Factors Associated with COVID-19 Breakthrough Infection in the Pre-Omicron Era Among Vaccinated Patients with Rheumatic Diseases: A Cohort Study
MD Naomi J Patel, MS Xiaosong Wang, MS Xiaoqing Fu, MD Yumeko Kawano, MPH Claire Cook, BA Kathleen M M Vanni, BA&Sc Grace Qian, BA Emily Banasiak, BS Emily Kowalski, ScD Yuqing Zhang, MD, MMSc Jeffrey A Sparks, MD, MSc Zachary S Wallace
doi:10.1101/2022.07.13.22277606
Objective: Rheumatic disease patients on certain immunomodulators are at increased risk of impaired humoral response to SARS-CoV-2 vaccines. We aimed to identify factors associated with breakthrough infection among patients with rheumatic diseases.
Methods: We identified patients with rheumatic diseases being treated with immunomodulators in a large healthcare system who received at least two doses of either the mRNA-1273 (Moderna) or BNT162b2 (Pfizer-BioNTech) vaccines or one dose of the Johnson & Johnson-Janssen (J&J) vaccine. We followed patients until SARS-CoV-2 infection, death, or December 15, 2021, when the Omicron variant became dominant in our region. We estimated the association of baseline characteristics with the risk of breakthrough infection using multivariable Cox regression.
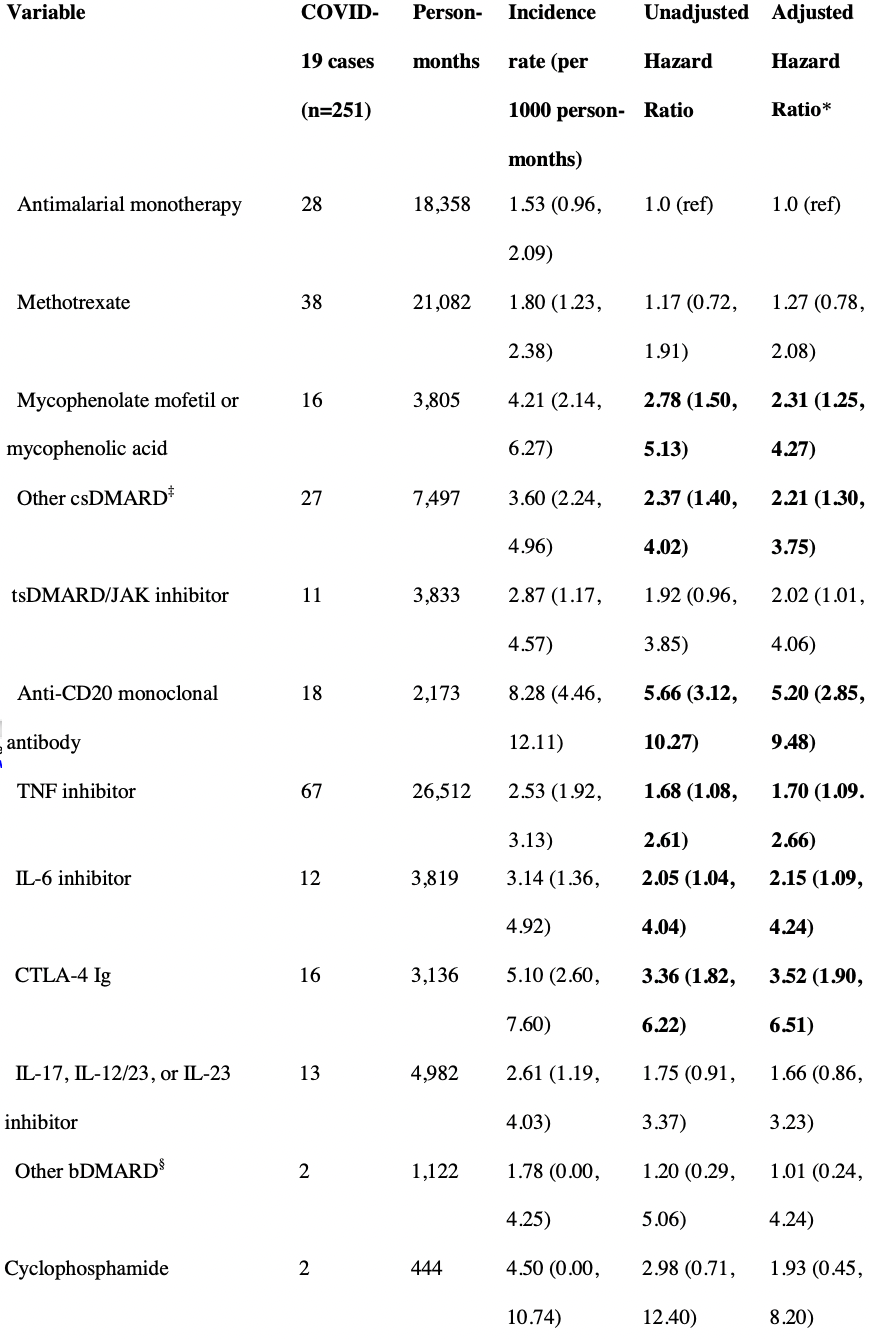
Results: We analyzed 11,468 patients (75% female, mean age 60 years). Compared to antimalarial monotherapy, multiple immunomodulators were associated with higher risk of infection: anti-CD20 monoclonal antibodies (aHR 5.20, 95% CI: 2.85, 9.48), CTLA-4 Ig (aHR 3.52, 95% CI: 1.90, 6.51), mycophenolate (aHR 2.31, 95% CI: 1.25, 4.27), IL-6 inhibitors (aHR 2.15, 95% CI: 1.09, 4.24), JAK inhibitors (aHR 2.02, 95% CI: 1.01, 4.06), and TNF inhibitors (aHR 1.70, 95% CI: 1.09, 2.66). mRNA-1273 recipients had a lower risk of breakthrough infection compared to BNT162b2 recipients (aHR 0.66, 95% CI: 0.50, 0.86). There was no association of sex, body mass index, smoking status, race, or ethnicity with risk of breakthrough infection.
Conclusion: Among patients with rheumatic diseases, multiple immunomodulators were associated with increased risk of breakthrough infection. These results highlight the need for additional mitigation strategies in this vulnerable population. .
week before the Moderna vaccine so Pfizer-BioNTech recipients may have experienced vaccine waning sooner, contributing to the observed higher risk. However, similar observations were made in a study by Hernan et al which rigorously accounted for these differences. 35 Additional studies in the SARD population are needed to determine the potential benefits associated with one vaccine type over another to guide future vaccine strategies in this vulnerable population. The most important way to prevent breakthrough infection is to get vaccinated with an effective vaccine. There were too few subjects who received Johnson & Johnson-Janssen to generate robust results and this vaccine is no longer recommended in the US. Our study has multiple strengths. First, we systematically identified patients with SARDs prescribed immunomodulatory medications prior to vaccination using a rigorous algorithm (90% PPV) applied in a large healthcare system's data warehouse. Second, we identified testconfirmed COVID-19 breakthrough infection using data from both PCR tests as well as antigen tests administered both in the healthcare setting and at home. Third, details regarding comorbidities and medications prescribed were available. Fourth, in contrast to previous studies, over 90% of patients in our cohort received an mRNA-based vaccine, with a similar proportion receiving Pfizer-BioNTech and Moderna. Despite these strengths, our study has certain limitations. First, our outcome of COVID-19..
References
Ahmed, Mehta, Paul, Postvaccination antibody titres predict protection against COVID-19 in patients with autoimmune diseases: survival analysis in a prospective cohort, Ann Rheum Dis
Apostolidis, Kakara, Painter, Cellular and humoral immune responses following SARS-CoV-2 mRNA vaccination in patients with multiple sclerosis on anti-CD20 therapy, Nat Med
Avouac, Drumez, Hachulla, COVID-19 outcomes in patients with inflammatory rheumatic and musculoskeletal diseases treated with rituximab: a cohort study, Lancet Rheumatol
Bajema, Dahl, Evener, Comparative Effectiveness and Antibody Responses to Moderna and Pfizer-BioNTech COVID-19 Vaccines among Hospitalized Veterans -Five Veterans Affairs Medical Centers, United States, February 1, MMWR Morb Mortal Wkly Rep
Boekel, Stalman, Wieske, Breakthrough SARS-CoV-2 infections with the delta (B.1.617.2) variant in vaccinated patients with immune-mediated inflammatory diseases using immunosuppressants: a substudy of two prospective cohort studies, Lancet Rheumatol
Brosh-Nissimov, Orenbuch-Harroch, Chowers, BNT162b2 vaccine breakthrough: clinical characteristics of 152 fully vaccinated hospitalized COVID-19 patients in Israel, Clin Microbiol Infect
Charlson, Pompei, Ales, Mackenzie, A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation, J Chron Dis
Cook, Patel, Silva, Clinical characteristics and outcomes of COVID-19 breakthrough infections among vaccinated patients with systemic autoimmune rheumatic diseases, Ann Rheum Dis
Cook, Patel, Silva, Clinical characteristics and outcomes of COVID-19 breakthrough infections among vaccinated patients with systemic autoimmune rheumatic diseases, Ann Rheum Dis
Cordtz, Kristensen, Westermann, COVID-19 infection and hospitalisation risk according to vaccination status and DMARD treatment in patients with rheumatoid arthritis, Rheumatology
Deepak, Kim, Paley, Glucocorticoids and B Cell Depleting Agents Substantially Impair Immunogenicity of mRNA Vaccines to SARS-CoV-2
Dickerman, Gerlovin, Madenci, Comparative Effectiveness of BNT162b2 and mRNA-1273 Vaccines in U.S. Veterans, N Engl J Med
Fragoulis, Karamanakos, Arida, Clinical outcomes of breakthrough COVID-19
Friedman, Curtis, Winthrop, Impact of disease-modifying antirheumatic drugs on vaccine immunogenicity in patients with inflammatory rheumatic and musculoskeletal diseases, Ann Rheum Dis
Furer, Eviatar, Zisman, Immunogenicity and safety of the BNT162b2 mRNA COVID-19 vaccine in adult patients with autoimmune inflammatory rheumatic diseases and in the general population: a multicentre study, Ann Rheum Dis
Fusco, Moran, Cane, Evaluation of COVID-19 vaccine breakthrough infections among immunocompromised patients fully vaccinated with BNT162b2, J Med Econ
Grainger, Kim, Conway, Yazdany, Robinson, COVID-19 in people with rheumatic diseases: risks, outcomes, treatment considerations, Nat Rev Rheumatol
Haberman, Herati, Simon, Methotrexate hampers immunogenicity to BNT162b2 mRNA COVID-19 vaccine in immune-mediated inflammatory disease, Ann Rheum Dis
Kim, Servi, Polinski, Validation of rheumatoid arthritis diagnoses in health care utilization data, Arthritis Res Ther
Lawson-Tovey, Hyrich, Gossec, SARS-CoV-2 infection after vaccination in patients with inflammatory rheumatic and musculoskeletal diseases, Ann Rheum Dis
Liew, Gianfrancesco, Harrison, Results From the COVID-19 Global Rheumatology Alliance Provider Registry
Liew, Gianfrancesco, Harrison, SARS-CoV-2 breakthrough infections among vaccinated individuals with rheumatic disease: results from the COVID-19 Global Rheumatology Alliance provider registry, RMD Open
Mrak, Tobudic, Koblischke, SARS-CoV-2 vaccination in rituximab-treated patients: B cells promote humoral immune responses in the presence of T-cell-mediated immunity, Ann Rheum Dis
Nalichowski, Keogh, Chueh, Murphy, Calculating the Benefits of a Research Patient Data Repository, AMIA Annu Symp Proc
Paik, Sparks, Kim, Immunogenicity, breakthrough infection, and underlying disease flare after SARS-CoV-2 vaccination among individuals with systemic autoimmune rheumatic diseases, Curr Opin Pharmacol
Serling-Boyd, Silva, Hsu, Coronavirus disease 2019 outcomes among patients with rheumatic diseases 6 months into the pandemic, Ann Rheum Dis
Silva, Boyd, Wallwork, Clinical characteristics and outcomes of patients with coronavirus disease 2019 (COVID-19) and rheumatic disease: a comparative cohort study from a US 'hot spot', Ann Rheum Dis
Simon, Tascilar, Schmidt, Humoral and Cellular Immune Responses to SARS-CoV-2 Infection and Vaccination in Autoimmune Disease Patients With B Cell Depletion, Arthritis Rheumatol
Sparks, Wallace, Seet, Associations of baseline use of biologic or targeted synthetic DMARDs with COVID-19 severity in rheumatoid arthritis: Results from the COVID-19 Global Rheumatology Alliance physician registry, Ann Rheum Dis
Sun, Zheng, Madhira, Association Between Immune Dysfunction and COVID-19
Widdifield, Kwong, Chen, Vaccine effectiveness against SARS-CoV-2 infection and severe outcomes among individuals with immune-mediated inflammatory diseases tested between March 1 and Nov 22, 2021, in Ontario, Canada: a population-based analysis, Lancet Rheumatol
DOI record:
{
"DOI": "10.1101/2022.07.13.22277606",
"URL": "http://dx.doi.org/10.1101/2022.07.13.22277606",
"abstract": "<jats:p>Objective: Rheumatic disease patients on certain immunomodulators are at increased risk of impaired humoral response to SARS-CoV-2 vaccines. We aimed to identify factors associated with breakthrough infection among patients with rheumatic diseases.\nMethods: We identified patients with rheumatic diseases being treated with immunomodulators in a large healthcare system who received at least two doses of either the mRNA-1273 (Moderna) or BNT162b2 (Pfizer-BioNTech) vaccines or one dose of the Johnson & Johnson-Janssen (J&J) vaccine. We followed patients until SARS-CoV-2 infection, death, or December 15, 2021, when the Omicron variant became dominant in our region. We estimated the association of baseline characteristics with the risk of breakthrough infection using multivariable Cox regression.\nResults: We analyzed 11,468 patients (75% female, mean age 60 years). Compared to antimalarial monotherapy, multiple immunomodulators were associated with higher risk of infection: anti-CD20 monoclonal antibodies (aHR 5.20, 95% CI: 2.85, 9.48), CTLA-4 Ig (aHR 3.52, 95% CI: 1.90, 6.51), mycophenolate (aHR 2.31, 95% CI: 1.25, 4.27), IL-6 inhibitors (aHR 2.15, 95% CI: 1.09, 4.24), JAK inhibitors (aHR 2.02, 95% CI: 1.01, 4.06), and TNF inhibitors (aHR 1.70, 95% CI: 1.09, 2.66). mRNA-1273 recipients had a lower risk of breakthrough infection compared to BNT162b2 recipients (aHR 0.66, 95% CI: 0.50, 0.86). There was no association of sex, body mass index, smoking status, race, or ethnicity with risk of breakthrough infection.\nConclusion: Among patients with rheumatic diseases, multiple immunomodulators were associated with increased risk of breakthrough infection. These results highlight the need for additional mitigation strategies in this vulnerable population.</jats:p>",
"accepted": {
"date-parts": [
[
2022,
7,
15
]
]
},
"author": [
{
"affiliation": [],
"family": "Patel",
"given": "Naomi J",
"sequence": "first"
},
{
"affiliation": [],
"family": "Wang",
"given": "Xiaosong",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Fu",
"given": "Xiaoqing",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kawano",
"given": "Yumeko",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cook",
"given": "Claire",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Vanni",
"given": "Kathleen MM",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Qiann",
"given": "Grace",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Banasiak",
"given": "Emily",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kowalski",
"given": "Emily",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zhang",
"given": "Yuqing",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-5556-4618",
"affiliation": [],
"authenticated-orcid": false,
"family": "Sparks",
"given": "Jeffrey A.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wallace",
"given": "Zachary S",
"sequence": "additional"
}
],
"container-title": [],
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2022,
7,
15
]
],
"date-time": "2022-07-15T20:00:32Z",
"timestamp": 1657915232000
},
"deposited": {
"date-parts": [
[
2022,
7,
15
]
],
"date-time": "2022-07-15T20:00:32Z",
"timestamp": 1657915232000
},
"group-title": "Rheumatology",
"indexed": {
"date-parts": [
[
2022,
7,
15
]
],
"date-time": "2022-07-15T20:41:48Z",
"timestamp": 1657917708405
},
"institution": [
{
"name": "medRxiv"
}
],
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2022,
7,
15
]
]
},
"link": [
{
"URL": "https://syndication.highwire.org/content/doi/10.1101/2022.07.13.22277606",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "246",
"original-title": [],
"posted": {
"date-parts": [
[
2022,
7,
15
]
]
},
"prefix": "10.1101",
"published": {
"date-parts": [
[
2022,
7,
15
]
]
},
"publisher": "Cold Spring Harbor Laboratory",
"reference-count": 0,
"references-count": 0,
"relation": {},
"resource": {
"primary": {
"URL": "http://medrxiv.org/lookup/doi/10.1101/2022.07.13.22277606"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subtitle": [],
"subtype": "preprint",
"title": "Factors Associated with COVID-19 Breakthrough Infection in the Pre-Omicron Era Among Vaccinated Patients with Rheumatic Diseases: A Cohort Study",
"type": "posted-content"
}