The altered entry pathway and antigenic distance of the SARS-CoV-2 Omicron variant map to separate domains of spike protein
Thomas P Peacock, Jonathan C Brown, Jie Zhou, Nazia Thakur, Ksenia Sukhova, Joseph Newman, Ruthiran Kugathasan, Ada W C Yan, Wilhelm Furnon, Giuditta De Lorenzo, Vanessa M Cowton, Dorothee Reuss, Maya Moshe, Jessica L Quantrill, Olivia K Platt, Myrsini Kaforou, Arvind H Patel, Massimo Palmarini, Dalan Bailey, Wendy S Barclay
doi:10.1101/2021.12.31.474653
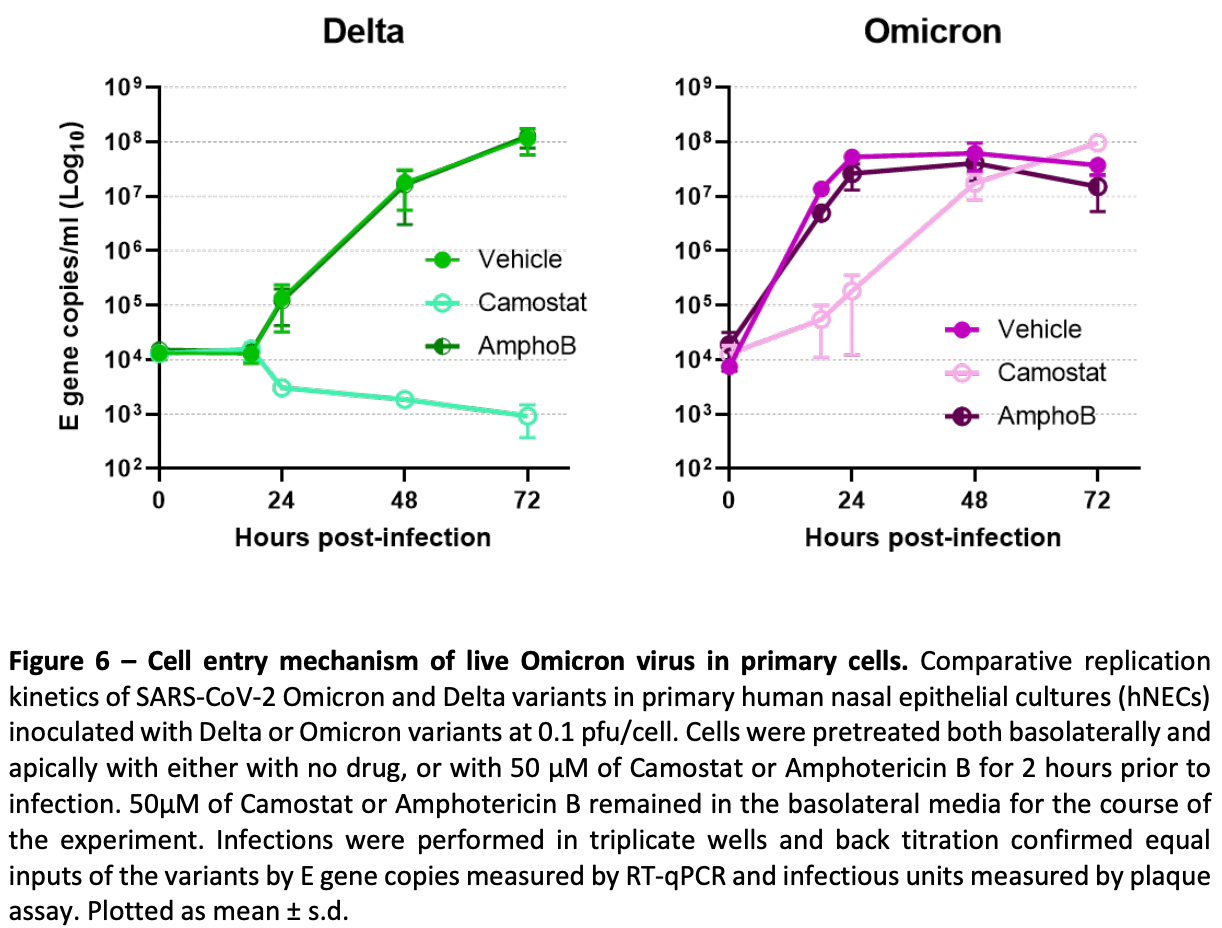
At the end of 2021 a new SARS-CoV-2 variant, Omicron, emerged and quickly spread across the world. It has been demonstrated that Omicron's high number of Spike mutations lead to partial immune evasion from even polyclonal antibody responses, allowing frequent re-infection and vaccine breakthroughs. However, it seems unlikely these antigenic differences alone explain its rapid growth; here we show Omicron replicates rapidly in human primary airway cultures, more so even than the previously dominant variant of concern, Delta. Omicron Spike continues to use human ACE2 as its primary receptor, to which it binds more strongly than other variants. Omicron Spike mediates enhanced entry into cells expressing several different animal ACE2s, including various domestic avian species, horseshoe bats and mice suggesting it has an increased propensity for reverse zoonosis and is more likely than previous variants to establish an animal reservoir of SARS-CoV-2. Unlike other SARS-CoV-2 variants, however, Omicron Spike has a diminished ability to induce syncytia formation. Furthermore, Omicron is capable of efficiently entering cells in a TMPRSS2-independent manner, via the endosomal route. We posit this enables Omicron to infect a greater number of cells in the respiratory epithelium, allowing it to be more infectious at lower exposure doses, and resulting in enhanced intrinsic transmissibility. .
Supplementary Figures Supplementary Figure S1
Supplementary Figure S2 -Different species ACE2 preference of different variants of concern. Receptor usage was screened using pseudoviruses expressing the indicated Spike proteins into 293Ts expressing the indicated ACE2 protein. Viral entry was measured by assaying luciferase activity (RLU) using the BrightGlo reagent (Promega).
References
Bentley, SARS-CoV-2 Omicron-B.1.1.529 Variant leads to less severe disease than Pango B and Delta variants strains in a mouse model of severe COVID-19, bioRxiv,
doi:10.1101/2021.12.26.474085Cele, SARS-CoV-2 Omicron has extensive but incomplete escape of Pfizer BNT162b2 elicited neutralization and requires ACE2 for infection, medRxiv,
doi:10.1101/2021.12.08.21267417Edie, Survey of human chromosome 21 gene expression effects on early development in Danio rerio. G3: Genes, Genomes, Genetics
Ferguson, Report 49 -Growth, population distribution and immune escape of Omicron in England
Heurich, TMPRSS2 and ADAM17 cleave ACE2 differentially and only proteolysis by TMPRSS2 augments entry driven by the severe acute respiratory syndrome coronavirus spike protein, J Virol,
doi:10.1128/jvi.02202-13Hoffmann, Kleine-Weber, Pöhlmann, A Multibasic Cleavage Site in the Spike Protein of SARS-CoV-2 Is Essential for Infection of Human Lung Cells, Mol Cell,
doi:10.1016/j.molcel.2020.04.022Hoffmann, The Omicron variant is highly resistant against antibody-mediated neutralization -implications for control of the COVID-19 pandemic, Cell,
doi:10.1016/j.cell.2021.12.032Ishikawa, Meng, Kondo, Iwamoto, Matsuda, Generation of a dual-functional split-reporter protein for monitoring membrane fusion using self-associating split GFP, Protein Eng Des Sel,
doi:10.1093/protein/gzs051Mckay, Self-amplifying RNA SARS-CoV-2 lipid nanoparticle vaccine candidate induces high neutralizing antibody titers in mice, Nat Commun,
doi:10.1038/s41467-020-17409-9Newman, Neutralising antibody activity against SARS-CoV-2 variants, including Omicron, in an elderly cohort vaccinated with BNT162b2, medRxiv,
doi:10.1101/2021.12.23.21268293Ni, Structural analysis of the Spike of the Omicron SARS-COV-2 variant by Cryo-EM and implications for immune evasion, bioRxiv,
doi:10.1101/2021.12.27.474250Peacock, The SARS-CoV-2 variants associated with infections in India, B.1.617, show enhanced spike cleavage by furin, bioRxiv,
doi:10.1101/2021.05.28.446163Peacock, The furin cleavage site in the SARS-CoV-2 spike protein is required for transmission in ferrets, Nat Microbiol,
doi:10.1038/s41564-021-00908-wPulliam, Increased risk of SARS-CoV-2 reinfection associated with emergence of the Omicron variant in South Africa, medRxiv,
doi:10.1101/2021.11.11.21266068Thakur, Micro-fusion inhibition tests: quantifying antibody neutralization of virusmediated cell-cell fusion, Journal of General Virology,
doi:10.1099/jgv.0.001506Thakur, SARS-CoV-2 variants of concern Alpha, Beta, Gamma and Delta have extended ACE2 receptor host-ranges, bioRxiv,
doi:10.1101/2021.11.23.469663Winstone, The polybasic cleavage site in the SARS-CoV-2 spike modulates viral sensitivity to Type I interferon and IFITM2, J Virol,
doi:10.1128/JVI.02422-20Zhou, Investigating SARS-CoV-2 surface and air contamination in an acute healthcare setting during the peak of the COVID-19 pandemic in London, Clin Infect Dis,
doi:10.1093/cid/ciaa905DOI record:
{
"DOI": "10.1101/2021.12.31.474653",
"URL": "http://dx.doi.org/10.1101/2021.12.31.474653",
"abstract": "<jats:p>At the end of 2021 a new SARS-CoV-2 variant, Omicron, emerged and quickly spread across the world. It has been demonstrated that Omicrons high number of Spike mutations lead to partial immune evasion from even polyclonal antibody responses, allowing frequent re-infection and vaccine breakthroughs. However, it seems unlikely these antigenic differences alone explain its rapid growth; here we show Omicron replicates rapidly in human primary airway cultures, more so even than the previously dominant variant of concern, Delta. Omicron Spike continues to use human ACE2 as its primary receptor, to which it binds more strongly than other variants. Omicron Spike mediates enhanced entry into cells expressing several different animal ACE2s, including various domestic avian species, horseshoe bats and mice suggesting it has an increased propensity for reverse zoonosis and is more likely than previous variants to establish an animal reservoir of SARS-CoV-2. Unlike other SARS-CoV-2 variants, however, Omicron Spike has a diminished ability to induce syncytia formation. Furthermore, Omicron is capable of efficiently entering cells in a TMPRSS2-independent manner, via the endosomal route. We posit this enables Omicron to infect a greater number of cells in the respiratory epithelium, allowing it to be more infectious at lower exposure doses, and resulting in enhanced intrinsic transmissibility.</jats:p>",
"accepted": {
"date-parts": [
[
2022,
1,
3
]
]
},
"author": [
{
"ORCID": "http://orcid.org/0000-0001-7077-2928",
"affiliation": [],
"authenticated-orcid": false,
"family": "Peacock",
"given": "Thomas P.",
"sequence": "first"
},
{
"ORCID": "http://orcid.org/0000-0001-6849-3962",
"affiliation": [],
"authenticated-orcid": false,
"family": "Brown",
"given": "Jonathan C",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-6413-2454",
"affiliation": [],
"authenticated-orcid": false,
"family": "Zhou",
"given": "Jie",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-4450-5911",
"affiliation": [],
"authenticated-orcid": false,
"family": "Thakur",
"given": "Nazia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Newman",
"given": "Joseph",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-4591-6026",
"affiliation": [],
"authenticated-orcid": false,
"family": "Kugathasan",
"given": "Ruthiran",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sukhova",
"given": "Ksenia",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-9878-4007",
"affiliation": [],
"authenticated-orcid": false,
"family": "Kaforou",
"given": "Myrsini",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-5640-2266",
"affiliation": [],
"authenticated-orcid": false,
"family": "Bailey",
"given": "Dalan",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-3948-0895",
"affiliation": [],
"authenticated-orcid": false,
"family": "Barclay",
"given": "Wendy S",
"sequence": "additional"
}
],
"container-title": [],
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2022,
1,
4
]
],
"date-time": "2022-01-04T02:25:13Z",
"timestamp": 1641263113000
},
"deposited": {
"date-parts": [
[
2022,
1,
4
]
],
"date-time": "2022-01-04T02:25:13Z",
"timestamp": 1641263113000
},
"group-title": "Microbiology",
"indexed": {
"date-parts": [
[
2022,
1,
4
]
],
"date-time": "2022-01-04T05:54:12Z",
"timestamp": 1641275652490
},
"institution": [
{
"name": "bioRxiv"
}
],
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2022,
1,
3
]
]
},
"link": [
{
"URL": "https://syndication.highwire.org/content/doi/10.1101/2021.12.31.474653",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "246",
"original-title": [],
"posted": {
"date-parts": [
[
2022,
1,
3
]
]
},
"prefix": "10.1101",
"published": {
"date-parts": [
[
2022,
1,
3
]
]
},
"publisher": "Cold Spring Harbor Laboratory",
"reference-count": 0,
"references-count": 0,
"relation": {},
"score": 1,
"short-container-title": [],
"short-title": [],
"source": "Crossref",
"subtitle": [],
"subtype": "preprint",
"title": [
"The SARS-CoV-2 variant, Omicron, shows rapid replication in human primary nasal epithelial cultures and efficiently uses the endosomal route of entry."
],
"type": "posted-content"
}