150300000_2021.10.25.21265218v1.21',
'doi-asserted-by': 'publisher',
'DOI': '10.1080/15548627.2019.1586248'},
{ 'key': '2021102804150300000_2021.10.25.21265218v1.22',
'doi-asserted-by': 'publisher',
'DOI': '10.1038/s41598-017-04968-z'},
{ 'key': '2021102804150300000_2021.10.25.21265218v1.23',
'doi-asserted-by': 'publisher',
'DOI': '10.1371/journal.pone.0106533'},
{ 'key': '2021102804150300000_2021.10.25.21265218v1.24',
'doi-asserted-by': 'publisher',
'DOI': '10.1080/14728222.2021.1952987'},
{ 'key': '2021102804150300000_2021.10.25.21265218v1.25',
'doi-asserted-by': 'publisher',
'DOI': '10.1007/s00406-020-01231-x'},
{ 'key': '2021102804150300000_2021.10.25.21265218v1.26',
'doi-asserted-by': 'publisher',
'DOI': '10.1002/phar.1992'},
{ 'key': '2021102804150300000_2021.10.25.21265218v1.27',
'doi-asserted-by': 'publisher',
'DOI': '10.1097/CCM.0000000000002308'},
{ 'key': '2021102804150300000_2021.10.25.21265218v1.28',
'doi-asserted-by': 'publisher',
'DOI': '10.31887/DCNS.2007.9.1/dhalperin'},
{ 'issue': '4',
'key': '2021102804150300000_2021.10.25.21265218v1.29',
'first-page': '242',
'article-title': 'A controlled comparison of adjuvant lithium carbonate or thyroid '
'hormone in clomipramine-treated patients with obsessive-compulsive '
'disorder',
'volume': '11',
'year': '1991',
'journal-title': 'Journal of Clinical Psychopharmacology'},
{ 'key': '2021102804150300000_2021.10.25.21265218v1.30',
'doi-asserted-by': 'publisher',
'DOI': '10.1038/s41569-020-00469-1'},
{ 'key': '2021102804150300000_2021.10.25.21265218v1.31',
'doi-asserted-by': 'publisher',
'DOI': '10.1186/s12933-021-01336-0'},
{ 'key': '2021102804150300000_2021.10.25.21265218v1.32',
'doi-asserted-by': 'publisher',
'DOI': '10.1001/jama.2020.25864'},
{ 'issue': '1',
'key': '2021102804150300000_2021.10.25.21265218v1.33',
'doi-asserted-by': 'crossref',
'first-page': 'e100337',
'DOI': '10.1136/bmjhci-2021-100337',
'article-title': 'Utility of routinely collected electronic health records data to '
'support effectiveness evaluations in inflammatory bowel disease: a '
'pilot study of tofacitinib',
'volume': '28',
'year': '2021',
'journal-title': 'BMJ Health & Care Informatics'}],
'container-title': [],
'original-title': [],
'link': [ { 'URL': 'https://syndication.highwire.org/content/doi/10.1101/2021.10.25.21265218',
'content-type': 'unspecified',
'content-version': 'vor',
'intended-application': 'similarity-checking'}],
'deposited': { 'date-parts': [[2021, 10, 28]],
'date-time': '2021-10-28T11:15:21Z',
'timestamp': 1635419721000},
'score': 1,
'resource': {'primary': {'URL': 'http://medrxiv.org/lookup/doi/10.1101/2021.10.25.21265218'}},
'subtitle': [],
'short-title': [],
'issued': {'date-parts': [[2021, 10, 26]]},
'references-count': 33,
'URL': 'http://dx.doi.org/10.1101/2021.10.25.21265218',
'relation': {},
'published': {'date-parts': [[2021, 10, 26]]},
'subtype': 'preprint'}