Effectiveness of Molnupiravir Treatment in Patients with COVID-19 in Korea: A Propensity Score Matched Study
Hye Rim Park, Min-Gyu Yoo, Jong Mu Kim, Soon Jong Bae, Hyungmin Lee, PhD Jungyeon Kim
Infection & Chemotherapy, doi:10.3947/ic.2023.0087
Background: The MOVe-OUT (efficacy and safety of molnupiravir in non-hospitalized adult participants with ) trial reported that the administration of molnupiravir in unvaccinated patients with coronavirus disease 2019 (COVID-19) before the Omicron epidemic showed a preventive effect of 31% against hospitalization and death. However, studies on the preventive effect of molnupiravir against progression to severe disease and death in patients with COVID-19 during the Omicron epidemic are limited. This study aimed to evaluate the preventive effect of molnupiravir against severe/critical illness or death and death in Korean patients with COVID-19 who were vaccinated mostly during the Omicron epidemic.
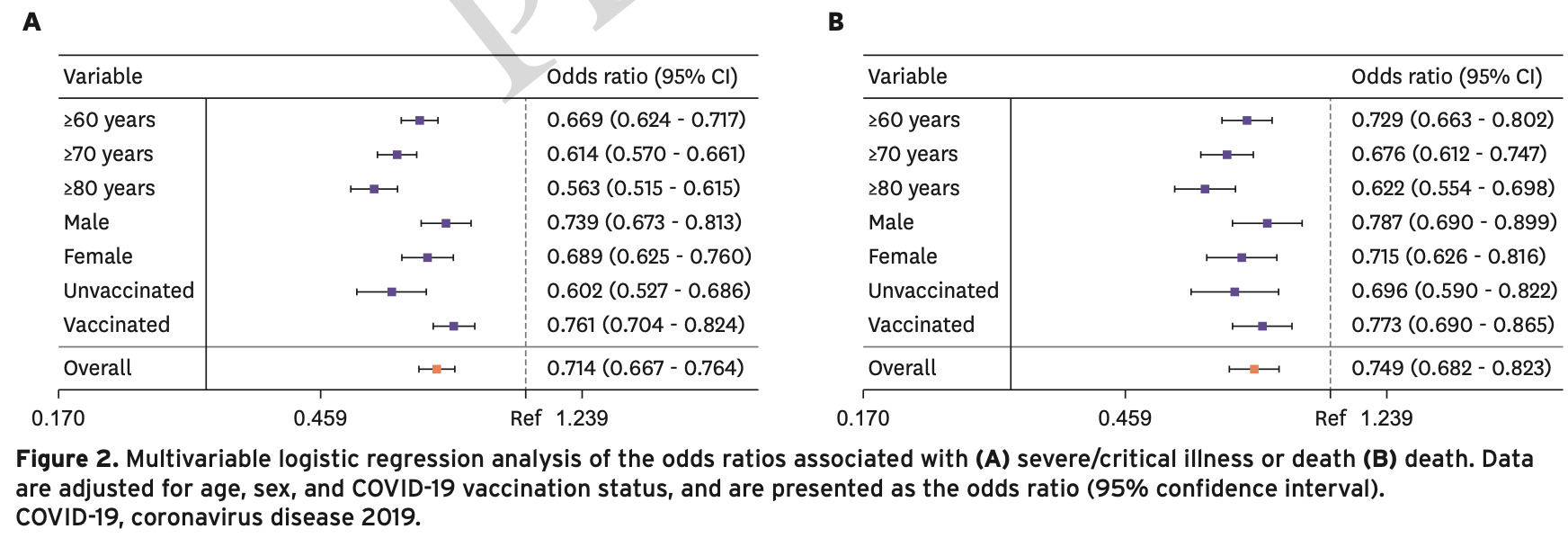
Materials and Methods: This study used large-scale retrospective cohort data to select patients with COVID-19 who were either treated or not treated with molnupiravir, between August 2022 and March 2023, at a ratio of 1 : 4 using the propensity score matching method. In total, 762,768 patients comprised the non-administered group, and 190,692 patients comprised the molnupiravir-administered group. The preventive effect of molnupiravir against severe/ critical illness or death and death was analyzed using logistic regression analysis. Results: The preventive effect of molnupiravir against severe/critical illness or death and death, represented by the odds ratio (OR) and 95% confidence interval (CI), in the molnupiravir-administered and non-administered group was (OR: 0.714; CI: 0.667 -0.764) and (OR: 0.749; CI: 0.682 -0.823), respectively. As age increased, the preventive effect against severe/critical illness or death and death increased. The preventive effect against severe/critical illness or death at ≥60 years was (OR: 0.
Conflict of interest No conflict of interest.
Author Contributions
References
Arbel, Sagy, Hoshen, Battat, Lavie et al., Nirmatrelvir use and severe Covid-19 outcomes during the omicron surge, N Engl J Med,
doi:10.1056/NEJMoa2204919Bajema, Berry, Streja, Rajeevan, Li et al., Effectiveness of COVID-19 treatment with nirmatrelvir-ritonavir or molnupiravir among U.S. Veterans: Target trial emulation studies with one-month and six-month outcomes, Ann Intern Med,
doi:10.7326/M22-3565Bernal, Da Silva, Musungaie, Kovalchuk, Gonzalez et al., Williams-8/10 icjournal
Bestetti, Furlan-Daniel, Silva, Pharmacological Treatment of Patients with Mild to Moderate COVID-19: a comprehensive review, Int J Environ Res Public Health,
doi:10.3390/ijerph18137212Butler, Hobbs, Gbinigie, Rahman, Hayward et al., Molnupiravir plus usual care versus usual care alone as early treatment for adults with COVID-19 at increased risk of adverse outcomes (PANORAMIC): an openlabel, platform-adaptive randomised controlled trial, Lancet,
doi:10.1016/S0140-6736(22)02597-1Diaz, Brown, Du, Pedley, Assaid et al., Molnupiravir for oral treatment of Covid-19 in nonhospitalized patients, N Engl J Med,
doi:10.1056/NEJMoa2116044Fiolet, Kherabi, Macdonald, Ghosn, Peiffer-Smadja, Comparing COVID-19 vaccines for their characteristics, efficacy and effectiveness against SARS-CoV-2 and variants of concern: a narrative review, Clin Microbiol Infect,
doi:10.1016/j.cmi.2021.10.005Guan, Liang, Zhao, Liang, Chen et al., China Medical Treatment Expert Group for COVID-19. Comorbidity and its impact on 1590 patients with COVID-19 in China: a nationwide analysis, Eur Respir J,
doi:10.1183/13993003.00547-2020Hammond, Leister-Tebbe, Gardner, Abreu, Wisemandle et al., Oral nirmatrelvir for high-risk, nonhospitalized adults with Covid-19, N Engl J Med,
doi:10.1056/NEJMoa2118542Hoertel, Boulware, Sánchez-Rico, Burgun, Limosin, Prevalence of contraindications to nirmatrelvir-ritonavir among hospitalized patients with COVID-19 at risk for progression to severe disease, JAMA Netw Open,
doi:10.1001/jamanetworkopen.2022.42140Ito, Piantham, Nishiura, Relative instantaneous reproduction number of Omicron SARS-CoV-2 variant with respect to the Delta variant in Denmark, J Med Virol,
doi:10.1002/jmv.27560Kim, Yoo, Bae, Kim, Lee, Effectiveness of paxlovid, an oral antiviral drug, against the Omicron BA.5 variant in Korea: severe progression and death between July and November 2022, J Korean Med Sci,
doi:10.3346/jkms.2023.38.e211Lv, Lv, Clinical characteristics and analysis of risk factors for disease progression of COVID-19: a retrospective Cohort Study, Int J Biol Sci,
doi:10.7150/ijbs.50654Najjar-Debbiny, Gronich, Weber, Khoury, Amar et al., Effectiveness of molnupiravir in highrisk patients: a propensity score matched analysis, Clin Infect Dis,
doi:10.1093/cid/ciac781Painter, Holman, Bush, Almazedi, Malik et al., Human safety, tolerability, and pharmacokinetics of molnupiravir, a novel broad-spectrum oral antiviral agent with activity against SARS-CoV-2, Antimicrob Agents Chemother,
doi:10.1128/AAC.02428-20Paraskevis, Gkova, Mellou, Gerolymatos, Psalida et al., Real-world effectiveness of molnupiravir and nirmatrelvir/ritonavir as treatments for COVID-19 in high-risk patients, J Infect Dis
Park, Park, Lee, Yu, Song et al., The effectiveness of Paxlovid treatment in long-term care facilities in South Korea during the outbreak of the Omicron variant of SARS-CoV-2, Osong Public Health Res Perspect,
doi:10.24171/j.phrp.2022.0262Rosenbaum, Db, The central role of the propensity score in observational studies for causal effects, Biometrika
Ross, Bortolussi-Courval, Hanula, Lee, Wilson et al., Drug interactions with nirmatrelvirritonavir in older adults using multiple medications, JAMA Netw Open,
doi:10.1001/jamanetworkopen.2022.20184Siddiqi, Mehra, COVID-19 illness in native and immunosuppressed states: a clinical-therapeutic staging proposal, J Heart Lung Transplant,
doi:10.1016/j.healun.2020.03.012Soy, Keser, Atagündüz, Pathogenesis and treatment of cytokine storm in COVID-19, Turk J Biol,
doi:10.3906/biy-2105-37Suzuki, Shibata, Minemura, Nikaido, Tanino et al., Real-world clinical outcomes of treatment with molnupiravir for patients with mild-to-moderate coronavirus disease 2019 during the Omicron variant pandemic, Clin Exp Med,
doi:10.1007/s10238-022-00949-3Takashita, Kinoshita, Yamayoshi, Sakai-Tagawa, Fujisaki et al., Efficacy of antibodies and antiviral drugs against Covid-19 omicron variant, N Engl J Med,
doi:10.1056/NEJMc2119407Takashita, Yamayoshi, Simon, Van Bakel, Sordillo et al., Efficacy of antibodies and antiviral drugs against omicron BA.2.12.1, BA.4, and BA.5 subvariants, N Engl J Med,
doi:10.1056/NEJMc2207519Watson, Barnsley, Toor, Hogan, Winskill et al., Global impact of the first year of COVID-19 vaccination: a mathematical modelling study, Lancet Infect Dis,
doi:10.1016/S1473-3099(22)00320-6Wen, Chen, Tang, Wang, Zhou et al., Efficacy and safety of three new oral antiviral treatment (molnupiravir, fluvoxamine and Paxlovid) for COVID-19:a meta-analysis, Ann Med,
doi:10.1080/07853890.2022.2034936Wong, Au, Lau, Lau, Cowling et al., Real-world effectiveness of early molnupiravir or nirmatrelvirritonavir in hospitalised patients with COVID-19 without supplemental oxygen requirement on admission during Hong Kong's omicron BA.2 wave: a retrospective cohort study, Lancet Infect Dis,
doi:10.1016/S1473-3099(22)00507-2Wong, Au, Lau, Lau, Cowling et al., Real-world effectiveness of molnupiravir and nirmatrelvir plus ritonavir against mortality, hospitalisation, and in-hospital outcomes among community-dwelling, ambulatory patients with confirmed SARS-CoV-2 infection during the omicron wave in Hong Kong: an observational study, Lancet,
doi:10.1016/S0140-6736(22)01586-0Wu, Chen, Cai, Xia, Zhou et al., Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China, JAMA Intern Med,
doi:10.1001/jamainternmed.2020.0994Xie, Bowe, Al-Aly, Molnupiravir and risk of hospital admission or death in adults with covid-19: emulation of a randomized target trial using electronic health records, BMJ,
doi:10.1136/bmj-2022-072705Yang, Zheng, Gou, Pu, Chen et al., Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: a systematic review and meta-analysis, Int J Infect Dis,
doi:10.1016/j.ijid.2020.03.017Ying-Hao, Yuan-Yuan, Dong, Qiu-Hua, Xue-Ran et al., Clinical characteristics and analysis of risk factors for disease progression of patients with SARS-CoV-2 omicron variant infection: a retrospective study of 25207 cases in a Fangcang hospital, Front Cell Infect Microbiol,
doi:10.3389/fcimb.2022.1009894{ 'indexed': { 'date-parts': [[2023, 11, 29]],
'date-time': '2023-11-29T00:23:14Z',
'timestamp': 1701217394293},
'reference-count': 40,
'publisher': 'XMLink',
'license': [ { 'start': { 'date-parts': [[2023, 1, 1]],
'date-time': '2023-01-01T00:00:00Z',
'timestamp': 1672531200000},
'content-version': 'tdm',
'delay-in-days': 0,
'URL': 'https://creativecommons.org/licenses/by-nc/4.0/'}],
'content-domain': {'domain': ['icjournal.org'], 'crossmark-restriction': False},
'DOI': '10.3947/ic.2023.0087',
'type': 'journal-article',
'created': { 'date-parts': [[2023, 11, 23]],
'date-time': '2023-11-23T01:52:36Z',
'timestamp': 1700704356000},
'update-policy': 'http://dx.doi.org/10.3947/crossmark_policy',
'source': 'Crossref',
'is-referenced-by-count': 0,
'title': 'Effectiveness of Molnupiravir Treatment in Patients with COVID-19 in Korea: A Propensity Score '
'Matched Study',
'prefix': '10.3947',
'volume': '55',
'author': [ { 'ORCID': 'http://orcid.org/0000-0002-8719-9387',
'authenticated-orcid': False,
'given': 'Hye Rim',
'family': 'Park',
'sequence': 'first',
'affiliation': [ { 'name': 'Patient Management Team, Central Disease Control Headquarters '
'for COVID-19, Korea Disease Control and Prevention Agency, '
'Cheongju, Korea.'}]},
{ 'ORCID': 'http://orcid.org/0000-0002-8153-6813',
'authenticated-orcid': False,
'given': 'Min-Gyu',
'family': 'Yoo',
'sequence': 'additional',
'affiliation': [ { 'name': 'Patient Management Team, Central Disease Control Headquarters '
'for COVID-19, Korea Disease Control and Prevention Agency, '
'Cheongju, Korea.'},
{ 'name': 'Division of Public Health Emergency Response Research, Bureau of '
'Public Health Emergency Preparedness, Korea Disease Control and '
'Prevention Agency, Cheongju, Korea.'}]},
{ 'ORCID': 'http://orcid.org/0000-0002-5840-6965',
'authenticated-orcid': False,
'given': 'Jong Mu',
'family': 'Kim',
'sequence': 'additional',
'affiliation': [ { 'name': 'Patient Management Team, Central Disease Control Headquarters '
'for COVID-19, Korea Disease Control and Prevention Agency, '
'Cheongju, Korea.'}]},
{ 'ORCID': 'http://orcid.org/0000-0002-9031-4825',
'authenticated-orcid': False,
'given': 'Soon Jong',
'family': 'Bae',
'sequence': 'additional',
'affiliation': [ { 'name': 'Patient Management Team, Central Disease Control Headquarters '
'for COVID-19, Korea Disease Control and Prevention Agency, '
'Cheongju, Korea.'}]},
{ 'ORCID': 'http://orcid.org/0000-0003-3478-3704',
'authenticated-orcid': False,
'given': 'Hyungmin',
'family': 'Lee',
'sequence': 'additional',
'affiliation': [ { 'name': 'Patient Management Team, Central Disease Control Headquarters '
'for COVID-19, Korea Disease Control and Prevention Agency, '
'Cheongju, Korea.'},
{ 'name': 'Division of Emerging Infectious Disease, Bureau of Infectious '
'Disease Risk Response, Korea Disease Control and Prevention '
'Agency, Cheongju, Korea.'},
{ 'name': 'Division of Vaccine-Prevetable Diseases Control and National '
'Immunization Program, Korea Disease Control and Prevention '
'Agency, Cheongju, Korea.'}]},
{ 'ORCID': 'http://orcid.org/0000-0003-2732-1544',
'authenticated-orcid': False,
'given': 'Jungyeon',
'family': 'Kim',
'sequence': 'additional',
'affiliation': [ { 'name': 'Patient Management Team, Central Disease Control Headquarters '
'for COVID-19, Korea Disease Control and Prevention Agency, '
'Cheongju, Korea.'},
{ 'name': 'Division of Emerging Infectious Disease, Bureau of Infectious '
'Disease Risk Response, Korea Disease Control and Prevention '
'Agency, Cheongju, Korea.'},
{ 'name': 'Division of Clinical Research, Center for Emerging Virus '
'Research, Natinal Institute of Infectious Disease, Korea Disease '
'Control and Prevention Agency, Cheongju, Korea.'}]}],
'member': '18617',
'published-online': {'date-parts': [[2023]]},
'reference': [ { 'key': '10.3947/ic.2023.0087_ref1',
'unstructured': 'World Health Organization (WHO). WHO coronavirus (COVID-19) dashboard. '
'Accessed 24 May 2023. Available at: https://covid19.who.int'},
{ 'key': '10.3947/ic.2023.0087_ref2',
'unstructured': 'Korea Disease Control and Prevention Agency (KDCA). Coronavirus disease '
'19 (COVID-19). Accessed 27 May 2023. Available at: '
'https://ncov.kdca.go.kr'},
{ 'key': '10.3947/ic.2023.0087_ref3',
'doi-asserted-by': 'crossref',
'first-page': '2000547',
'DOI': '10.1183/13993003.00547-2020',
'volume': '55',
'author': 'Guan',
'year': '2020',
'journal-title': 'Eur Respir J'},
{ 'key': '10.3947/ic.2023.0087_ref4',
'doi-asserted-by': 'crossref',
'first-page': '91',
'DOI': '10.1016/j.ijid.2020.03.017',
'volume': '94',
'author': 'Yang',
'year': '2020',
'journal-title': 'Int J Infect Dis'},
{ 'key': '10.3947/ic.2023.0087_ref5',
'doi-asserted-by': 'crossref',
'first-page': '934',
'DOI': '10.1001/jamainternmed.2020.0994',
'volume': '180',
'author': 'Wu',
'year': '2020',
'journal-title': 'JAMA Intern Med'},
{ 'key': '10.3947/ic.2023.0087_ref6',
'doi-asserted-by': 'crossref',
'first-page': '2265',
'DOI': '10.1002/jmv.27560',
'volume': '94',
'author': 'Ito',
'year': '2022',
'journal-title': 'J Med Virol'},
{ 'key': '10.3947/ic.2023.0087_ref7',
'doi-asserted-by': 'crossref',
'first-page': '7212',
'DOI': '10.3390/ijerph18137212',
'volume': '18',
'author': 'Bestetti',
'year': '2021',
'journal-title': 'Int J Environ Res Public Health'},
{ 'key': '10.3947/ic.2023.0087_ref8',
'doi-asserted-by': 'crossref',
'first-page': '405',
'DOI': '10.1016/j.healun.2020.03.012',
'volume': '39',
'author': 'Siddiqi',
'year': '2020',
'journal-title': 'J Heart Lung Transplant'},
{ 'key': '10.3947/ic.2023.0087_ref9',
'doi-asserted-by': 'crossref',
'first-page': '1757',
'DOI': '10.1056/NEJMcp2009249',
'volume': '383',
'author': 'Gandhi',
'year': '2020',
'journal-title': 'N Engl J Med'},
{ 'key': '10.3947/ic.2023.0087_ref10',
'doi-asserted-by': 'crossref',
'first-page': '1',
'DOI': '10.7150/ijbs.50654',
'volume': '17',
'author': 'Lv',
'year': '2021',
'journal-title': 'Int J Biol Sci'},
{ 'key': '10.3947/ic.2023.0087_ref11',
'doi-asserted-by': 'crossref',
'first-page': '1009894',
'DOI': '10.3389/fcimb.2022.1009894',
'volume': '12',
'author': 'Ying-Hao',
'year': '2022',
'journal-title': 'Front Cell Infect Microbiol'},
{ 'key': '10.3947/ic.2023.0087_ref12',
'doi-asserted-by': 'crossref',
'first-page': '372',
'DOI': '10.3906/biy-2105-37',
'volume': '45',
'author': 'Soy',
'year': '2021',
'journal-title': 'Turk J Biol'},
{ 'key': '10.3947/ic.2023.0087_ref13',
'doi-asserted-by': 'crossref',
'first-page': '516',
'DOI': '10.1080/07853890.2022.2034936',
'volume': '54',
'author': 'Wen',
'year': '2022',
'journal-title': 'Ann Med'},
{ 'key': '10.3947/ic.2023.0087_ref14',
'doi-asserted-by': 'crossref',
'first-page': '509',
'DOI': '10.1056/NEJMoa2116044',
'volume': '386',
'author': 'Jayk Bernal',
'year': '2022',
'journal-title': 'N Engl J Med'},
{ 'key': '10.3947/ic.2023.0087_ref15',
'doi-asserted-by': 'crossref',
'first-page': '1397',
'DOI': '10.1056/NEJMoa2118542',
'volume': '386',
'author': 'Hammond',
'year': '2022',
'journal-title': 'N Engl J Med'},
{ 'key': '10.3947/ic.2023.0087_ref16',
'doi-asserted-by': 'crossref',
'first-page': '1294',
'DOI': '10.3390/antibiotics10111294',
'volume': '10',
'author': 'Lee',
'year': '2021',
'journal-title': 'Antibiotics (Basel)'},
{ 'key': '10.3947/ic.2023.0087_ref17',
'first-page': 'e02428',
'volume': '65',
'author': 'Painter',
'year': '2021',
'journal-title': 'Antimicrob Agents Chemother'},
{ 'key': '10.3947/ic.2023.0087_ref18',
'unstructured': 'US Food and Drug Administration (FDA). Fact sheet for healthcare '
'providers: Emergency use authorization for Lagevrio™ (molnupiravir) '
'capsules. Accessed 1 February 2023. Available at: '
'https://www.fda.gov/media/155054/download'},
{ 'key': '10.3947/ic.2023.0087_ref19',
'unstructured': 'Korea Disease Control and Prevention Agency (KDCA). Guide to use of '
'coronavirus infectious diseases-19 treatment in South Korea (edition '
'11). Accessed 1 September 2023. Available at: '
'https://ncov.kdca.go.kr/extendedCareBoardList.jsp?brdId=8&brdGubun=81'},
{ 'key': '10.3947/ic.2023.0087_ref20',
'doi-asserted-by': 'crossref',
'first-page': '995',
'DOI': '10.1056/NEJMc2119407',
'volume': '386',
'author': 'Takashita',
'year': '2022',
'journal-title': 'N Engl J Med'},
{ 'key': '10.3947/ic.2023.0087_ref21',
'doi-asserted-by': 'crossref',
'first-page': '468',
'DOI': '10.1056/NEJMc2207519',
'volume': '387',
'author': 'Takashita',
'year': '2022',
'journal-title': 'N Engl J Med'},
{ 'key': '10.3947/ic.2023.0087_ref22',
'doi-asserted-by': 'crossref',
'first-page': '790',
'DOI': '10.1056/NEJMoa2204919',
'volume': '387',
'author': 'Arbel',
'year': '2022',
'journal-title': 'N Engl J Med'},
{ 'key': '10.3947/ic.2023.0087_ref23',
'doi-asserted-by': 'crossref',
'first-page': '281',
'DOI': '10.1016/S0140-6736(22)02597-1',
'volume': '401',
'author': 'Butler',
'year': '2023',
'journal-title': 'Lancet'},
{ 'key': '10.3947/ic.2023.0087_ref24',
'doi-asserted-by': 'crossref',
'first-page': '453',
'DOI': '10.1093/cid/ciac781',
'volume': '76',
'author': 'Najjar-Debbiny',
'year': '2023',
'journal-title': 'Clin Infect Dis'},
{ 'key': '10.3947/ic.2023.0087_ref25',
'doi-asserted-by': 'crossref',
'first-page': '1681',
'DOI': '10.1016/S1473-3099(22)00507-2',
'volume': '22',
'author': 'Wong',
'year': '2022',
'journal-title': 'Lancet Infect Dis'},
{ 'key': '10.3947/ic.2023.0087_ref26',
'doi-asserted-by': 'crossref',
'first-page': '1213',
'DOI': '10.1016/S0140-6736(22)01586-0',
'volume': '400',
'author': 'Wong',
'year': '2022',
'journal-title': 'Lancet'},
{ 'key': '10.3947/ic.2023.0087_ref27',
'doi-asserted-by': 'crossref',
'first-page': '807',
'DOI': '10.7326/M22-3565',
'volume': '176',
'author': 'Bajema',
'year': '2023',
'journal-title': 'Ann Intern Med'},
{ 'key': '10.3947/ic.2023.0087_ref28',
'doi-asserted-by': 'crossref',
'first-page': 'e072705',
'DOI': '10.1136/bmj-2022-072705',
'volume': '380',
'author': 'Xie',
'year': '2023',
'journal-title': 'BMJ'},
{ 'key': '10.3947/ic.2023.0087_ref29',
'doi-asserted-by': 'crossref',
'first-page': '2715',
'DOI': '10.1007/s10238-022-00949-3',
'volume': '23',
'author': 'Suzuki',
'year': '2023',
'journal-title': 'Clin Exp Med'},
{ 'key': '10.3947/ic.2023.0087_ref30',
'doi-asserted-by': 'crossref',
'first-page': '443',
'DOI': '10.24171/j.phrp.2022.0262',
'volume': '13',
'author': 'Park',
'year': '2022',
'journal-title': 'Osong Public Health Res Perspect'},
{ 'key': '10.3947/ic.2023.0087_ref31',
'doi-asserted-by': 'crossref',
'first-page': '41',
'DOI': '10.1093/biomet/70.1.41',
'volume': '70',
'author': 'Rosenbaum',
'year': '1983',
'journal-title': 'Biometrika'},
{ 'key': '10.3947/ic.2023.0087_ref32',
'unstructured': 'Korea Disease Control and Prevention Agency (KDCA). Coronavirus disease '
'2019 response and management guidelines for local governments in South '
'Korea (edition 13-1). Accessed 22 August 2023. Available at: '
'https://ncov.kdca.go.kr/duBoardList.do?brdId=2&brdGubun=28'},
{ 'key': '10.3947/ic.2023.0087_ref33',
'doi-asserted-by': 'crossref',
'first-page': '202',
'DOI': '10.1016/j.cmi.2021.10.005',
'volume': '28',
'author': 'Fiolet',
'year': '2022',
'journal-title': 'Clin Microbiol Infect'},
{ 'key': '10.3947/ic.2023.0087_ref34',
'doi-asserted-by': 'crossref',
'first-page': '1293',
'DOI': '10.1016/S1473-3099(22)00320-6',
'volume': '22',
'author': 'Watson',
'year': '2022',
'journal-title': 'Lancet Infect Dis'},
{ 'key': '10.3947/ic.2023.0087_ref35',
'unstructured': 'Our world in Data. Share of people who received at least one dose of '
'COVID-19 vaccine. Accessed 1 February 2023. Available at: '
'https://ourworldindata.org/grapher/share-people-vaccinated-covid'},
{ 'key': '10.3947/ic.2023.0087_ref36',
'doi-asserted-by': 'crossref',
'first-page': 'e2242140',
'DOI': '10.1001/jamanetworkopen.2022.42140',
'volume': '5',
'author': 'Hoertel',
'year': '2022',
'journal-title': 'JAMA Netw Open'},
{ 'key': '10.3947/ic.2023.0087_ref37',
'doi-asserted-by': 'crossref',
'first-page': 'e2220184',
'DOI': '10.1001/jamanetworkopen.2022.20184',
'volume': '5',
'author': 'Ross',
'year': '2022',
'journal-title': 'JAMA Netw Open'},
{ 'key': '10.3947/ic.2023.0087_ref38',
'unstructured': 'Korea Disease Control and Prevention Agency (KDCA). Press reference '
'data. Accessed 29 December 2022. Available at: '
'https://www.kdca.go.kr/board/board.es?mid=a20501010000&bid=0015&list_no=721541&cg_code=&act=view&nPage=16'},
{ 'key': '10.3947/ic.2023.0087_ref39',
'doi-asserted-by': 'crossref',
'first-page': 'e211',
'DOI': '10.3346/jkms.2023.38.e211',
'volume': '38',
'author': 'Kim',
'year': '2023',
'journal-title': 'J Korean Med Sci'},
{ 'key': '10.3947/ic.2023.0087_ref40',
'doi-asserted-by': 'crossref',
'first-page': 'jiad324',
'author': 'Paraskevis',
'year': '2023',
'journal-title': 'J Infect Dis',
'DOI': '10.1093/infdis/jiad324'}],
'container-title': 'Infection & Chemotherapy',
'original-title': [],
'language': 'en',
'link': [ { 'URL': 'https://icjournal.org/pdf/10.3947/ic.2023.0087',
'content-type': 'application/pdf',
'content-version': 'vor',
'intended-application': 'text-mining'},
{ 'URL': 'https://icjournal.org/DOIx.php?id=10.3947/ic.2023.0087',
'content-type': 'unspecified',
'content-version': 'vor',
'intended-application': 'text-mining'},
{ 'URL': 'https://icjournal.org/DOIx.php?id=10.3947/ic.2023.0087',
'content-type': 'unspecified',
'content-version': 'vor',
'intended-application': 'similarity-checking'}],
'deposited': { 'date-parts': [[2023, 11, 28]],
'date-time': '2023-11-28T04:34:30Z',
'timestamp': 1701146070000},
'score': 1,
'resource': {'primary': {'URL': 'https://icjournal.org/DOIx.php?id=10.3947/ic.2023.0087'}},
'subtitle': [],
'short-title': [],
'issued': {'date-parts': [[2023]]},
'references-count': 40,
'URL': 'http://dx.doi.org/10.3947/ic.2023.0087',
'relation': {},
'ISSN': ['2093-2340', '2092-6448'],
'subject': ['Pharmacology (medical)', 'Infectious Diseases'],
'container-title-short': 'Infect Chemother',
'published': {'date-parts': [[2023]]},
'assertion': [ { 'value': '2023-09-13',
'name': 'received',
'label': 'Received',
'group': {'name': 'publication_history', 'label': 'Publication History'}},
{ 'value': '2023-11-10',
'name': 'accepted',
'label': 'Accepted',
'group': {'name': 'publication_history', 'label': 'Publication History'}},
{ 'value': '2023-11-22',
'name': 'published_online',
'label': 'Published online',
'group': {'name': 'publication_history', 'label': 'Publication History'}},
{ 'value': 'Copyright © 2023 by The Korean Society of Infectious Diseases, Korean Society '
'for Antimicrobial Therapy, and The Korean Society for AIDS',
'name': 'copyright',
'label': 'Copyright',
'group': {'name': 'Copyright_and_licensing', 'label': 'Copyright and Licensing'}},
{ 'value': 'This is an Open Access article distributed under the terms of the Creative '
'Commons Attribution Non-Commercial License '
'(https://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted '
'non-commercial use, distribution, and reproduction in any medium, provided the '
'original work is properly cited.',
'name': 'license',
'label': 'License',
'explanation': {'URL': 'https://creativecommons.org/licenses/by-nc/4.0/'},
'group': {'name': 'Copyright_and_licensing', 'label': 'Copyright and Licensing'}}]}